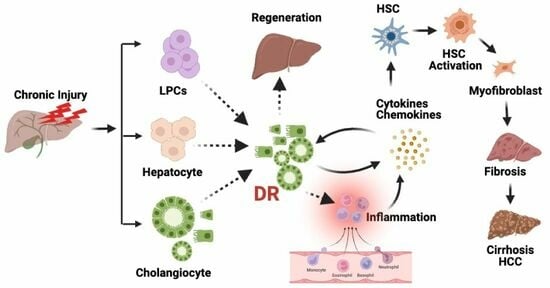
Ductular Reactions in Liver Injury, Regeneration, and Disease Progression—An Overview
Abstract
:1. Introduction
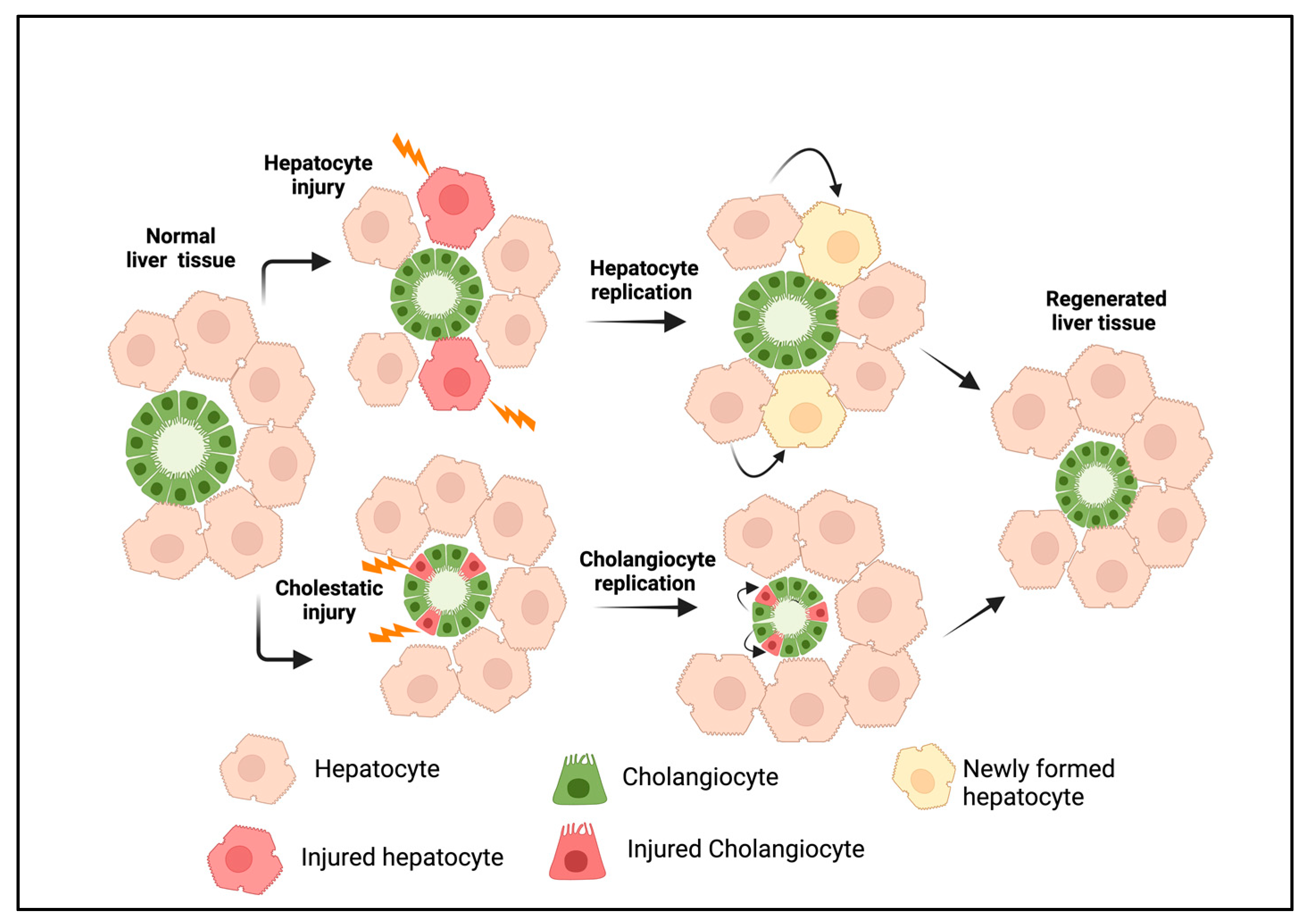
2. Origin and Cellular Heterogeneity in DRs
2.1. Cholangiocytes
2.2. Hepatocyte-Derived Cholangiocytes
2.3. Liver Progenitor Cells
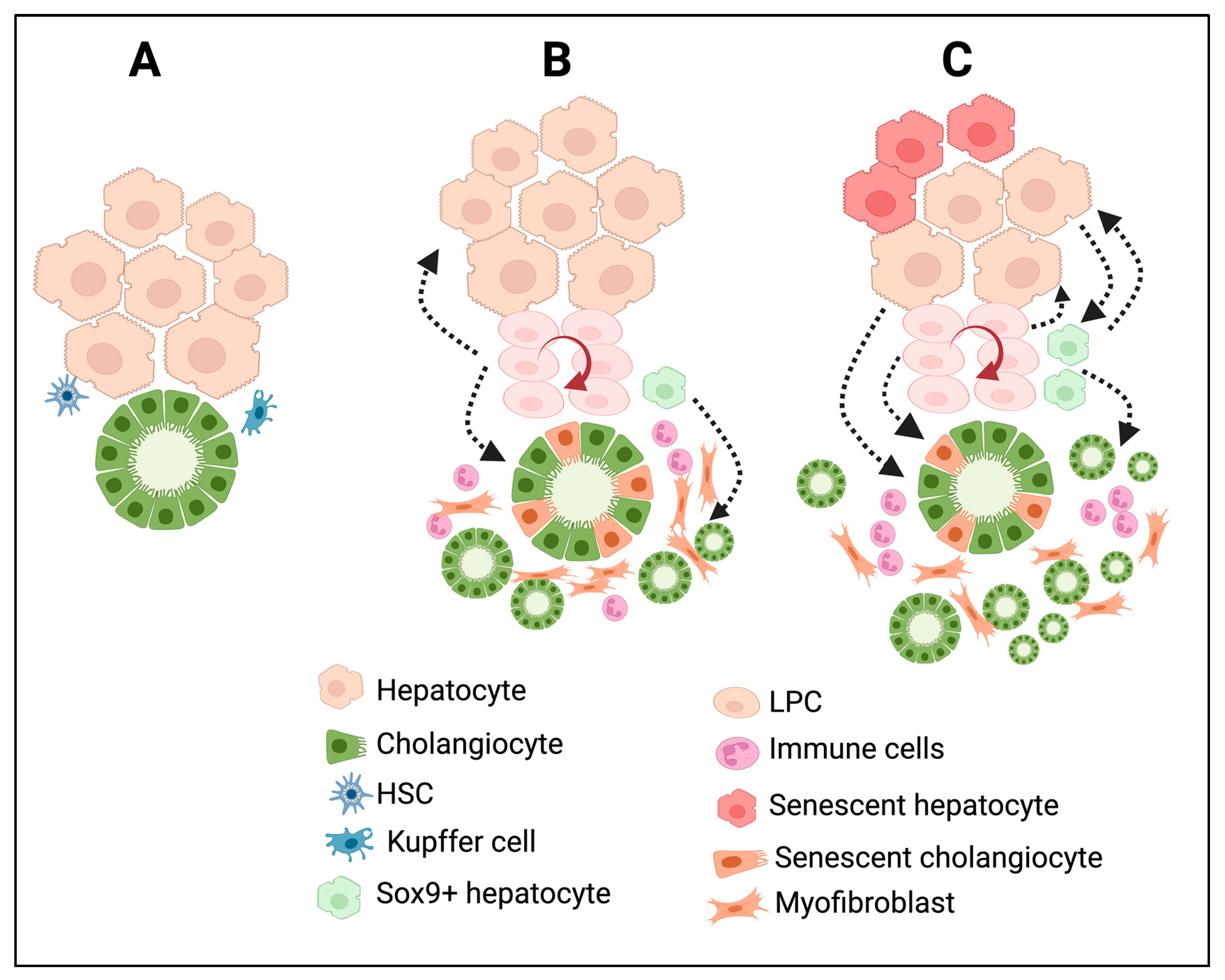
3. Types of Ductular Reactions
4. Experimental Liver Disease Models Associated with DRs
| Experimental Model | Relevant Human Disease Phenotype | References |
|---|---|---|
| Thioacetamide (TAA) | Liver fibrosis, cirrhosis, HCC, CCA | [81] |
| Bile duct ligation | Obstructive cholestasis, fibrosis | [83,84] |
| 3,5-Diethoxycarbonyl-1,4-dihydrocollidine-diet-induced (DDC) diet | Primary sclerosing cholangitis | [85,86] |
| CCl4 injection | Fibrosis, cirrhosis | [87] |
| Mdr2 knockout mice | Primary sclerosing cholangitis | [88,89] |
| Rhesus rotavirus (RRV) infection | Biliary atresia | [90,91] |
| Diethyl nitrosamine (DEN) | Liver fibrosis, cirrhosis, HCC | [92] |
| Methionine–choline-deficient (MCD) diet | Steatohepatitis | [82,93] |
| Choline-deficient, ethionine-supplemented (CDE) diet | Steatohepatitis | [94,95] |
5. Molecular Regulation of DRs
| Pathway | Injury Model | Effect on DRs | References |
|---|---|---|---|
| Notch | BDL, DDC, MCD | ↑ | [49,93,140] |
| cAMP/PKA | BDL | ↑ | [111,157] |
| Secretin–secretin receptor | BDL | ↑ | [112,113] |
| YAP | DDC, Mdr2−/−, CCl4 | ↑ | [95,132,158] |
| IL6R/HGF | BDL | ↑ | [106] |
| Serotonin | BDL | ↓ | [107] |
| 5HT/5HTR2A/2B/2C axis | BDL, Mdr2−/− | ↑ | [108] |
| TGF-beta | Mdr2−/− | ↑ | [119] |
| FXR | Mdr2−/−, DDC | ↑ | [120,159] |
| EGFR | DDC | ↑ | [126] |
| COX2/TGF beta | TAA | ↑ | [145] |
| CNN1/αvβ3/NfkB/Notch | BDL | ↑ | [160] |
| Hedgehog | BDL, CCl4 | ↑ | [158,161] |
| TWEAK/Fn14 pathway | CDE, RRV | ↑ | [134,162] |
| IL-33 | RRV | ↑ | [163] |
6. Translational Significance of DRs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Stanger, B.Z. Cellular homeostasis and repair in the mammalian liver. Annu. Rev. Physiol. 2015, 77, 179–200. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Liver regeneration. J. Cell Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017, 65, 1384–1392. [Google Scholar] [CrossRef]
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011, 458, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Roskams, T.A.; Theise, N.D.; Balabaud, C.; Bhagat, G.; Bhathal, P.S.; Bioulac-Sage, P.; Brunt, E.M.; Crawford, J.M.; Crosby, H.A.; Desmet, V.; et al. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology 2004, 39, 1739–1745. [Google Scholar] [CrossRef]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
- Mack, C.L.; Sokol, R.J. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr. Res. 2005, 57 Pt 2, 87R–94R. [Google Scholar] [CrossRef] [PubMed]
- Marakovits, C.; Francis, H. Unraveling the complexities of fibrosis and ductular reaction in liver disease: Pathogenesis, mechanisms, and therapeutic insights. Am. J. Physiol. Cell Physiol. 2024, 326, C698–C706. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.; Kronfli, R.; Makin, E. Advances in understanding of biliary atresia pathogenesis and progression—A riddle wrapped in a mystery inside an enigma. Expert. Rev. Gastroenterol. Hepatol. 2023, 17, 343–352. [Google Scholar] [CrossRef]
- Mavila, N.; James, D.; Shivakumar, P.; Nguyen, M.V.; Utley, S.; Mak, K.; Wu, A.; Zhou, S.; Wang, L.; Vendyres, C.; et al. Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology 2014, 60, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.K.; Karlsen, T.H.; Folseraas, T. Cholangiocytes in the pathogenesis of primary sclerosing cholangitis and development of cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1390–1400. [Google Scholar] [CrossRef]
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary sclerosing cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Pham, L.; Glaser, S.; Francis, H.; Alpini, G. Pathophysiological Roles of Ductular Reaction in Liver Inflammation and Hepatic Fibrogenesis. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, E.; Tirnitz-Parker, J.E.; Clouston, A.D.; Kayali, Z.; Lee, A.; Gan, E.K.; Ramm, G.A.; Kench, J.G.; Bowen, D.G.; Olynyk, J.K.; et al. Analysis of the intrahepatic ductular reaction and progenitor cell responses in hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2014, 20, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.R.; Aly, M.; Remih, K.; Tyson, L.D.; Guldiken, N.; Goldin, R.; Quaglia, A.; Thursz, M.; Strnad, P. Serum keratin 19 (CYFRA21-1) is a prognostic biomarker in severe alcoholic hepatitis. Liver Int. 2022, 42, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- LaRusso, N.F.; Ishii, M.; Vroman, B.T. The ins and outs of membrane movement in biliary epithelia. Trans. Am. Clin. Clim. Assoc. 1991, 102, 245–258; discussion 249–258. [Google Scholar]
- Glaser, S.; Francis, H.; Demorrow, S.; Lesage, G.; Fava, G.; Marzioni, M.; Venter, J.; Alpini, G. Heterogeneity of the intrahepatic biliary epithelium. World J. Gastroenterol. 2006, 12, 3523–3536. [Google Scholar] [CrossRef] [PubMed]
- Marzioni, M.; Glaser, S.S.; Francis, H.; Phinizy, J.L.; LeSage, G.; Alpini, G. Functional heterogeneity of cholangiocytes. Semin. Liver Dis. 2002, 22, 227–240. [Google Scholar] [CrossRef]
- Glaser, S.S.; Gaudio, E.; Rao, A.; Pierce, L.M.; Onori, P.; Franchitto, A.; Francis, H.L.; Dostal, D.E.; Venter, J.K.; DeMorrow, S.; et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab. Investig. 2009, 89, 456–469. [Google Scholar] [CrossRef]
- Maroni, L.; Haibo, B.; Ray, D.; Zhou, T.; Wan, Y.; Meng, F.; Marzioni, M.; Alpini, G. Functional and structural features of cholangiocytes in health and disease. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 368–380. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Masyuk, A.I.; Masyuk, T.V.; O’Hara, S.P.; LaRusso, N.F. Physiology of cholangiocytes. Compr. Physiol. 2013, 3, 541–565. [Google Scholar] [CrossRef] [PubMed]
- LeSage, G.D.; Glaser, S.S.; Marucci, L.; Benedetti, A.; Phinizy, J.L.; Rodgers, R.; Caligiuri, A.; Papa, E.; Tretjak, Z.; Jezequel, A.M.; et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am. J. Physiol. 1999, 276, G1289–G1301. [Google Scholar] [CrossRef] [PubMed]
- Kyritsi, K.; Wu, N.; Zhou, T.; Carpino, G.; Baiocchi, L.; Kennedy, L.; Chen, L.; Ceci, L.; Meyer, A.A.; Barupala, N.; et al. Knockout of secretin ameliorates biliary and liver phenotypes during alcohol-induced hepatotoxicity. Cell Biosci. 2023, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Syal, G.; Fausther, M.; Dranoff, J.A. Advances in cholangiocyte immunobiology. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1077–G1086. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Cholangiocyte primary cilia in liver health and disease. Dev. Dyn. 2008, 237, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Q.; Masyuk, T.V.; Muff, M.A.; Tietz, P.S.; Masyuk, A.I.; Larusso, N.F. Isolation and characterization of cholangiocyte primary cilia. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G500–G509. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, K.; Wu, N.; Zhou, T.; Huang, L.; Sato, K.; Venter, J.; Ceci, L.; Chen, D.; Ramos-Lorenzo, S.; Invernizzi, P.; et al. Amelioration of Ductular Reaction by Stem Cell Derived Extracellular Vesicles in MDR2 Knockout Mice via Lethal-7 microRNA. Hepatology 2019, 69, 2562–2578. [Google Scholar] [CrossRef]
- Olaizola, P.; Lee-Law, P.Y.; Arbelaiz, A.; Lapitz, A.; Perugorria, M.J.; Bujanda, L.; Banales, J.M. MicroRNAs and extracellular vesicles in cholangiopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1293–1307. [Google Scholar] [CrossRef]
- Katsumi, T.; Guicciardi, M.E.; Azad, A.; Bronk, S.F.; Krishnan, A.; Gores, G.J. Activated cholangiocytes release macrophage-polarizing extracellular vesicles bearing the DAMP S100A11. Am. J. Physiol. Cell Physiol. 2019, 317, C788–C799. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology 2016, 64, 2219–2233. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Meng, F.; Glaser, S.; Alpini, G. Exosomes in liver pathology. J. Hepatol. 2016, 65, 213–221. [Google Scholar] [CrossRef]
- Mancinelli, R.; Franchitto, A.; Gaudio, E.; Onori, P.; Glaser, S.; Francis, H.; Venter, J.; Demorrow, S.; Carpino, G.; Kopriva, S.; et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am. J. Pathol. 2010, 176, 1790–1800. [Google Scholar] [CrossRef]
- Alpini, G.; Glaser, S.S.; Ueno, Y.; Pham, L.; Podila, P.V.; Caligiuri, A.; LeSage, G.; LaRusso, N.F. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am. J. Physiol. 1998, 274, G767–G775. [Google Scholar] [CrossRef] [PubMed]
- LeSage, G.D.; Benedetti, A.; Glaser, S.; Marucci, L.; Tretjak, Z.; Caligiuri, A.; Rodgers, R.; Phinizy, J.L.; Baiocchi, L.; Francis, H.; et al. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology 1999, 29, 307–319. [Google Scholar] [CrossRef]
- Alpini, G.; Ueno, Y.; Glaser, S.S.; Marzioni, M.; Phinizy, J.L.; Francis, H.; Lesage, G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology 2001, 34, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Kamimoto, K.; Kaneko, K.; Kok, C.Y.; Okada, H.; Miyajima, A.; Itoh, T. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. Elife 2016, 5, e15034. [Google Scholar] [CrossRef]
- Gadd, V.L.; Aleksieva, N.; Forbes, S.J. Epithelial Plasticity during Liver Injury and Regeneration. Cell Stem Cell 2020, 27, 557–573. [Google Scholar] [CrossRef]
- Nejak-Bowen, K. If It Looks Like a Duct and Acts Like a Duct: On the Role of Reprogrammed Hepatocytes in Cholangiopathies. Gene Expr. 2020, 20, 19–23. [Google Scholar] [CrossRef]
- Limaye, P.B.; Alarcon, G.; Walls, A.L.; Nalesnik, M.A.; Michalopoulos, G.K.; Demetris, A.J.; Ochoa, E.R. Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Lab. Investig. 2008, 88, 865–872. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Pu, W.; Huang, X.; Qiu, L.; Li, Y.; Yu, W.; Zhao, H.; Liu, X.; He, L.; et al. Lineage Tracing Reveals the Bipotency of SOX9(+) Hepatocytes during Liver Regeneration. Stem Cell Rep. 2019, 12, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Kamimura, H.; Takamura, M.; Yamagiwa, S.; Matsuda, Y.; Sato, Y.; Nomoto, M.; Ichida, T.; Aoyagi, Y. Clinicopathological analysis of CD133 and NCAM human hepatic stem/progenitor cells in damaged livers and hepatocellular carcinomas. Hepatol. Res. 2009, 39, 1080–1090. [Google Scholar] [CrossRef]
- Zhou, H.; Rogler, L.E.; Teperman, L.; Morgan, G.; Rogler, C.E. Identification of hepatocytic and bile ductular cell lineages and candidate stem cells in bipolar ductular reactions in cirrhotic human liver. Hepatology 2007, 45, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K.; Barua, L.; Bowen, W.C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 2005, 41, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.R.; Lin, W.R. Periportal SRY (Sex Determining Region Y)-Box 9-Positive Hepatocytes: Progenitors with a Biliary Leaning. Hepatology 2019, 70, 1470–1473. [Google Scholar] [CrossRef]
- Xiao, J.C.; Jin, X.L.; Ruck, P.; Adam, A.; Kaiserling, E. Hepatic progenitor cells in human liver cirrhosis: Immunohistochemical, electron microscopic and immunofluorencence confocal microscopic findings. World J. Gastroenterol. 2004, 10, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430. [Google Scholar] [CrossRef]
- Font-Burgada, J.; Shalapour, S.; Ramaswamy, S.; Hsueh, B.; Rossell, D.; Umemura, A.; Taniguchi, K.; Nakagawa, H.; Valasek, M.A.; Ye, L.; et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell 2015, 162, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Yanger, K.; Zong, Y.; Maggs, L.R.; Shapira, S.N.; Maddipati, R.; Aiello, N.M.; Thung, S.N.; Wells, R.G.; Greenbaum, L.E.; Stanger, B.Z. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes. Dev. 2013, 27, 719–724. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.B.; Kim, K.H.; Lee, W.R.; Kim, J.Y.; An, H.J.; Park, K.K. Immunohistochemical study for the origin of ductular reaction in chronic liver disease. Int. J. Clin. Exp. Pathol. 2014, 7, 4076–4085. [Google Scholar]
- Tarlow, B.D.; Pelz, C.; Naugler, W.E.; Wakefield, L.; Wilson, E.M.; Finegold, M.J.; Grompe, M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 2014, 15, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Sone, M.; Chen, X.; Okada, Y.; Yamamoto, M.; Xin, B.; Matsuo, Y.; Komatsu, M.; Suzuki, A.; Enomoto, K.; et al. Contributions of hepatocytes and bile ductular cells in ductular reactions and remodeling of the biliary system after chronic liver injury. Am. J. Pathol. 2014, 184, 3001–3012. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; So, J.; Shin, D. Hepatocyte-to-cholangiocyte conversion occurs through transdifferentiation independently of proliferation in zebrafish. Hepatology 2023, 77, 1198–1210. [Google Scholar] [CrossRef]
- Kuwahara, R.; Kofman, A.V.; Landis, C.S.; Swenson, E.S.; Barendswaard, E.; Theise, N.D. The hepatic stem cell niche: Identification by label-retaining cell assay. Hepatology 2008, 47, 1994–2002. [Google Scholar] [CrossRef] [PubMed]
- Kordes, C.; Haussinger, D. Hepatic stem cell niches. J. Clin. Investig. 2013, 123, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Theise, N.D.; Saxena, R.; Portmann, B.C.; Thung, S.N.; Yee, H.; Chiriboga, L.; Kumar, A.; Crawford, J.M. The canals of Hering and hepatic stem cells in humans. Hepatology 1999, 30, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Theise, N. Canals of Hering: Recent insights and current knowledge. Semin. Liver Dis. 2004, 24, 43–48. [Google Scholar] [CrossRef]
- Roskams, T. Progenitor cell involvement in cirrhotic human liver diseases: From controversy to consensus. J. Hepatol. 2003, 39, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Roskams, T.A.; Libbrecht, L.; Desmet, V.J. Progenitor cells in diseased human liver. Semin. Liver Dis. 2003, 23, 385–396. [Google Scholar] [CrossRef]
- Raven, A.; Lu, W.Y.; Man, T.Y.; Ferreira-Gonzalez, S.; O’Duibhir, E.; Dwyer, B.J.; Thomson, J.P.; Meehan, R.R.; Bogorad, R.; Koteliansky, V.; et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 2017, 547, 350–354. [Google Scholar] [CrossRef]
- Schaub, J.R.; Malato, Y.; Gormond, C.; Willenbring, H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014, 8, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Yanger, K.; Knigin, D.; Zong, Y.; Maggs, L.; Gu, G.; Akiyama, H.; Pikarsky, E.; Stanger, B.Z. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell 2014, 15, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Russell, J.O.; Molina, L.M.; Monga, S.P. Liver Progenitors and Adult Cell Plasticity in Hepatic Injury and Repair: Knowns and Unknowns. Annu. Rev. Pathol. 2020, 15, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.; Lozoya, O.; Wang, Y.; Cardinale, V.; Gaudio, E.; Alpini, G.; Mendel, G.; Wauthier, E.; Barbier, C.; Alvaro, D.; et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011, 53, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Miyajima, A. Liver regeneration by stem/progenitor cells. Hepatology 2014, 59, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Crosby, H.A.; Kelly, D.A.; Strain, A.J. Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology 2001, 120, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Qiu, B.J.; Qi, X.S.; Chen, C.Y.; Liu, W.M.; Zhou, S.A.; Ding, M.; Lu, F.F.; Zhao, J.; Tang, D.; et al. CD24(+)LCN2(+) liver progenitor cells in ductular reaction contributed to macrophage inflammatory responses in chronic liver injury. Cell Biosci. 2023, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Wang, Y.; Carpino, G.; Reid, L.M.; Gaudio, E.; Alvaro, D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology 2012, 55, 2041–2042. [Google Scholar] [CrossRef]
- Carpino, G.; Cardinale, V.; Onori, P.; Franchitto, A.; Berloco, P.B.; Rossi, M.; Wang, Y.; Semeraro, R.; Anceschi, M.; Brunelli, R.; et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: An anatomical in situ study yielding evidence of maturational lineages. J. Anat. 2012, 220, 186–199. [Google Scholar] [CrossRef]
- de Jong, I.E.M.; van Leeuwen, O.B.; Lisman, T.; Gouw, A.S.H.; Porte, R.J. Repopulating the biliary tree from the peribiliary glands. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1524–1531. [Google Scholar] [CrossRef]
- Zhong, A.; Short, C.; Xu, J.; Fernandez, G.E.; Malkoff, N.; Noriega, N.; Yeo, T.; Wang, L.; Mavila, N.; Asahina, K.; et al. Prominin-1 promotes restitution of the murine extrahepatic biliary luminal epithelium following cholestatic liver injury. Hepatol. Commun. 2023, 7, e0018. [Google Scholar] [CrossRef] [PubMed]
- Clerbaux, L.A.; Manco, R.; Van Hul, N.; Bouzin, C.; Sciarra, A.; Sempoux, C.; Theise, N.D.; Leclercq, I.A. Invasive Ductular Reaction Operates Hepatobiliary Junctions upon Hepatocellular Injury in Rodents and Humans. Am. J. Pathol. 2019, 189, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, W.K.; Shu, Y.Y.; Ye, J. Mechanisms of ductular reaction in non-alcoholic steatohepatitis. World J. Gastroenterol. 2022, 28, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Bravo, B.; Arino, S.; Blaya, D.; Pose, E.; Martinez Garcia de la Torre, R.A.; Latasa, M.U.; Martinez-Sanchez, C.; Zanatto, L.; Sererols-Vinas, L.; Cantallops-Vila, P.; et al. Hepatocyte dedifferentiation profiling in alcohol-related liver disease identifies CXCR4 as a driver of cell reprogramming. J. Hepatol. 2023, 79, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Bravo, B.; Rodrigo-Torres, D.; Arino, S.; Coll, M.; Pose, E.; Blaya, D.; Graupera, I.; Perea, L.; Vallverdu, J.; Rubio-Tomas, T.; et al. Ductular Reaction Cells Display an Inflammatory Profile and Recruit Neutrophils in Alcoholic Hepatitis. Hepatology 2019, 69, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, J. Letter: Ductular reaction is a risk factor for prognosis of chronic hepatitis B complicated with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2023, 57, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Faraci, G.; Fabris, L.; Saccomanno, S.; Cadamuro, M.; Pierantonelli, I.; Trozzi, L.; Bugianesi, E.; Guido, M.; Strazzabosco, M.; et al. Insulin resistance and necroinflammation drives ductular reaction and epithelial-mesenchymal transition in chronic hepatitis C. Gut 2011, 60, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Clouston, A.D.; Powell, E.E.; Walsh, M.J.; Richardson, M.M.; Demetris, A.J.; Jonsson, J.R. Fibrosis correlates with a ductular reaction in hepatitis C: Roles of impaired replication, progenitor cells and steatosis. Hepatology 2005, 41, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Passman, A.M.; Strauss, R.P.; McSpadden, S.B.; Finch-Edmondson, M.L.; Woo, K.H.; Diepeveen, L.A.; London, R.; Callus, B.A.; Yeoh, G.C. A modified choline-deficient, ethionine-supplemented diet reduces morbidity and retains a liver progenitor cell response in mice. Dis. Model. Mech. 2015, 8, 1635–1641. [Google Scholar] [CrossRef]
- Kohn-Gaone, J.; Dwyer, B.J.; Grzelak, C.A.; Miller, G.; Shackel, N.A.; Ramm, G.A.; McCaughan, G.W.; Elsegood, C.L.; Olynyk, J.K.; Tirnitz-Parker, J.E.E. Divergent Inflammatory, Fibrogenic, and Liver Progenitor Cell Dynamics in Two Common Mouse Models of Chronic Liver Injury. Am. J. Pathol. 2016, 186, 1762–1774. [Google Scholar] [CrossRef]
- Wallace, M.C.; Hamesch, K.; Lunova, M.; Kim, Y.; Weiskirchen, R.; Strnad, P.; Friedman, S.L. Standard operating procedures in experimental liver research: Thioacetamide model in mice and rats. Lab. Anim. 2015, 49 (Suppl. S1), 21–29. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Michelotti, G.A.; Xie, G.; Almeida Pereira, T.; Boursier, J.; Bohnic, B.; Guy, C.D.; Diehl, A.M. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS ONE 2015, 10, e0127991. [Google Scholar] [CrossRef] [PubMed]
- Tag, C.G.; Sauer-Lehnen, S.; Weiskirchen, S.; Borkham-Kamphorst, E.; Tolba, R.H.; Tacke, F.; Weiskirchen, R. Bile duct ligation in mice: Induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J. Vis. Exp. 2015, 96, e52438. [Google Scholar] [CrossRef]
- Van Campenhout, S.; Van Vlierberghe, H.; Devisscher, L. Common Bile Duct Ligation as Model for Secondary Biliary Cirrhosis. Methods Mol. Biol. 2019, 1981, 237–247. [Google Scholar] [CrossRef]
- Pose, E.; Sancho-Bru, P.; Coll, M. 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine Diet: A Rodent Model in Cholestasis Research. Methods Mol. Biol. 2019, 1981, 249–257. [Google Scholar] [CrossRef]
- Fickert, P.; Stoger, U.; Fuchsbichler, A.; Moustafa, T.; Marschall, H.U.; Weiglein, A.H.; Tsybrovskyy, O.; Jaeschke, H.; Zatloukal, K.; Denk, H.; et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am. J. Pathol. 2007, 171, 525–536. [Google Scholar] [CrossRef]
- Scholten, D.; Trebicka, J.; Liedtke, C.; Weiskirchen, R. The carbon tetrachloride model in mice. Lab. Anim. 2015, 49, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mauad, T.H.; van Nieuwkerk, C.M.; Dingemans, K.P.; Smit, J.J.; Schinkel, A.H.; Notenboom, R.G.; van den Bergh Weerman, M.A.; Verkruisen, R.P.; Groen, A.K.; Oude Elferink, R.P.; et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am. J. Pathol. 1994, 145, 1237–1245. [Google Scholar]
- Popov, Y.; Patsenker, E.; Fickert, P.; Trauner, M.; Schuppan, D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J. Hepatol. 2005, 43, 1045–1054. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Donnelly, B.; Temple, H.; Tiao, G.M. A Rotavirus-Induced Mouse Model to Study Biliary Atresia and Neonatal Cholestasis. Methods Mol. Biol. 2019, 1981, 259–271. [Google Scholar] [CrossRef]
- Petersen, C.; Kuske, M.; Bruns, E.; Biermanns, D.; Wussow, P.V.; Mildenberger, H. Progress in developing animal models for biliary atresia. Eur. J. Pediatr. Surg. 1998, 8, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Tolba, R.; Kraus, T.; Liedtke, C.; Schwarz, M.; Weiskirchen, R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim. 2015, 49, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Morell, C.M.; Fiorotto, R.; Meroni, M.; Raizner, A.; Torsello, B.; Cadamuro, M.; Spagnuolo, G.; Kaffe, E.; Sutti, S.; Albano, E.; et al. Notch signaling and progenitor/ductular reaction in steatohepatitis. PLoS ONE 2017, 12, e0187384. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, B.; Croager, E.J.; Farley-Roche, C.A.; Ong, J.K.; Dumble, M.L.; Knight, B.; Yeoh, G.C. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology 2001, 34, 519–522. [Google Scholar] [CrossRef]
- Planas-Paz, L.; Sun, T.; Pikiolek, M.; Cochran, N.R.; Bergling, S.; Orsini, V.; Yang, Z.; Sigoillot, F.; Jetzer, J.; Syed, M.; et al. YAP, but Not RSPO-LGR4/5, Signaling in Biliary Epithelial Cells Promotes a Ductular Reaction in Response to Liver Injury. Cell Stem Cell 2019, 25, 39–53.e10. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, V.; Strazzabosco, M.; Fabris, L.; Calvisi, D.F. Animal models of biliary injury and altered bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt B, 1254–1261. [Google Scholar] [CrossRef]
- Liedtke, C.; Luedde, T.; Sauerbruch, T.; Scholten, D.; Streetz, K.; Tacke, F.; Tolba, R.; Trautwein, C.; Trebicka, J.; Weiskirchen, R. Experimental liver fibrosis research: Update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meyer, C.; Xu, C.; Weng, H.; Hellerbrand, C.; ten Dijke, P.; Dooley, S. Animal models of chronic liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G449–G468. [Google Scholar] [CrossRef] [PubMed]
- Gijbels, E.; Pieters, A.; De Muynck, K.; Vinken, M.; Devisscher, L. Rodent models of cholestatic liver disease: A practical guide for translational research. Liver Int. 2021, 41, 656–682. [Google Scholar] [CrossRef]
- Fickert, P.; Pollheimer, M.J.; Beuers, U.; Lackner, C.; Hirschfield, G.; Housset, C.; Keitel, V.; Schramm, C.; Marschall, H.U.; Karlsen, T.H.; et al. Characterization of animal models for primary sclerosing cholangitis (PSC). J. Hepatol. 2014, 60, 1290–1303. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Yang, Y.; Yang, Y.; Li, H.; Li, Y.; Zhang, F.; Wang, L. Animal models of primary biliary cholangitis: Status and challenges. Cell Biosci. 2023, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, V.; Cadamuro, M.; Spirli, C.; Fiorotto, R.; Strazzabosco, M.; Fabris, L. Animal models of cholestasis: An update on inflammatory cholangiopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, D.; Mancino, M.G.; Glaser, S.; Gaudio, E.; Marzioni, M.; Francis, H.; Alpini, G. Proliferating cholangiocytes: A neuroendocrine compartment in the diseased liver. Gastroenterology 2007, 132, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, D.; Gigliozzi, A.; Attili, A.F. Regulation and deregulation of cholangiocyte proliferation. J. Hepatol. 2000, 33, 333–340. [Google Scholar] [CrossRef] [PubMed]
- LeSage, G.; Glaser, S.; Alpini, G. Regulation of cholangiocyte proliferation. Liver 2001, 21, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sakamoto, T.; Ezure, T.; Yokomuro, S.; Murase, N.; Michalopoulos, G.; Demetris, A.J. Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in mice: Interactions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology 1998, 28, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Marzioni, M.; Glaser, S.; Francis, H.; Marucci, L.; Benedetti, A.; Alvaro, D.; Taffetani, S.; Ueno, Y.; Roskams, T.; Phinizy, J.L.; et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 2005, 128, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Kyritsi, K.; Chen, L.; O’Brien, A.; Francis, H.; Hein, T.W.; Venter, J.; Wu, N.; Ceci, L.; Zhou, T.; Zawieja, D.; et al. Modulation of the Tryptophan Hydroxylase 1/Monoamine Oxidase-A/5-Hydroxytryptamine/5-Hydroxytryptamine Receptor 2A/2B/2C Axis Regulates Biliary Proliferation and Liver Fibrosis During Cholestasis. Hepatology 2020, 71, 990–1008. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Huang, Z.; Gurley, E.C.; Wang, X.; Wang, J.; He, H.; Yang, H.; Lai, G.; Zhang, L.; et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology 2018, 68, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, X.; Zhu, W.; Wang, Y.; Zhao, D.; Wang, X.; Gurley, E.C.; Liang, G.; Chen, W.; Lai, G.; et al. Cholangiocyte-Derived Exosomal Long Noncoding RNA H19 Promotes Hepatic Stellate Cell Activation and Cholestatic Liver Fibrosis. Hepatology 2019, 70, 1317–1335. [Google Scholar] [CrossRef]
- Francis, H.; Glaser, S.; Ueno, Y.; Lesage, G.; Marucci, L.; Benedetti, A.; Taffetani, S.; Marzioni, M.; Alvaro, D.; Venter, J.; et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J. Hepatol. 2004, 41, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, M.; Attili, F.; Alpini, G.; Glaser, S. Prolonged administration of secretin to normal rats increases biliary proliferation and secretin-induced ductal secretory activity. Hepatobiliary Surg. Nutr. 2014, 3, 118–125. [Google Scholar] [PubMed]
- Glaser, S.; Lam, I.P.; Franchitto, A.; Gaudio, E.; Onori, P.; Chow, B.K.; Wise, C.; Kopriva, S.; Venter, J.; White, M.; et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 2010, 52, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhou, T.; Carpino, G.; Baiocchi, L.; Kyritsi, K.; Kennedy, L.; Ceci, L.; Chen, L.; Wu, C.; Kundu, D.; et al. Prolonged administration of a secretin receptor antagonist inhibits biliary senescence and liver fibrosis in Mdr2 −/− mice. Hepatology 2023, 77, 1849–1865. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; Franchitto, A.; Demorrow, S.; Onori, P.; Gaudio, E.; Wise, C.; Francis, H.; Venter, J.; Kopriva, S.; Mancinelli, R.; et al. Activation of alpha(1)-adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology 2011, 53, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Franchitto, A.; Glaser, S.; Meng, F.; Onori, P.; Demorrow, S.; Francis, H.; Venter, J.; Carpino, G.; Baker, K.; et al. GABA induces the differentiation of small into large cholangiocytes by activation of Ca(2+)/CaMK I-dependent adenylyl cyclase 8. Hepatology 2013, 58, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Tanimizu, N.; Ichinohe, N.; Mitaka, T. beta-adrenergic receptor agonist promotes ductular expansion during 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced chronic liver injury. Sci. Rep. 2023, 13, 7084. [Google Scholar] [CrossRef]
- Bernard, J.K.; Marakovits, C.; Smith, L.G.; Francis, H. Mast Cell and Innate Immune Cell Communication in Cholestatic Liver Disease. Semin. Liver Dis. 2023, 43, 226–233. [Google Scholar] [CrossRef]
- Kyritsi, K.; Kennedy, L.; Meadows, V.; Hargrove, L.; Demieville, J.; Pham, L.; Sybenga, A.; Kundu, D.; Cerritos, K.; Meng, F.; et al. Mast Cells Induce Ductular Reaction Mimicking Liver Injury in Mice Through Mast Cell-Derived Transforming Growth Factor Beta 1 Signaling. Hepatology 2021, 73, 2397–2410. [Google Scholar] [CrossRef]
- Meadows, V.; Kennedy, L.; Ekser, B.; Kyritsi, K.; Kundu, D.; Zhou, T.; Chen, L.; Pham, L.; Wu, N.; Demieville, J.; et al. Mast Cells Regulate Ductular Reaction and Intestinal Inflammation in Cholestasis Through Farnesoid X Receptor Signaling. Hepatology 2021, 74, 2684–2698. [Google Scholar] [CrossRef]
- Zhou, T.; Meadows, V.; Kundu, D.; Kyritsi, K.; Owen, T.; Ceci, L.; Carpino, G.; Onori, P.; Gaudio, E.; Wu, N.; et al. Mast cells selectively target large cholangiocytes during biliary injury via H2HR-mediated cAMP/pERK1/2 signaling. Hepatol. Commun. 2022, 6, 2715–2731. [Google Scholar] [CrossRef] [PubMed]
- Arino, S.; Aguilar-Bravo, B.; Coll, M.; Lee, W.Y.; Peiseler, M.; Cantallops-Vila, P.; Sererols-Vinas, L.; Martinez-Garcia de la Torre, R.A.; Martinez-Sanchez, C.; Pedragosa, J.; et al. Ductular reaction-associated neutrophils promote biliary epithelium proliferation in chronic liver disease. J. Hepatol. 2023, 79, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Godbole, N.; Nyholm, I.; Hukkinen, M.; Davidson, J.R.; Tyraskis, A.; Eloranta, K.; Andersson, N.; Lohi, J.; Heikkila, P.; Kyronlahti, A.; et al. Prognostic and Pathophysiologic Significance of IL-8 (CXCL8) in Biliary Atresia. J. Clin. Med. 2021, 10, 2705. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Fujii, H.; Michalopoulos, G.; Fung, J.J.; Demetris, A.J. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: Interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology 1994, 20, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Joplin, R.; Hishida, T.; Tsubouchi, H.; Daikuhara, Y.; Ayres, R.; Neuberger, J.M.; Strain, A.J. Human intrahepatic biliary epithelial cells proliferate in vitro in response to human hepatocyte growth factor. J. Clin. Investig. 1992, 90, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Kitade, M.; Factor, V.M.; Andersen, J.B.; Tomokuni, A.; Kaji, K.; Akita, H.; Holczbauer, A.; Seo, D.; Marquardt, J.U.; Conner, E.A.; et al. Specific fate decisions in adult hepatic progenitor cells driven by MET and EGFR signaling. Genes. Dev. 2013, 27, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bessho, K.; Shivakumar, P.; Mourya, R.; Mohanty, S.K.; Dos Santos, J.L.; Miura, I.K.; Porta, G.; Bezerra, J.A. Th2 signals induce epithelial injury in mice and are compatible with the biliary atresia phenotype. J. Clin. Investig. 2011, 121, 4244–4256. [Google Scholar] [CrossRef] [PubMed]
- Landi, A.; Weismuller, T.J.; Lankisch, T.O.; Santer, D.M.; Tyrrell, D.L.; Manns, M.P.; Houghton, M. Differential serum levels of eosinophilic eotaxins in primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis. J. Interferon Cytokine Res. 2014, 34, 204–214. [Google Scholar] [CrossRef]
- Napoli, J.; Prentice, D.; Niinami, C.; Bishop, G.A.; Desmond, P.; McCaughan, G.W. Sequential increases in the intrahepatic expression of epidermal growth factor, basic fibroblast growth factor, and transforming growth factor beta in a bile duct ligated rat model of cirrhosis. Hepatology 1997, 26, 624–633. [Google Scholar] [CrossRef]
- Gaudio, E.; Barbaro, B.; Alvaro, D.; Glaser, S.; Francis, H.; Ueno, Y.; Meininger, C.J.; Franchitto, A.; Onori, P.; Marzioni, M.; et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 2006, 130, 1270–1282. [Google Scholar] [CrossRef]
- Alvaro, D.; Alpini, G.; Onori, P.; Perego, L.; Svegliata Baroni, G.; Franchitto, A.; Baiocchi, L.; Glaser, S.S.; Le Sage, G.; Folli, F.; et al. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology 2000, 119, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lyu, Z.; Li, B.; You, Z.; Cui, N.; Li, Y.; Li, Y.; Huang, B.; Chen, R.; Chen, Y.; et al. P4HA2 induces hepatic ductular reaction and biliary fibrosis in chronic cholestatic liver diseases. Hepatology 2023, 78, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.G.; Lu, W.Y.; Boulter, L.; Gordon-Keylock, S.; Ridgway, R.A.; Williams, M.J.; Taube, J.; Thomas, J.A.; Wojtacha, D.; Gambardella, A.; et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 6542–6547. [Google Scholar] [CrossRef] [PubMed]
- Short, C.; Zhong, A.; Xu, J.; Mahdi, E.; Glazier, A.; Malkoff, N.; Noriega, N.; Yeo, T.; Asahina, K.; Wang, K.S. TWEAK/FN14 promotes profibrogenic pathway activation in Prominin-1-expressing hepatic progenitor cells in biliary atresia. Hepatology 2023, 77, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.V.; Zagory, J.A.; Dietz, W.H.; Park, A.; Fenlon, M.; Zhao, M.; Xu, J.; Lua, I.; Mavila, N.; Asahina, K.; et al. Hepatic Prominin-1 expression is associated with biliary fibrosis. Surgery 2017, 161, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Zagory, J.A.; Fenlon, M.; Dietz, W.; Zhao, M.; Nguyen, M.V.; Trinh, P.; Adoumie, M.; Park, A.; Xu, J.; Mahdi, E.; et al. Prominin-1 Promotes Biliary Fibrosis Associated With Biliary Atresia. Hepatology 2019, 69, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.; Strazzabosco, M. Emerging roles of Notch signaling in liver disease. Hepatology 2015, 61, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Jafar-Nejad, H. The Roles of Notch Signaling in Liver Development and Disease. Biomolecules 2019, 9, 608. [Google Scholar] [CrossRef]
- Strazzabosco, M.; Fabris, L. Development of the bile ducts: Essentials for the clinical hepatologist. J. Hepatol. 2012, 56, 1159–1170. [Google Scholar] [CrossRef]
- Zagory, J.A.; Dietz, W.; Park, A.; Fenlon, M.; Xu, J.; Utley, S.; Mavila, N.; Wang, K.S. Notch signaling promotes ductular reactions in biliary atresia. J. Surg. Res. 2017, 215, 250–256. [Google Scholar] [CrossRef]
- Fabris, L.; Cadamuro, M.; Guido, M.; Spirli, C.; Fiorotto, R.; Colledan, M.; Torre, G.; Alberti, D.; Sonzogni, A.; Okolicsanyi, L.; et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am. J. Pathol. 2007, 171, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Bales, C.; Nelson, A.; Gonzalez, D.M.; Underkoffler, L.; Segalov, M.; Wilson-Rawls, J.; Cole, S.E.; Moran, J.L.; Russo, P.; et al. Bile duct proliferation in Jag1/fringe heterozygous mice identifies candidate modifiers of the Alagille syndrome hepatic phenotype. Hepatology 2008, 48, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Boulter, L.; Govaere, O.; Bird, T.G.; Radulescu, S.; Ramachandran, P.; Pellicoro, A.; Ridgway, R.A.; Seo, S.S.; Spee, B.; Van Rooijen, N.; et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 2012, 18, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guldiken, N.; Spurny, M.; Mohammed, H.H.; Haybaeck, J.; Pollheimer, M.J.; Fickert, P.; Gassler, N.; Jeon, M.K.; Trautwein, C.; et al. Loss of keratin 19 favours the development of cholestatic liver disease through decreased ductular reaction. J. Pathol. 2015, 237, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Tai, Y.; Zhao, C.; Xiao, Y.; Yang, Z.; Zhang, L.; Gan, C.; Dai, W.; Tong, H.; Tang, C.; et al. Atypical cholangiocytes derived from hepatocyte-cholangiocyte transdifferentiation mediated by COX-2: A kind of misguided liver regeneration. Inflamm. Regen. 2023, 43, 37. [Google Scholar] [CrossRef]
- Schaub, J.R.; Huppert, K.A.; Kurial, S.N.T.; Hsu, B.Y.; Cast, A.E.; Donnelly, B.; Karns, R.A.; Chen, F.; Rezvani, M.; Luu, H.Y.; et al. De novo formation of the biliary system by TGFbeta-mediated hepatocyte transdifferentiation. Nature 2018, 557, 247–251. [Google Scholar] [CrossRef]
- Pi, L.; Robinson, P.M.; Jorgensen, M.; Oh, S.H.; Brown, A.R.; Weinreb, P.H.; Trinh, T.L.; Yianni, P.; Liu, C.; Leask, A.; et al. Connective tissue growth factor and integrin alphavbeta6: A new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology 2015, 61, 678–691. [Google Scholar] [CrossRef]
- Hu, S.; Russell, J.O.; Liu, S.; Cao, C.; McGaughey, J.; Rai, R.; Kosar, K.; Tao, J.; Hurley, E.; Poddar, M.; et al. beta-Catenin-NF-kappaB-CFTR interactions in cholangiocytes regulate inflammation and fibrosis during ductular reaction. Elife 2021, 10. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, X.; Shen, H.; Sheng, L.; Liangpunsakul, S.; Lok, A.S.; Omary, M.B.; Wang, S.; Rui, L. Biliary NIK promotes ductular reaction and liver injury and fibrosis in mice. Nat. Commun. 2022, 13, 5111. [Google Scholar] [CrossRef]
- Alpini, G.; Glaser, S.; Robertson, W.; Phinizy, J.L.; Rodgers, R.E.; Caligiuri, A.; LeSage, G. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am. J. Physiol. 1997, 273 Pt 1, G518–G529. [Google Scholar] [CrossRef]
- Alpini, G.; Glaser, S.S.; Ueno, Y.; Rodgers, R.; Phinizy, J.L.; Francis, H.; Baiocchi, L.; Holcomb, L.A.; Caligiuri, A.; LeSage, G.D. Bile acid feeding induces cholangiocyte proliferation and secretion: Evidence for bile acid-regulated ductal secretion. Gastroenterology 1999, 116, 179–186. [Google Scholar] [CrossRef]
- Alpini, G.; Glaser, S.; Alvaro, D.; Ueno, Y.; Marzioni, M.; Francis, H.; Baiocchi, L.; Stati, T.; Barbaro, B.; Phinizy, J.L.; et al. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology 2002, 123, 1226–1237. [Google Scholar] [CrossRef]
- Camargo, F.D.; Gokhale, S.; Johnnidis, J.B.; Fu, D.; Bell, G.W.; Jaenisch, R.; Brummelkamp, T.R. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 2007, 17, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Yimlamai, D.; Christodoulou, C.; Galli, G.G.; Yanger, K.; Pepe-Mooney, B.; Gurung, B.; Shrestha, K.; Cahan, P.; Stanger, B.Z.; Camargo, F.D. Hippo pathway activity influences liver cell fate. Cell 2014, 157, 1324–1338. [Google Scholar] [CrossRef]
- Pepe-Mooney, B.J.; Dill, M.T.; Alemany, A.; Ordovas-Montanes, J.; Matsushita, Y.; Rao, A.; Sen, A.; Miyazaki, M.; Anakk, S.; Dawson, P.A.; et al. Single-Cell Analysis of the Liver Epithelium Reveals Dynamic Heterogeneity and an Essential Role for YAP in Homeostasis and Regeneration. Cell Stem Cell 2019, 25, 23–38.e28. [Google Scholar] [CrossRef]
- Thompson, M.D.; Awuah, P.; Singh, S.; Monga, S.P. Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am. J. Pathol. 2010, 177, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- LeSag, E.G.; Alvaro, D.; Benedetti, A.; Glaser, S.; Marucci, L.; Baiocchi, L.; Eisel, W.; Caligiuri, A.; Phinizy, J.L.; Rodgers, R.; et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology 1999, 117, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Huang, H.; Ni, J.; Shen, J.; Liu, Z.; Li, L.; Fu, S.; Yan, J.; Hu, B. Shh-Yap signaling controls hepatic ductular reactions in CCl(4)-induced liver injury. Env. Toxicol. 2021, 36, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.E.; Jungwirth, E.; Panzitt, K.; Wagner, M.; Anakk, S. Hepatic farnesoid X receptor is necessary to facilitate ductular reaction and expression of heme biosynthetic genes. Hepatol. Commun. 2023, 7, e0213. [Google Scholar] [CrossRef]
- Kim, K.H.; Chen, C.C.; Alpini, G.; Lau, L.F. CCN1 induces hepatic ductular reaction through integrin alphavbeta(5)-mediated activation of NF-kappaB. J. Clin. Investig. 2015, 125, 1886–1900. [Google Scholar] [CrossRef]
- Omenetti, A.; Yang, L.; Li, Y.X.; McCall, S.J.; Jung, Y.; Sicklick, J.K.; Huang, J.; Choi, S.; Suzuki, A.; Diehl, A.M. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab. Investig. 2007, 87, 499–514. [Google Scholar] [CrossRef]
- Tirnitz-Parker, J.E.; Viebahn, C.S.; Jakubowski, A.; Klopcic, B.R.; Olynyk, J.K.; Yeoh, G.C.; Knight, B. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology 2010, 52, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Razumilava, N.; Gores, G.J.; Walters, S.; Mizuochi, T.; Mourya, R.; Bessho, K.; Wang, Y.H.; Glaser, S.S.; Shivakumar, P.; et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J. Clin. Investig. 2014, 124, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Omenetti, A.; Choi, S.; Michelotti, G.; Diehl, A.M. Hedgehog signaling in the liver. J. Hepatol. 2011, 54, 366–373. [Google Scholar] [CrossRef]
- Omenetti, A.; Diehl, A.M. Hedgehog signaling in cholangiocytes. Curr. Opin. Gastroenterol. 2011, 27, 268–275. [Google Scholar] [CrossRef]
- Jung, Y.; McCall, S.J.; Li, Y.X.; Diehl, A.M. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology 2007, 45, 1091–1096. [Google Scholar] [CrossRef]
- Omenetti, A.; Bass, L.M.; Anders, R.A.; Clemente, M.G.; Francis, H.; Guy, C.D.; McCall, S.; Choi, S.S.; Alpini, G.; Schwarz, K.B.; et al. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology 2011, 53, 1246–1258. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, I.; Park, J.; Bram, Y.; Schwartz, R.E. Hedgehog Signaling Demarcates a Niche of Fibrogenic Peribiliary Mesenchymal Cells. Gastroenterology 2020, 159, 624–638.e629. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Corcuera, A.; Sehrawat, T.S.; Jalan-Sakrikar, N.; Gibbons, H.R.; Pirius, N.E.; Khanal, S.; Hamdan, F.H.; Aseem, S.O.; Cao, S.; Banales, J.M.; et al. Long non-coding RNA ACTA2-AS1 promotes ductular reaction by interacting with the p300/ELK1 complex. J. Hepatol. 2022, 76, 921–933. [Google Scholar] [CrossRef]
- Passman, A.M.; Haughey, M.J.; Carlotti, E.; Williams, M.J.; Cereser, B.; Lin, M.L.; Devkumar, S.; Gabriel, J.P.; Gringeri, E.; Cillo, U.; et al. Hepatocytes undergo punctuated expansion dynamics from a periportal stem cell niche in normal human liver. J. Hepatol. 2023, 79, 417–432. [Google Scholar] [CrossRef]
- Saviano, A.; Henderson, N.C.; Baumert, T.F. Single-cell genomics and spatial transcriptomics: Discovery of novel cell states and cellular interactions in liver physiology and disease biology. J. Hepatol. 2020, 73, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Malato, Y.; Calvisi, D.F.; Naqvi, S.; Razumilava, N.; Ribback, S.; Gores, G.J.; Dombrowski, F.; Evert, M.; Chen, X.; et al. Cholangiocarcinomas can originate from hepatocytes in mice. J. Clin. Investig. 2012, 122, 2911–2915. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.S.; Lim, W.T.; Choi, H.S. Biology of Cholangiocytes: From Bench to Bedside. Gut Liver 2016, 10, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Gringeri, E.; Burra, P.; Gambato, M. Primary Sclerosing Cholangitis-Associated Cholangiocarcinoma: From Pathogenesis to Diagnostic and Surveillance Strategies. Cancers 2023, 15, 4947. [Google Scholar] [CrossRef] [PubMed]
- Saca, D.; Flamm, S.L. Cholangiocarcinoma Surveillance Recommendations in Patients with Primary Sclerosing Cholangitis. Clin. Liver Dis. 2024, 28, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Nanjundappa, R.H.; Christen, U.; Umeshappa, C.S. Distinct immune surveillance in primary biliary cholangitis and primary sclerosing cholangitis is linked with discrete cholangiocarcinoma risk. Hepatol. Commun. 2023, 7. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Arab, J.P.; Reyes, D.; Lapitz, A.; Moshage, H.; Banales, J.M.; Arrese, M. Extracellular Vesicles in NAFLD/ALD: From Pathobiology to Therapy. Cells 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Campello, E.; Cadamuro, M.; Simioni, P. The evil relationship between liver fibrosis and cardiovascular disease in metabolic dysfunction-associated fatty liver disease (MAFLD): Looking for the culprit. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166763. [Google Scholar] [CrossRef]
- Russell, J.O.; Lu, W.Y.; Okabe, H.; Abrams, M.; Oertel, M.; Poddar, M.; Singh, S.; Forbes, S.J.; Monga, S.P. Hepatocyte-Specific beta-Catenin Deletion During Severe Liver Injury Provokes Cholangiocytes to Differentiate Into Hepatocytes. Hepatology 2019, 69, 742–759. [Google Scholar] [CrossRef]
- Lu, W.Y.; Bird, T.G.; Boulter, L.; Tsuchiya, A.; Cole, A.M.; Hay, T.; Guest, R.V.; Wojtacha, D.; Man, T.Y.; Mackinnon, A.; et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015, 17, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Espanol-Suner, R.; Carpentier, R.; Van Hul, N.; Legry, V.; Achouri, Y.; Cordi, S.; Jacquemin, P.; Lemaigre, F.; Leclercq, I.A. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 2012, 143, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Momen-Heravi, F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 455–466. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavila, N.; Siraganahalli Eshwaraiah, M.; Kennedy, J. Ductular Reactions in Liver Injury, Regeneration, and Disease Progression—An Overview. Cells 2024, 13, 579. https://doi.org/10.3390/cells13070579
Mavila N, Siraganahalli Eshwaraiah M, Kennedy J. Ductular Reactions in Liver Injury, Regeneration, and Disease Progression—An Overview. Cells. 2024; 13(7):579. https://doi.org/10.3390/cells13070579
Chicago/Turabian StyleMavila, Nirmala, Mallikarjuna Siraganahalli Eshwaraiah, and Jaquelene Kennedy. 2024. "Ductular Reactions in Liver Injury, Regeneration, and Disease Progression—An Overview" Cells 13, no. 7: 579. https://doi.org/10.3390/cells13070579
APA StyleMavila, N., Siraganahalli Eshwaraiah, M., & Kennedy, J. (2024). Ductular Reactions in Liver Injury, Regeneration, and Disease Progression—An Overview. Cells, 13(7), 579. https://doi.org/10.3390/cells13070579