1. Introduction
Insect hemocytes constitute the cellular elements of a powerful innate immune response that shows many similarities to the innate immune system of vertebrates [
1,
2,
3]. Our understanding of insect and vertebrate innate immunity largely comes from the analysis of the model organism,
Drosophila melanogaster. Hemocytes of
D. melanogaster phagocytose microbes, produce antimicrobial peptides, encapsulate parasitoids and contribute to tissue remodeling during development. These processes are generated by three main hemocyte classes: the plasmatocytes, the crystal cells and the lamellocytes, which are localized in three hematopoietic compartments: the circulation, the lymph gland and the sessile tissue [
4,
5,
6]. The most prevalent cells in the circulation are the plasmatocytes. They are spherical cells that phagocytose microbes, produce antimicrobial peptides and matrix proteins, and contribute to the elimination of parasitoids as they attach to the chorion at the beginning of capsule formation [
7]. After cuticle damage or following parasitoid infection, plasmatocytes transdifferentiate into large, flattened non-phagocytic, encapsulating cells, termed lamellocytes [
4,
8]. Lamellocytes differentiate in circulation, sessile tissue and the main hematopoietic organ, the lymph gland [
9]. The encapsulation reaction starts with the attachment of plasmatocytes to the parasitoids; then, lamellocytes form several cellular layers around eggs and larvae of the wasp to envelop and isolate the developing intruder [
7]. Lamellocytes, the characteristic encapsulating cell type in the species of the
melanogaster subgroup, produce prophenoloxidase 3 (PPO3), a key enzyme in the melanization of the capsule and in killing the parasitoid [
10]. The crystal cells carry prophenoloxydases and represent a minor subpopulation of hemocytes [
11,
12]. These cells contain components of the melanization system that is activated upon wounding and the encapsulation reaction. Based on transcriptomic studies in
D. melanogaster, a new class of hemocytes, called primocytes, have been identified. These cells are present in every hematopoietic compartment and are likely to be involved in the activation of effector hemocytes [
13,
14].
Certain non-model species of
Drosophilidae utilize other effector hemocyte types to encapsulate parasitoids. Non-phagocytic, multicellular, web-like structures have been found in species of the
ananassae subgroup, in
Zaprionus indianus,
Drosophila falleni,
Drosophila phalerata and
Drosophila grimshawi [
15,
16] upon infection by a variety of parasitoid wasps. Species differentiating multinucleated hemocytes are highly resistant to endoparasitoids [
15,
17]. Multinucleation provides a metabolic advantage for the cells as the increased genetic material within one cytoplasm leads to higher protein expression levels and intensified metabolism [
18,
19], which may also contribute to the effective anti-parasitoid immune response [
15,
17]. Hemocytes with more than one nucleus have been described in naïve
Drosophila willistoni and were classified as plasmatocytes [
20]. In this species, hemocytes have been grouped on the basis of their morphological features as plasmatocytes, podocytes, spheroidocytes, oenocytoids, nematocytes and crystalloid cells [
20].
The aim of the present study was to systematically group and characterize hemocytes of D. willistoni by a combination of immunological and functional analyses. In particular, our focus is directed toward the multinucleated hemocyte population, and we assess their function in terms of defense against endoparasitoid wasps.
2. Materials and Methods
2.1. Insect Stocks and Culturing
D. willistoni wild type (14030-0811.24) was obtained from UC San Diego Drosophila stock center. D. melanogaster wild type (Oregon-R) was acquired from Bloomington Drosophila Stock Center. Each strain was maintained at 25 °C on standard yeast-cornmeal food. The Leptopilina boulardi (G486), Leptopilina heterotoma 14 and Leptopilina victoriae UNK parasitoid wasps were provided by Prof. Todd Schlenke (University of Arizona, Tucson, AZ, USA) and maintained on D. melanogaster wild type Oregon-R. Wasps were kept at 25 °C on 1.5% agar supplemented with 10% glucose, with additional honey placed on blotting-paper.
2.2. Generation of Wasp-Induced Samples and Parasitization Assay
A total of 60 early second instar D. melanogaster or D. willistoni larvae were exposed to 15 female Leptopilina boulardi, Leptopilina heterotoma or Leptopilina victoriae parasitoid wasps for 6 h at 25 °C. Then, 48 h after the infection, the parasitized larvae were selected under dissecting microscope based on the melanized sites of the oviposition, showing that the respective larva was subjected to successful parasitoid attack. The selected infected larvae were used in the respective experiments.
To test parasitization of D. willistoni and D. melanogaster by the three parasitoid wasp species, four independent experiments were carried out; one representative image is presented. To generate the 1:1 ratio of D. melanogaster and D. willistoni larvae in the respective parasitization experiments, we applied 30-30 early second instar larvae of both fly species into the vials.
For parasitization assay 48 h after the infection, 10 of the 60 infected larvae were dissected to test the parasitization success, and a vial was considered available for the assay when each larva carried at least one parasitoid [
17]. For each combination, the results of four independent experiments were cropped.
2.3. Production of Monoclonal Antibodies
A total of 3 one-month-old female BALB/C mice were immunized three times at 3-week intervals with 105 hemocytes isolated in PBS from L. victoriae parasitoid wasp infected D. willistoni larvae. For hybridoma generation, the spleen cells were fused with Sp2/0 myeloma cells using polyethylene glycol (PEG 1450). Hybridomas were maintained and selected by Kohler and Milstein’s hybridoma technology (1976). Supernatants of individual cultures were screened by indirect immunofluorescence, and positive clones were maintained further. The hybridoma lines producing antibodies reacting with a fraction of cells or with all hemocytes were cloned at 0.3 cell/culture to ensure homogeneity. Tissue culture supernatants of individual clones were selected, including three discriminative (10C5, 1C1 and 8G5) and one panhemocyte antibody (1F1).
2.4. Hemocyte and Parasitoid Isolation, Preparation of the Lymph Gland
All procedures were carried out at room temperature. Individual
D. willistoni larvae were dissected in Schneider’s medium (Lonza, Basel, Switzerland) supplemented with 5% fetal bovine serum (GIBCO, Grand Island, NY, USA) and 0.01% 1-phenyl-2-thiourea (Sigma, St. Louis, MO, USA) (CSM) in 30 µL volume on multispot microscope slides (Hendley-Essex, Loughton, UK). The released hemocytes were allowed to settle and adhere to the spots for 1 h, and then were processed further, as described in
Section 2.5. Parasitoids were harvested by micropipette from the hemolymph of the dissected larvae and processed for immunostaining. The lymph glands were prepared from wasp-infected larvae 24 h after parasitization and from age matched naïve animals. The larvae were dissected along the longitudinal axis on the ventral part, and the digestive tract and fat body were removed.
2.5. Indirect Immunofluorescence and Image Analysis
The adhered hemocytes were fixed for 6 min with acetone, dried for 3 min, and blocked with PBS supplemented with 0.1% BSA for 20 min. The parasitoids and lymph glands were fixed with 2% paraformaldehyde for 10 min, blocked and permeabilized with 0.1% BSA in PBS supplemented with 0.1% Triton X-100. Samples were incubated with the respective hemocyte specific primary monoclonal antibodies in the form of undiluted hybridoma supernatants (1F1, 10C5, 1C1 or 8G5) and T2/48 as negative control. The anti-phospho-histoneH3 (aH3P) was a rabbit polyclonal primary antibody (Sigma, 1:1000 dilution), and the anti-bromodeoxyuridine (aBrdU) was an Alexa Fluor 488-conjugated mouse monoclonal primary antibody (Invitrogen (Waltham, MA, USA), 1:10 dilution). Samples were washed 3 times for 5 min in PBS, and then incubated with the secondary antibodies and DAPI for 45 min. Secondary antibodies were: anti-mouse Alexa Fluor 488 goat antibody (Invitrogen, 1:1000 dilution), anti-mouse CF 568 goat antibody (Biotium (Fremont, CA, USA), 1:1000 dilution) and anti-rabbit Alexa Fluor 568 goat antibody (Invitrogen, 1:1000 dilution). Samples were washed 3 times with PBS for 5 min and covered with Fluoromount G medium and coverslip. Analysis was performed with an Olympus (Tokyo, Japan) FV1000 confocal LSM microscope or an epifluorescence microscope (Zeiss Axioscope (Jena, Germany) 2 MOT). Hemocytes were counted with the ImageJ program (version 1.52a) based on nuclear DAPI fluorescence.
2.6. BrdU Labeling of the Larvae and Ex Vivo Detection of Cell Fusion
The flies laid eggs in vials containing standard fly food supplemented with 1 mM BrdU, in the dark. Second instar larvae were parasitized by L. victoriae for 6 h in the dark. Hemocytes were isolated from the larvae 48 h after parasitization. Hemocytes of BrdU-treated and untreated larvae were mixed in CSM, then allowed to settle at room temperature for 1 h on a microscopic slide. The attached cells were fixed with acetone for 6 min, treated with 2 M HCl for 15 min, washed 4 times in PBS, and blocked in PBS containing 0.1% BSA for 20 min. As primary reagent, the mouse anti-BrdU Alexa Fluor 488-conjugated antibody was used for 1 h, then the samples were washed 3 times in PBS, and anti-mouse Alexa Fluor 488 goat antibody (Invitrogen, 1:1000 dilution) was applied for 45 min. Nuclei were visualized with DAPI (Sigma, 1:400). The slides were washed 3 times in PBS, covered with Fluoromount G medium and coverslip, then analyzed with an epifluorescence microscope (Zeiss Axioskope 2 MOT).
2.7. Phagocytosis Assays
Fluorescein isothiocyanate (FITC)-labeled Escherichia coli bacteria (SzMC 0582) (Szeged Microbial Collection, University of Szeged, Szeged, Hungary) were used. For ex vivo phagocytosis assay, hemocytes of 4 larvae were pooled in 100 μL CSM. Six μL E. coli-FITC conjugate was added from a 10% bacterial suspension in sterile PBS to the hemocytes, which were incubated for 45 min at room temperature. The fluorescence of the non-phagocytosed bacteria was quenched with trypan blue in 0.2% final concentration. Fluorescence of the engulfed bacteria was visualized with an epifluorescence microscope (Zeiss Axioscope 2 MOT). The in vivo phagocytosis assay was carried out by injecting naïve or infected larvae (72 h after parasitization) with 0.1 μL E. coli-FITC from a 4% bacterial suspension in sterile PBS. Injected larvae were incubated at room temperature for 1 h under sterile conditions in petri dishes on Whatman paper steeped with Drosophila Ringer solution. Hemocytes were isolated, adhered on microscopic slides for 1 h, fixed in acetone and reacted with the respective antibodies as described above for indirect immunofluorescence analysis. To detect phagocytic ability of hemocytes settled on the parasitoids, 42 µL of 10% FITC-labeled bacteria was added to 700 µL CSM containing the isolated parasitoids, mixed and incubated for 1 h. Then, parasitoids were washed 3 times with PBS, and indirect immunofluorescence analysis was performed as described above. The samples were analyzed with an Olympus FV1000 confocal LSM microscope, and images in the nucleus plane were used.
4. Discussion
Insects deploy a continuously developing, robust and complex immune response to keep up with the co-evolution of pathogens. This is manifested in a multifaceted cellular immune response with a substantial morphological and functional diversity of effector cells using a variety of molecules against the pathogens. The immune defense reactions of insects show interesting similarities to those of the mammals; the antimicrobial peptides and proteolytic cascades are regulated by similar regulatory networks, and the effector cells operate on the same principles. The concerted action of the cellular and humoral components of the immune system provides prompt elimination of microbes by phagocytic cells and encapsulation of larger particles by specialized cell types transdifferentiating from phagocytic cells [
1,
2,
3]. Lamellocytes have been described in numerous species of the so-called “oriental” subgroups of the
melanogaster group, including the
melanogaster,
eugracilis,
suzukii,
ficusphila,
elegans,
rhopaloa and
oshimai subgroups within the
Drosophilidae family [
14]. A combination of antigen expression analysis with functional assays has defined a variety of other encapsulating cell types in the species of the
ananassae subgroup [
15] and in
Zaprionus indianus [
22]. These species possess a network of elongated, multiform cells and anucleated cell fragments, which are involved in the formation of multinucleated syncytia with variable morphological features by homotypic cell–cell fusion [
15,
16,
22]. All these species have a highly efficient immune defense reaction against parasitoids [
15,
17]. In this work, we describe another multinucleated encapsulating cell type in
D. willistoni, which possesses a binary function, as these cells are also phagocytic. Previously, hemocytes of
D. willistoni have been classified based on their distinctive morphological features [
20], but here, we combined immunological and functional assays to follow the dynamics and role of hemocyte subpopulations in the naïve state and following parasitoid infections.
First, we analyzed the defense against three closely related parasitoid wasp species,
L. victoriae,
L. heterotoma and
L. boulardi, and found that only the first two parasitized
D. willistoni larvae, while
L. boulardi was not attracted. The fact that
L. boulardi wasps readily attacked a mixture of
D. melanogaster and
D. willistoni larvae suggested that the lack of parasitization was due to an absence of sensing and not by a repellent substance produced by
D. willistoni. Both,
L. victoriae and
L. heterotoma inject venom into the hosts to block effective immune responses and the killing of their larvae; however, there are substantial differences in the effect of the venom [
23,
24,
25]. We showed that
L. heterotoma infection suppressed the immune response as it caused lysis of hemocytes.
L. victoriae infection stimulated the immune reaction as infection induced a substantial increase in the total hemocyte count and did not affect hemocyte activation or the successful encapsulation reaction in
D. willistoni. In contrast, we previously showed that oviposition of
L. victoriae actively suppressed the immune reaction of
D. ananassae as it caused a strong decrease in hemocyte count [
15]. These two species show different abilities to encapsulate the same parasitoid,
L. victoriae, which suggests that the co-evolution of parasites and hosts in different geographical regions evolved differently. The native range of
D. ananassae is South and Southeast Asia and the Indo and South Pacific [
26], while
D. willistoni geographically originates from South America [
27]. The parasitization assays also highlighted a more effective immune defense in
D. willistoni than in
D. melanogaster against both
L. heterotoma and
L. victoriae (
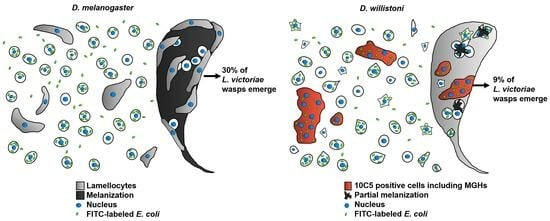
Figure 1B).
The immune defense reaction of
D. willistoni to
L. victoriae was further characterized by the use of discriminative monoclonal antibodies. The cell subpopulation, which showed an impressive shift after parasitization with
L. victoriae, reacted with the 10C5 antibody. These were larger, flattened cells that were also present in the naïve state, and the subpopulation was mainly composed of mononuclear cells. After
L. victoriae infection, the ratio of the 10C5 positive cells showed an extensive increase, and multinucleated cells differentiated (
Figure 3 and
Figure 4A). The multinucleated cells all expressed the 10C5 antigen and lacked the 1C1 antigen. Similarly, to the MGHs of
D. ananassae and
Z. indianus, we found that the 10C5 positive hemocytes of
D. willistoni settled on the parasitoid, suggesting their involvement in the capsule formation and anti-parasitoid defense reactions (
Figure 4B,C). Furthermore, differentiation of the MGHs varies across species because, while MGHs are formed by cell fusion during circulation in
D. ananassae and
Z. indianus [
15,
22], in
D. willistoni, nuclear division could also be involved in the formation of these cells (
Figure 5C). Although mitotic events were detected in the 10C5 positive cells, we observed that cell fusion was a more frequent phenomenon for differentiating MGHs.
Surprisingly, we found that, in both naïve and parasitoid wasp-infected
D. willistoni about 30% of the hemocytes were non-phagocytic, while in
D. melanogaster and in
Z. indianus, the ratio of these cells was about 5% of the total hemocyte population [
11,
12,
22]. The role of the large number of non-phagocytic cells in
D. willistoni is not clear. This group of cells also includes the 8G5 positive subpopulation, but the 8G5 positive cells only represent a maximum of 4% of the hemocytes (
Figure 3B), which means that there are other non-phagocytic cells in about 26%, which do not carry the 8G5 specific antigen. We found that, among the 1C1 negative hemocyte subpopulation, the ratio of the non-phagocytic cells doubled after
L. victoriae infection, suggesting that this group of hemocytes might have acquired another defense function while losing their phagocytic ability. Furthermore, we found that the majority (about 77%) of the 10C5 positive cells, including all multinucleated cells, phagocytosed bacteria (
Figure 4), while MGHs of
D. ananassae and
Z. indianus and lamellocytes of
D. melanogaster were non-phagocytic [
15,
22]. We found that 10C5 positive cells of
D. willistoni, including multinucleated hemocytes, kept their phagocytic capacity (
Figure 4B) even when localized on the parasitoid wasp (
Figure 4C). Similar to the 10C5 positive cells of
D. willistoni, in three species of the
obscura group,
Drosophila tolteca,
Drosophila affinis and
Drosophila obscura, pseudopodocytes differentiated to encapsulate parasitoids, and they also kept their phagocytic ability [
28,
29]. It is not clear yet whether the binary function of the 10C5 positive cell type of
D. willistoni serves as an evolutionary advantage, contributing to a highly effective, multifaceted cellular immune response. In conclusion, the large number of non-phagocytic cells in
D. willistoni may explain why the 10C5 positive hemocytes, including the multinucleated cell population, maintained their phagocytic ability and hence retained the number of the phagocytic cells at a respectable level.
D. willistoni has evolved a more effective defense reaction against
L. victoriae and
L. heterotoma parasitoids than
D. melanogaster (
Figure 1B). The genome of
D. willistoni does not have an ortholog of PPO3, a gene specifically associated with lamellocytes which are restricted to a limited number of drosophilids and essential for melanization in the capsule in these species [
10,
14]. This observation suggests that
D. willistoni has evolved a distinct defense mechanism against parasitoids. However, we observed partial, spotty melanization in the capsule (
Figure 2C and
Figure 4B,C). Although
L. heterotoma infection caused a drastic decrease in the number of circulating hemocytes (
Figure 2A,B) and the cells did not attach to the developing wasp larvae (
Figure 2C), some adult flies eclosed after wasp infection, suggesting the involvement of humoral defense factors in anti-parasitoid immunity. We found that the
D. willistoni LOC124460379 gene (NCBI database) encodes for a Hemolysin E-like protein (XP_046866967.1), and another gene, localized 4 kb upstream of the LOC124460379, which has not been annotated in GeneBank, encodes for a homolog protein, showing 64% identity with XP_046866967.1. Hemolysin E proteins are pore-forming toxins produced by certain microbial species belonging to the
Enterobacteriaceae family and used to attack eukaryotic cells [
30,
31,
32]. Such genes were detected in
D. ananassae and are supposed to be involved in the elimination of parasitoids [
33]. Moreover, other genes captured by horizontal gene transfer encode for humoral factors produced by the fat body and are functional modules in the anti-parasitoid defense of
D. ananassae subgroup species [
34]. The possible presence of a humoral anti-parasitoid immune defense reaction in
D. willistoni is also supported by the observation that this species is resistant to
Asobara tabida parasitoid infection through mechanisms other than encapsulation [
21].
This study provides insight into the immune defense reactions of D. willistoni, involving multinucleated effector cells, which are able to both encapsulate parasitoid wasps and phagocytose bacteria. The high ratio of circulating non-phagocytic cells indicates that hemocyte subpopulations with alternative functions could also be involved in the efficient anti-parasitoid defense reaction. These data extend our knowledge and highlight evolutionary advantages regarding hemocytes of Drosophilidae.