An Automated Imaging-Based Screen for Genetic Modulators of ER Organisation in Cultured Human Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
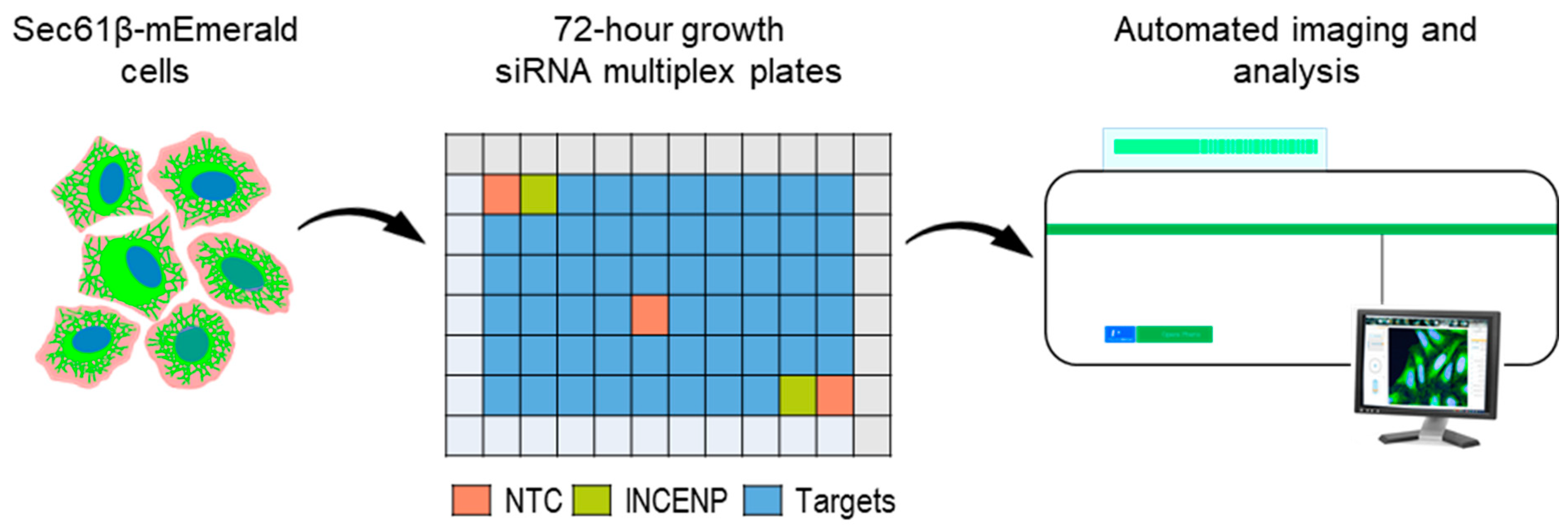
2.2. Multiplex siRNA Screen
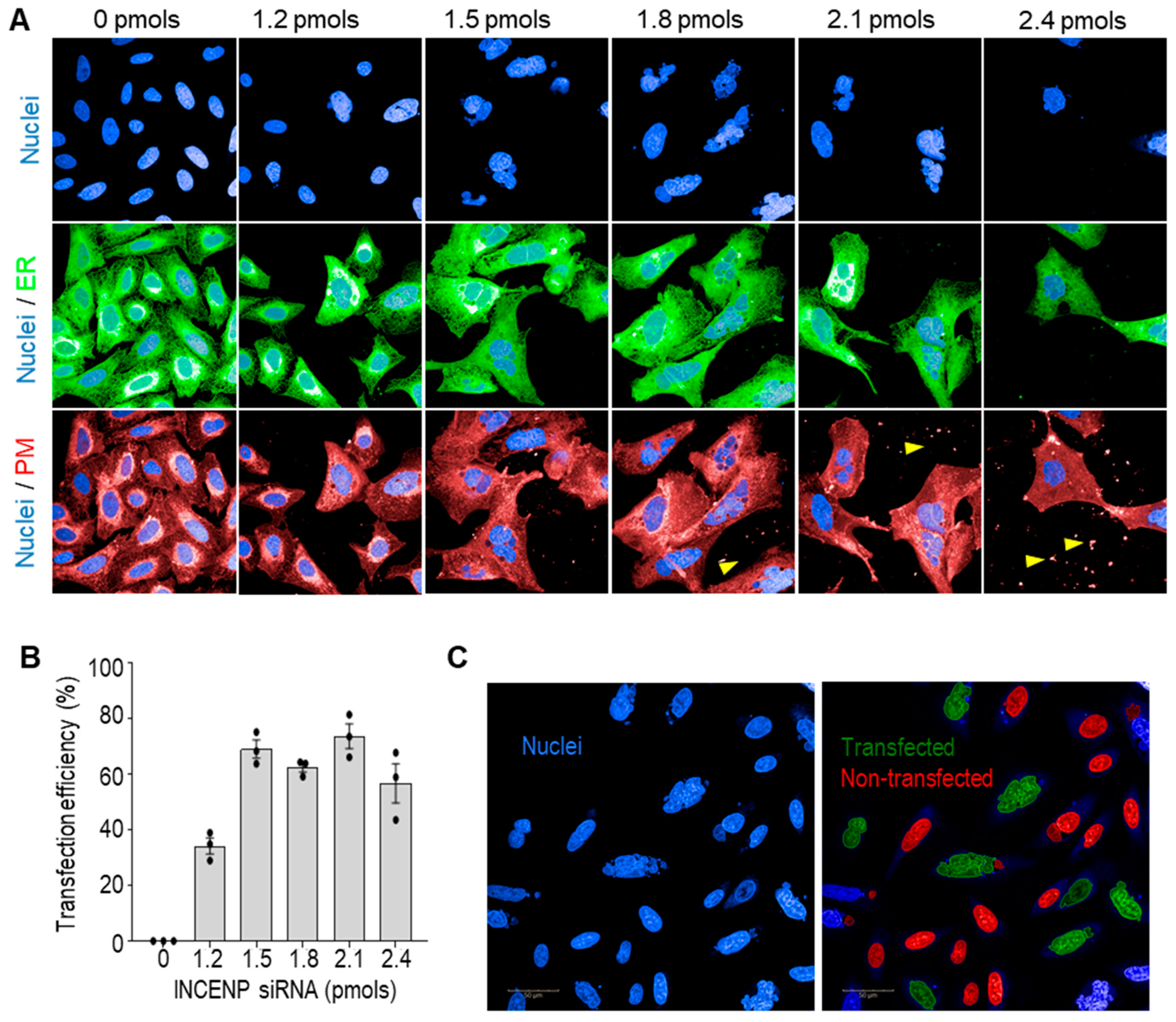
2.2.1. Preparation of Solid-Phase Transfection siRNA Assay Plates
2.2.2. siRNA Screen
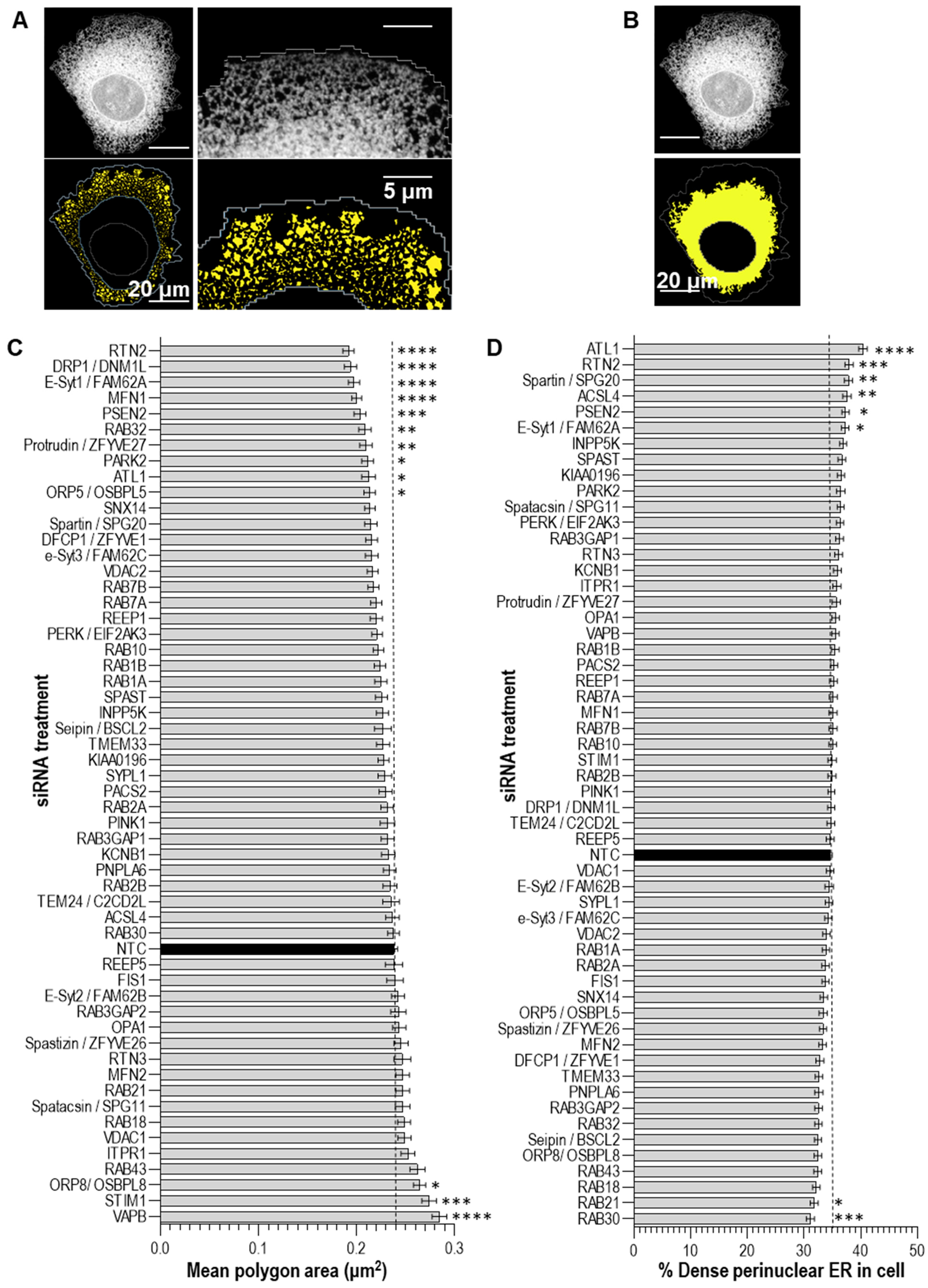
2.2.3. Automated Confocal Imaging and ER Analysis
2.2.4. Liquid-Phase siRNA Transfection
2.3. Quantitative PCR
2.4. Statistical Analysis
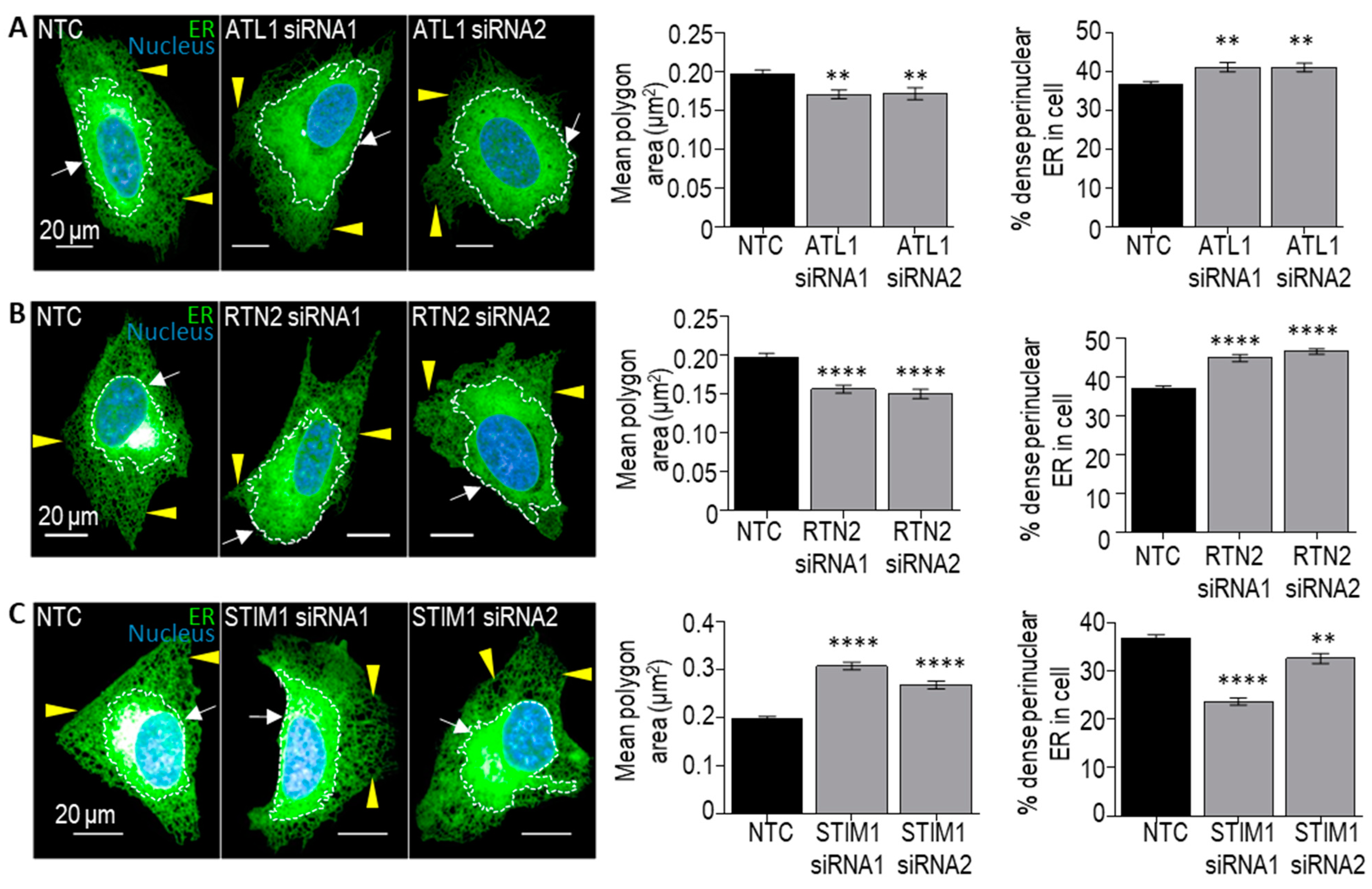
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackstone, C. Converging Cellular Themes for the Hereditary Spastic Paraplegias. Curr. Opin. Neurobiol. 2018, 51, 139–146. [Google Scholar] [CrossRef]
- Meyyazhagan, A.; Orlacchio, A. Hereditary Spastic Paraplegia: An Update. Int. J. Mol. Sci. 2022, 23, 1697. [Google Scholar] [CrossRef]
- Shibata, Y.; Hu, J.; Kozlov, M.M.; Rapoport, T.A. Mechanisms Shaping the Membranes of Cellular Organelles. Annu. Rev. Cell Dev. Biol. 2009, 25, 329–354. [Google Scholar] [CrossRef]
- Voeltz, G.K.; Prinz, W.A.; Shibata, Y.; Rist, J.M.; Rapoport, T.A. A Class of Membrane Proteins Shaping the Tubular Endoplasmic Reticulum. Cell 2006, 124, 573–586. [Google Scholar] [CrossRef]
- Hu, J.; Shibata, Y.; Voss, C.; Shemesh, T.; Li, Z.; Coughlin, M.; Kozlov, M.M.; Rapoport, T.A.; Prinz, W.A. Membrane Proteins of the Endoplasmic Reticulum Induce High-Curvature Tubules. Science 2008, 319, 1247–1250. [Google Scholar] [CrossRef]
- Yang, Y.S.; Strittmatter, S.M. The Reticulons: A Family of Proteins with Diverse Functions. Genome Biol. 2007, 8, 234. [Google Scholar] [CrossRef]
- Rao, K.; Stone, M.C.; Weiner, A.T.; Gheres, K.W.; Zhou, C.; Deitcher, D.L.; Levitan, E.S.; Rolls, M.M. Spastin, Atlastin, and ER Relocalization Are Involved in Axon but Not Dendrite Regeneration. Mol. Biol. Cell 2016, 27, 3245–3256. [Google Scholar] [CrossRef]
- Rizo, T.; Gebhardt, L.; Riedlberger, J.; Eberhardt, E.; Fester, L.; Alansary, D.; Winkler, J.; Turan, S.; Arnold, P.; Niemeyer, B.A.; et al. Store-Operated Calcium Entry Is Reduced in Spastin-Linked Hereditary Spastic Paraplegia. Brain 2022, 145, 3131–3146. [Google Scholar] [CrossRef]
- Van Tienhoven, M.; Atkins, J.; Li, Y.; Glynn, P. Human Neuropathy Target Esterase Catalyzes Hydrolysis of Membrane Lipids. J. Biol. Chem. 2002, 277, 20942–20948. [Google Scholar] [CrossRef]
- Hou, W.-Y.; Song, X.; Wang, Y.; Chang, P.; Chen, R.; Wu, Y.-J. The Alteration of the Expression Level of Neuropathy Target Esterase in Human Neuroblastoma SK-N-SH Cells Disrupts Cellular Phospholipids Homeostasis. Toxicol. Vitr. 2023, 86, e105509. [Google Scholar] [CrossRef]
- Wang, H.; Becuwe, M.; Housden, B.E.; Chitraju, C.; Porras, A.J.; Graham, M.M.; Liu, X.N.; Thiam, A.R.; Savage, D.B.; Agarwal, A.K.; et al. Seipin Is Required for Converting Nascent to Mature Lipid Droplets. eLife 2016, 5, e16582. [Google Scholar] [CrossRef]
- Zoni, V.; Khaddaj, R.; Lukmantara, I.; Shinoda, W.; Yang, H.; Schneiter, R.; Vanni, S. Seipin Accumulates and Traps Diacylglycerols and Triglycerides in Its Ring-like Structure. Proc. Natl. Acad. Sci. USA 2021, 118, e2017205118. [Google Scholar] [CrossRef]
- Eastman, S.W.; Yassaee, M.; Bieniasz, P.D. A Role for Ubiquitin Ligases and Spartin/SPG20 in Lipid Droplet Turnover. J. Cell Biol. 2009, 184, 881–894. [Google Scholar] [CrossRef]
- Renvoisé, B.; Stadler, J.; Singh, R.; Bakowska, J.C.; Blackstone, C. Spg20−/− Mice Reveal Multimodal Functions for Troyer Syndrome Protein Spartin in Lipid Droplet Maintenance, Cytokinesis and BMP Signaling. Hum. Mol. Genet. 2012, 21, 3604–3618. [Google Scholar] [CrossRef]
- Byrne, D.J.; Garcia-Pardo, M.E.; Cole, N.B.; Batnasan, B.; Heneghan, S.; Sohail, A.; Blackstone, C.; O’Sullivan, N.C. Liver X Receptor-Agonist Treatment Rescues Degeneration in a Drosophila Model of Hereditary Spastic Paraplegia. Acta Neuropathol. Commun. 2022, 10, 40. [Google Scholar] [CrossRef]
- Mou, Y.; Dong, Y.; Chen, Z.; Denton, K.R.; Duff, M.O.; Blackstone, C.; Zhang, S.-C.; Li, X.-J. Impaired Lipid Metabolism in Astrocytes Underlies Degeneration of Cortical Projection Neurons in Hereditary Spastic Paraplegia. Acta Neuropathol. Commun. 2020, 8, 214. [Google Scholar] [CrossRef]
- Synofzik, M.; Rugarli, E.; Reid, E.; Schüle, R. Ataxia and Spastic Paraplegia in Mitochondrial Disease. In Handbook of Clinical Neurology; Horvath, R., Hirano, M., Chinnery, P.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 194, Chapter 6; pp. 79–98. [Google Scholar]
- Denton, K.; Mou, Y.; Xu, C.C.; Shah, D.; Chang, J.; Blackstone, C.; Li, X.J. Impaired Mitochondrial Dynamics Underlie Axonal Defects in Hereditary Spastic Paraplegias. Hum. Mol. Genet. 2018, 27, 2517–2530. [Google Scholar] [CrossRef]
- Fowler, P.C.; O’Sullivan, N.C. ER-Shaping Proteins Are Required for ER and Mitochondrial Network Organization in Motor Neurons. Hum. Mol. Genet. 2016, 25, 2827–2837. [Google Scholar] [CrossRef]
- Zhu, P.P.; Hung, H.F.; Batchenkova, N.; Nixon-Abell, J.; Henderson, J.; Zheng, P.; Renvoisé, B.; Pang, S.; Xu, C.S.; Saalfeld, S.; et al. Transverse Endoplasmic Reticulum Expansion in Hereditary Spastic Paraplegia Corticospinal Axons. Hum. Mol. Genet. 2022, 31, 2779–2795. [Google Scholar] [CrossRef]
- Liu, X.; Guo, X.; Niu, L.; Li, X.; Sun, F.; Hu, J.; Wang, X.; Shen, K. Atlastin-1 Regulates Morphology and Function of Endoplasmic Reticulum in Dendrites. Nat. Commun. 2019, 10, 568. [Google Scholar] [CrossRef]
- Marchesi, L.T.; Leblanc, M.; Stevanin, G. Current Knowledge of Endolysosomal and Autophagy Defects in Hereditary Spastic Paraplegia. Cells 2021, 10, 1678. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Lee, S.; Blackstone, C. Spastic Paraplegia Proteins Spastizin and Spatacsin Mediate Autophagic Lysosome Reformation. J. Clin. Investig. 2014, 124, 5249–5262. [Google Scholar] [CrossRef]
- Khundadze, M.; Kollmann, K.; Koch, N.; Biskup, C.; Nietzsche, S. A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/ SPASTIZIN for the Endolysosomal System. PLoS Genet. 2013, 9, e1003988. [Google Scholar] [CrossRef] [PubMed]
- Renvoisé, B.; Chang, J.; Singh, R.; Yonekawa, S.; FitzGibbon, E.J.; Mankodi, A.; Vanderver, A.; Schindler, A.B.; Toro, C.; Gahl, W.A.; et al. Lysosomal Abnormalities in Hereditary Spastic Paraplegia Types SPG15 and SPG11. Ann. Clin. Transl. Neurol. 2014, 1, 379–389. [Google Scholar] [CrossRef]
- Allison, R.; Edgar, J.R.; Pearson, G.; Rizo, T.; Newton, T.; Günther, S.; Berner, F.; Hague, J.; Connell, J.W.; Winkler, J.; et al. Defects in ER-Endosome Contacts Impact Lysosome Function in Hereditary Spastic Paraplegia. J. Cell Biol. 2017, 216, 1337–1355. [Google Scholar] [CrossRef]
- Fowler, P.C.; Garcia-Pardo, M.E.; Simpson, J.C.; O’Sullivan, N.C. NeurodegenERation: The Central Role for ER Contacts in Neuronal Function and Axonopathy, Lessons From Hereditary Spastic Paraplegias and Related Diseases. Front. Neurosci. 2019, 13, 1051. [Google Scholar] [CrossRef]
- Westrate, L.M.; Lee, J.E.; Prinz, W.A.; Voeltz, G.K. Form Follows Function: The Importance of Endoplasmic Reticulum Shape. Annu. Rev. Biochem. 2015, 84, 791–811. [Google Scholar] [CrossRef]
- Shibata, Y.; Voeltz, G.K.; Rapoport, T.A. Rough Sheets and Smooth Tubules. Cell 2006, 126, 435–439. [Google Scholar] [CrossRef]
- Voeltz, G.K.; Prinz, W.A. Sheets, Ribbons and Tubules—How Organelles Get Their Shape. Nat. Rev. Mol. Cell Biol. 2007, 8, 258–264. [Google Scholar] [CrossRef]
- Garcia-Pardo, M.E.; Simpson, J.C.; O’Sullivan, N.C. A Novel Automated Image Analysis Pipeline for Quantifying Morphological Changes to the Endoplasmic Reticulum in Cultured Human Cells. BMC Bioinform. 2021, 22, 427. [Google Scholar] [CrossRef]
- Zheng, P.; Chen, Q.; Tian, X.; Qian, N.; Chai, P.; Liu, B.; Hu, J.; Blackstone, C.; Zhu, D.; Teng, J.; et al. DNA Damage Triggers Tubular Endoplasmic Reticulum Extension to Promote Apoptosis by Facilitating ER-Mitochondria Signaling. Cell Res. 2018, 28, 833–854. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.; Simpson, J.C. High-Content Screening and Analysis of the Golgi Complex. Methods Cell Biol. 2013, 118, 281–295. [Google Scholar] [PubMed]
- Erfle, H.; Neumann, B.; Rogers, P.; Bulkescher, J.; Ellenberg, J.; Pepperkok, R. Work Flow for Multiplexing SiRNA Assays by Solid-Phase Reverse Transfection in Multiwell Plates. SLAS Discov. 2008, 13, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.A.; Heck, M.M.S.; Earnshaw, W.C. The Inner Centromere Protein (INCENP) Antigens: Movement from Inner Centromere to Midbody during Mitosis. J. Cell Biol. 1987, 105, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Mansoury, M.; Hamed, M.; Karmustaji, R.; Al Hannan, F.; Safrany, S.T. The Edge Effect: A Global Problem. The Trouble with Culturing Cells in 96-Well Plates. Biochem. Biophys. Rep. 2021, 26, e100987. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, I.; Gouveia, S.M.; van der Vaart, B.; Demmers, J.; Smyth, J.T.; Honnappa, S.; Splinter, D.; Steinmetz, M.O.; Putney, J.W.; Hoogenraad, C.C.; et al. STIM1 Is a MT-plus-End-Tracking Protein Involved in Remodeling of the ER. Curr. Biol. 2008, 18, 177–182. [Google Scholar] [CrossRef]
- Pavez, M.; Thompson, A.C.; Arnott, H.J.; Mitchell, C.B.; DAtri, I.; Don, E.K.; Chilton, J.K.; Scott, E.K.; Lin, J.Y.; Young, K.M.; et al. STIM1 Is Required for Remodeling of the Endoplasmic Reticulum and Microtubule Cytoskeleton in Steering Growth Cones. J. Neurosci. 2019, 39, 5095–5114. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Pardo, M.E.; Simpson, J.C.; O’Sullivan, N.C. An Automated Imaging-Based Screen for Genetic Modulators of ER Organisation in Cultured Human Cells. Cells 2024, 13, 577. https://doi.org/10.3390/cells13070577
Garcia-Pardo ME, Simpson JC, O’Sullivan NC. An Automated Imaging-Based Screen for Genetic Modulators of ER Organisation in Cultured Human Cells. Cells. 2024; 13(7):577. https://doi.org/10.3390/cells13070577
Chicago/Turabian StyleGarcia-Pardo, M. Elena, Jeremy C. Simpson, and Niamh C. O’Sullivan. 2024. "An Automated Imaging-Based Screen for Genetic Modulators of ER Organisation in Cultured Human Cells" Cells 13, no. 7: 577. https://doi.org/10.3390/cells13070577






