ERβ Regulation of Indian Hedgehog Expression in the First Wave of Ovarian Follicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Histological Evaluation of Ovarian Phenotypes
2.3. Total Follicle Counting in the Serial Sections of the Whole Ovary
2.4. Detection of Differentially Expressed Genes
2.5. Evaluation of Gene Expression Using RT-qPCR
2.6. RNAScope In Situ Hybridization
2.7. Statistical Analysis
3. Results
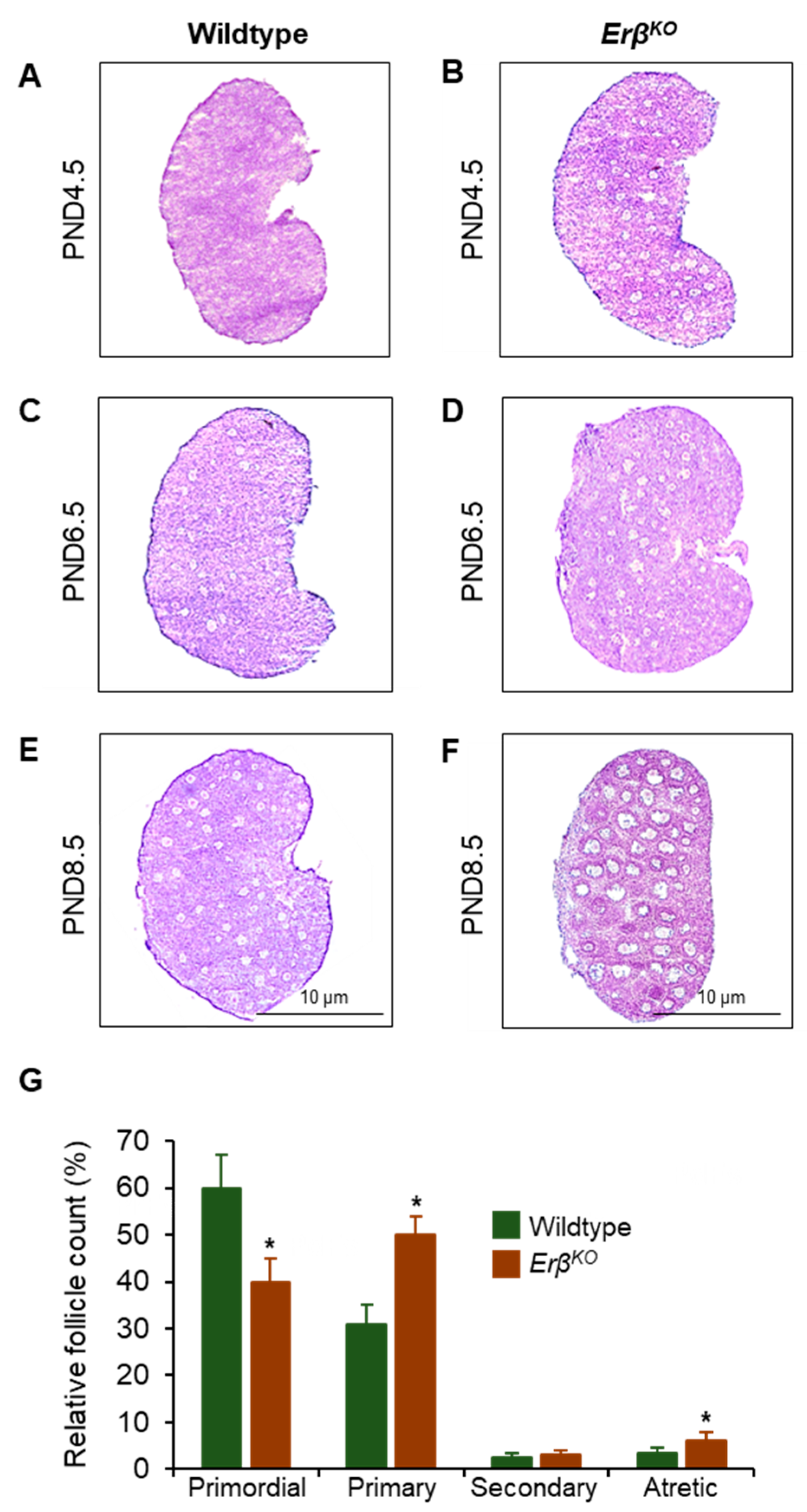
3.1. Increased Primordial Follicle Growth Activation in ErβKO Rats
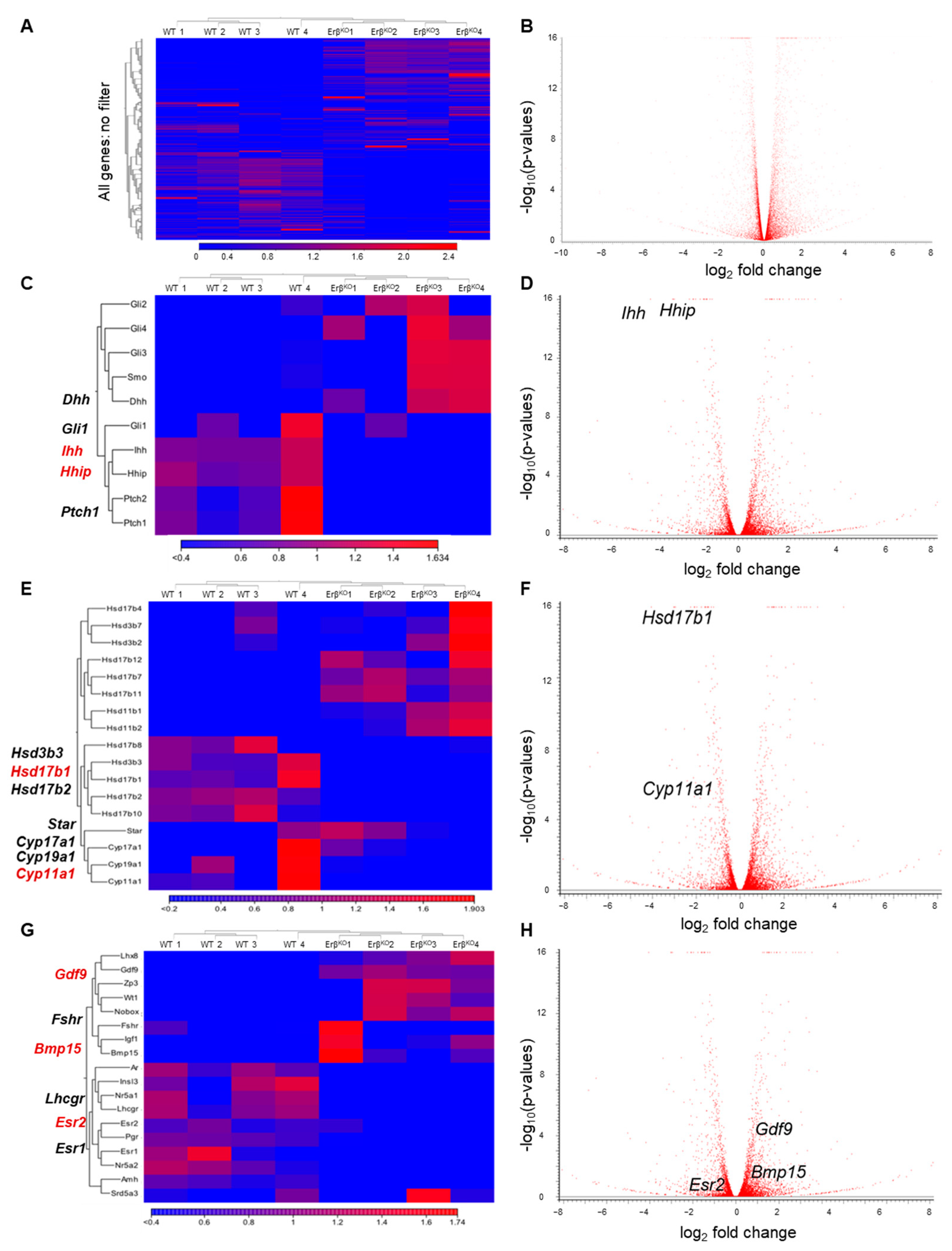
3.2. ERβ Regulates Genes in Neonatal Rat Ovaries
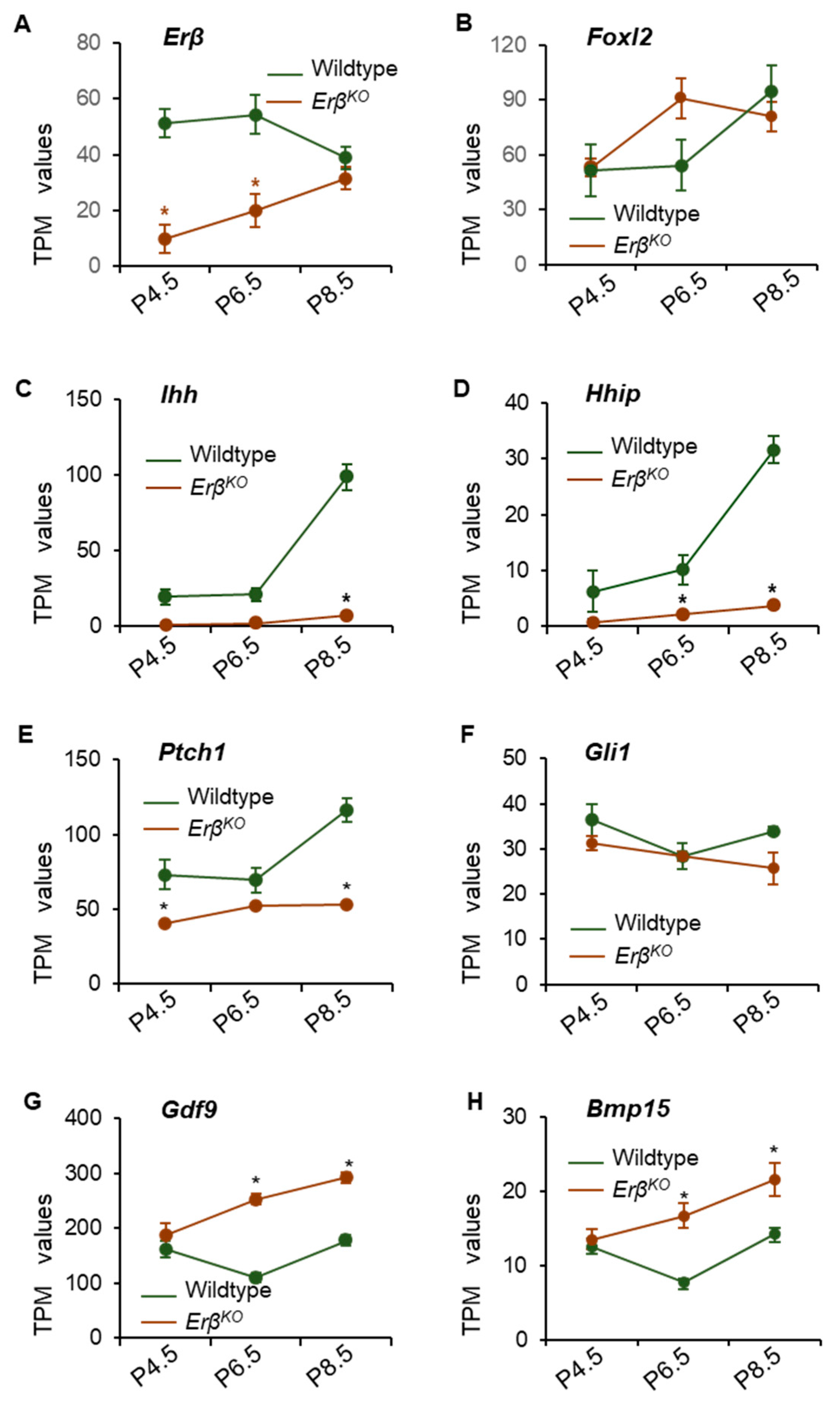
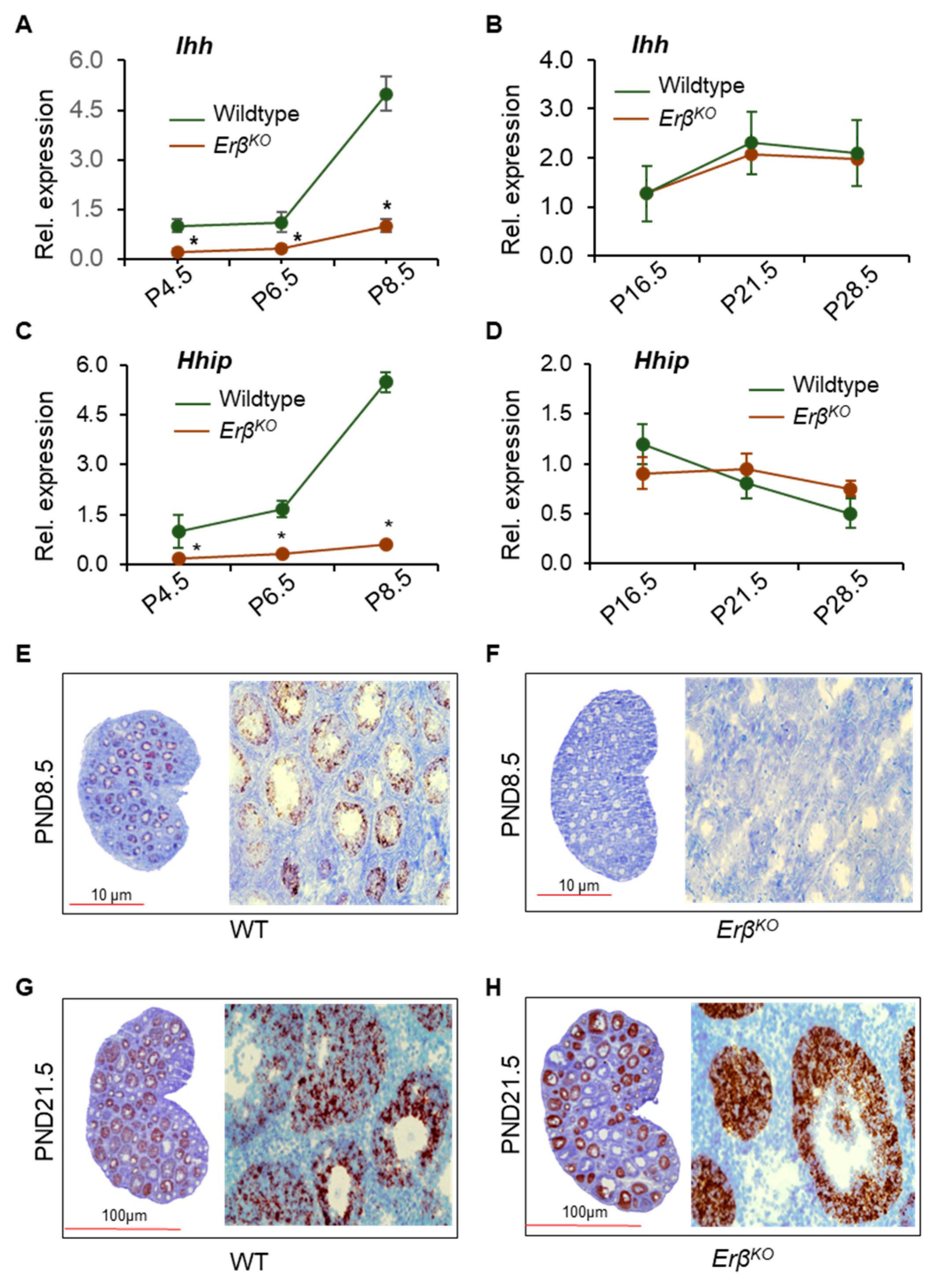
3.3. ERβ Regulates Indian Hedgehog Signaling
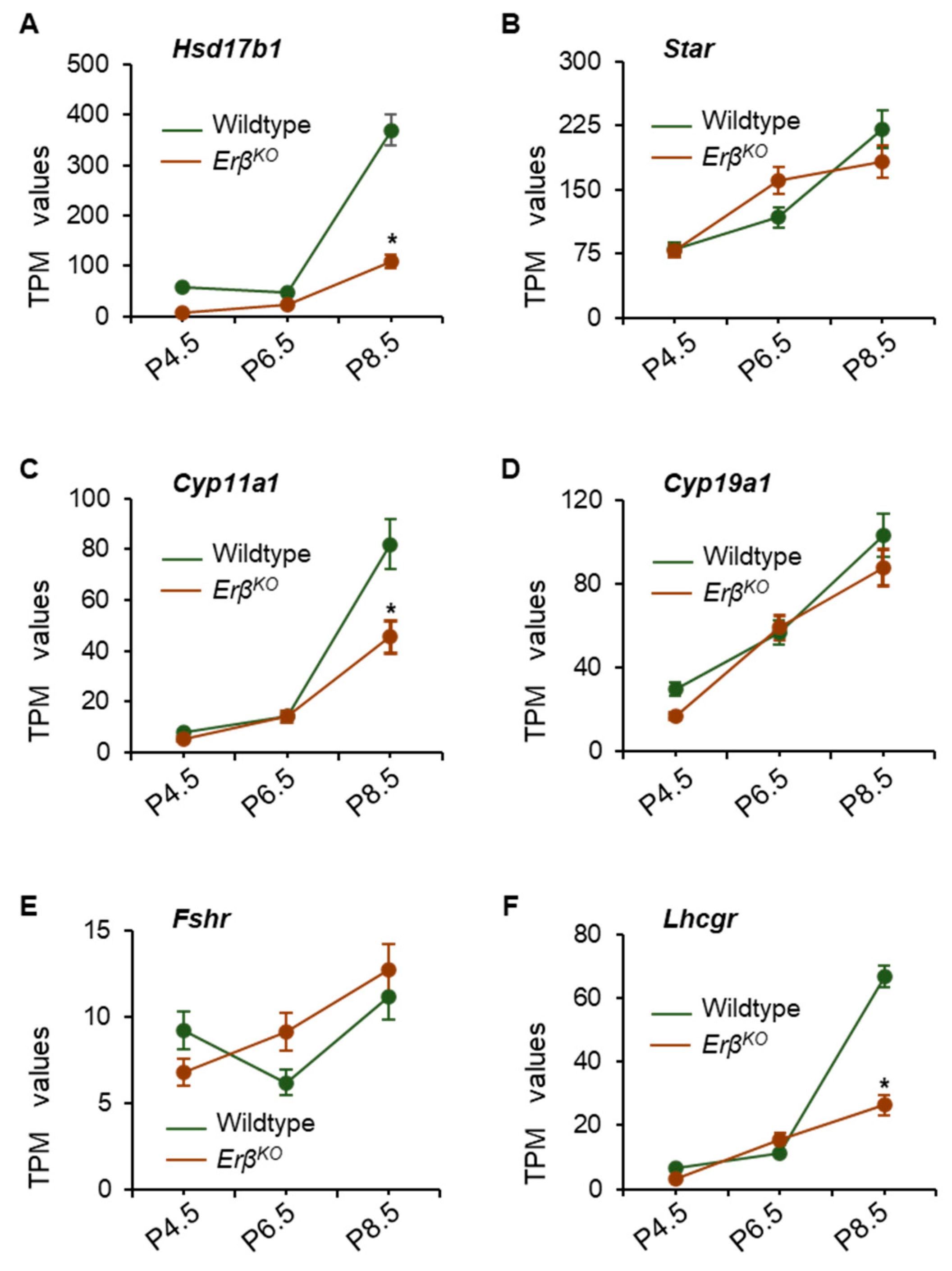
3.4. Altered Expression of Steroidogenic Enzyme Genes in ErβKO Ovaries
3.5. ERβ Regulates Ihh Only during the Early Neonatal Period
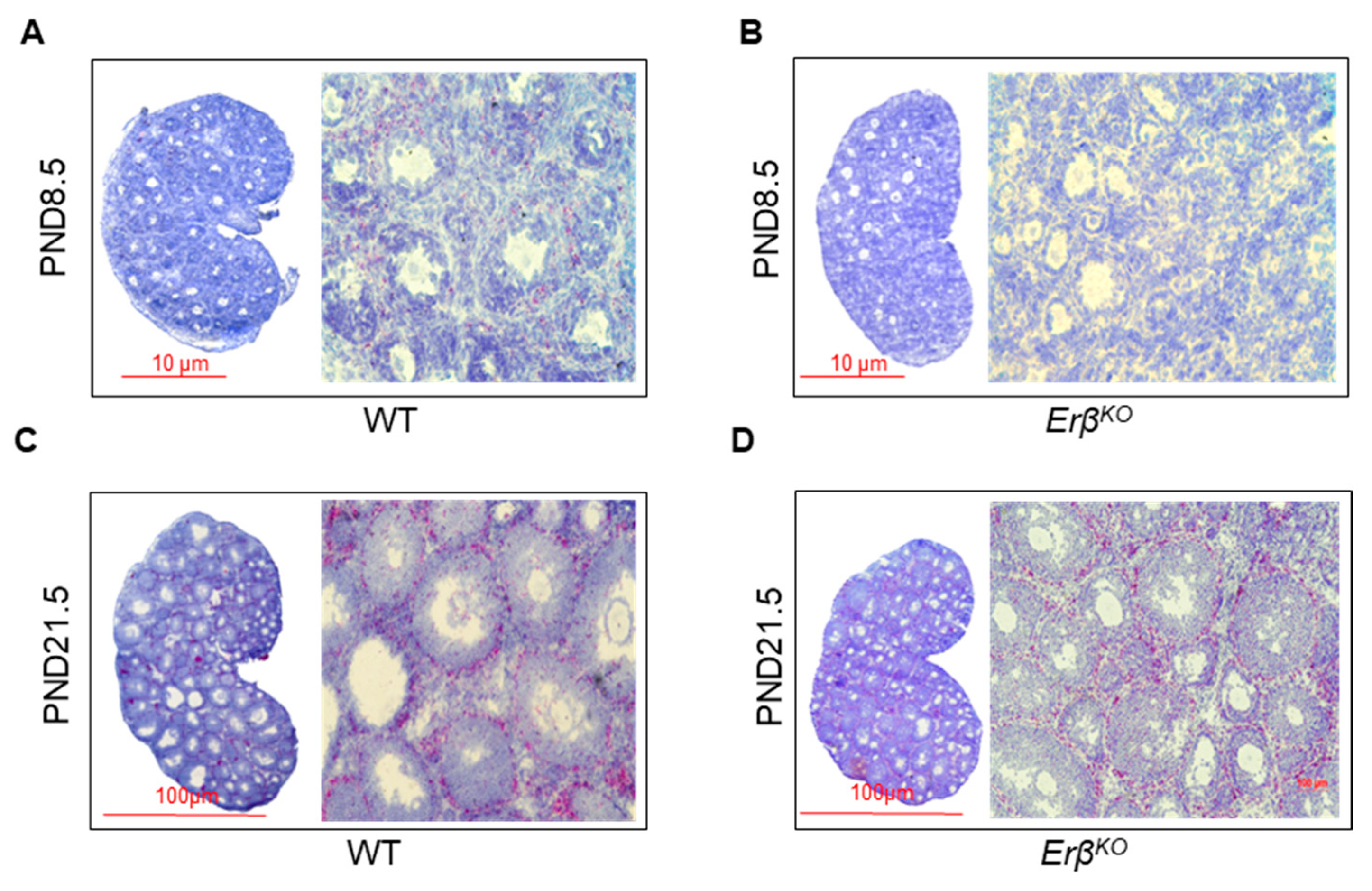
3.6. ERβ-Regulated Ihh Impacts Theca Cell Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, H.; Liu, K. The two classes of primordial follicles in the mouse ovary: Their development, physiological functions and implications for future research. Mol. Hum. Reprod. 2014, 20, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, H.; Gorre, N.; Risal, S.; Shen, Y.; Liu, K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet. 2014, 23, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, M.; Zhang, J.; Tong, Y.; Chen, T.; Wang, C.; Pan, W.; Xiao, Z. Mechanisms of primordial follicle activation and new pregnancy opportunity for premature ovarian failure patients. Front. Physiol. 2023, 14, 1113684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Liu, R.; Yang, C.; Wang, X.; Ran, Z.; Zhou, S.; Li, X.; He, C. A Prepubertal Mice Model to Study the Growth Pattern of Early Ovarian Follicles. Int. J. Mol. Sci. 2021, 22, 5130. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Murata, C.; Harikae, K.; Suzuki, H.; Kanai-Azuma, M.; Kurohmaru, M.; Tsunekawa, N.; Kanai, Y. Defects in the first wave of folliculogenesis in mouse XO ovaries. J. Reprod. Dev. 2017, 63, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Vaz, B.; El Mansouri, F.; Liu, X.; Taketo, T. Premature ovarian insufficiency in the XO female mouse on the C57BL/6J genetic background. Mol. Hum. Reprod. 2020, 26, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Rumi, M.A.K.; Singh, P.; Roby, K.F.; Zhao, X.; Iqbal, K.; Ratri, A.; Lei, T.; Cui, W.; Borosha, S.; Dhakal, P.; et al. Defining the Role of Estrogen Receptor beta in the Regulation of Female Fertility. Endocrinology 2017, 158, 2330–2343. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, V.P.; Ratri, A.; Masumi, S.; Borosha, S.; Ghosh, S.; Christenson, L.K.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. Granulosa cell genes that regulate ovarian follicle development beyond the antral stage: The role of estrogen receptor β. Mol. Cell Endocrinol. 2021, 528, 111212. [Google Scholar] [CrossRef]
- Khristi, V.; Chakravarthi, V.P.; Singh, P.; Ghosh, S.; Pramanik, A.; Ratri, A.; Borosha, S.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. ESR2 regulates granulosa cell genes essential for follicle maturation and ovulation. Mol. Cell Endocrinol. 2018, 474, 214–226. [Google Scholar] [CrossRef]
- Chakravarthi, V.P.; Ghosh, S.; Roby, K.F.; Wolfe, M.W.; Rumi, M.K. A Gatekeeping Role of ESR2 to Maintain the Primordial Follicle Reserve. Endocrinology 2020, 161, bqaa037. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Liu, K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 2009, 30, 438–464. [Google Scholar] [CrossRef]
- Ford, E.; Beckett, E.L.; Roman, S.; McLaughlin, E.A.; Sutherland, J. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 2019, 159, R15–R29. [Google Scholar] [CrossRef]
- Russell, M.C.; Cowan, R.G.; Harman, R.M.; Walker, A.L.; Quirk, S.M. The hedgehog signaling pathway in the mouse ovary. Biol. Reprod. 2007, 77, 226–236. [Google Scholar] [CrossRef]
- Wijgerde, M.; Ooms, M.; Hoogerbrugge, J.W.; Grootegoed, J.A. Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology 2005, 146, 3558–3566. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Peng, J.; Matzuk, M.M.; Yao, H.H. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat. Commun. 2015, 6, 6934. [Google Scholar] [CrossRef]
- Liu, C.; Rodriguez, K.F.; Brown, P.R.; Yao, H.H. Reproductive, Physiological, and Molecular Outcomes in Female Mice Deficient in Dhh and Ihh. Endocrinology 2018, 159, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- Finco, I.; LaPensee, C.R.; Krill, K.T.; Hammer, G.D. Hedgehog signaling and steroidogenesis. Annu. Rev. Physiol. 2015, 77, 105–129. [Google Scholar] [CrossRef]
- Ren, Y.; Cowan, R.G.; Harman, R.M.; Quirk, S.M. Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol. Endocrinol. 2009, 23, 711–723. [Google Scholar] [CrossRef]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and mechanisms. Genes Dev. 2008, 22, 2454–2472. [Google Scholar] [CrossRef]
- Franco, H.L.; Yao, H.H. Sex and hedgehog: Roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2012, 20, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.E.; Fuller, P.J. The importance of ERbeta signalling in the ovary. J. Endocrinol. 2010, 205, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, V.P.; Khristi, V.; Ghosh, S.; Yerrathota, S.; Dai, E.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. ESR2 Is Essential for Gonadotropin-Induced Kiss1 Expression in Granulosa Cells. Endocrinology 2018, 159, 3860–3873. [Google Scholar] [CrossRef] [PubMed]
- Picut, C.A.; Swanson, C.L.; Scully, K.L.; Roseman, V.C.; Parker, R.F.; Remick, A.K. Ovarian follicle counts using proliferating cell nuclear antigen (PCNA) and semi-automated image analysis in rats. Toxicol. Pathol. 2008, 36, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Roby, K.F.; Son, D.S.; Terranova, P.F. Alterations of events related to ovarian function in tumor necrosis factor receptor type I knockout mice. Biol. Reprod. 1999, 61, 1616–1621. [Google Scholar] [CrossRef]
- Roby, K.F. Alterations in follicle development, steroidogenesis, and gonadotropin receptor binding in a model of ovulatory blockade. Endocrinology 2001, 142, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Roby, K.F.; Son, D.S.; Taylor, C.C.; Montgomery-Rice, V.; Kirchoff, J.; Tang, S.; Terranova, P.F. Alterations in reproductive function in SRC tyrosine kinase knockout mice. Endocrine 2005, 26, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968, 17, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Reverchon, M.; Cloix, L.; Froment, P.; Ramé, C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 2012, 56, 959–967. [Google Scholar] [CrossRef]
- Hughes, C.H.K.; Smith, O.E.; Meinsohn, M.C.; Brunelle, M.; Gévry, N.; Murphy, B.D. Steroidogenic factor 1 (SF-1; Nr5a1) regulates the formation of the ovarian reserve. Proc. Natl. Acad. Sci. USA 2023, 120, e2220849120. [Google Scholar] [CrossRef]
- Russell, D.L.; Doyle, K.M.; Gonzales-Robayna, I.; Pipaon, C.; Richards, J.S. Egr-1 induction in rat granulosa cells by follicle-stimulating hormone and luteinizing hormone: Combinatorial regulation by transcription factors cyclic adenosine 3′,5′-monophosphate regulatory element binding protein, serum response factor, sp1, and early growth response factor-1. Mol. Endocrinol. 2003, 17, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Ovitt, C.E.; Anlag, K.; Fehsenfeld, S.; Gredsted, L.; Treier, A.C.; Treier, M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 2004, 131, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, A.; Hughes, C.H.K.; Murphy, B.D. Orphan nuclear receptors in angiogenesis and follicular development. Reproduction 2021, 162, R35–R54. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Yao, Z.; Lee, T.; Yamamoto, S.; Erickson, G.F.; Shimasaki, S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J. Biol. Chem. 2000, 275, 39523–39528. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Spradling, A.C. Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc. Natl. Acad. Sci. USA 2020, 117, 20015–20026. [Google Scholar] [CrossRef] [PubMed]
- Pisarska, M.D.; Barlow, G.; Kuo, F.T. Minireview: Roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology. Endocrinology 2011, 152, 1199–1208. [Google Scholar] [CrossRef]
- Nicol, B.; Grimm, S.A.; Chalmel, F.; Lecluze, E.; Pannetier, M.; Pailhoux, E.; Dupin-De-Beyssat, E.; Guiguen, Y.; Capel, B.; Yao, H.H. RUNX1 maintains the identity of the fetal ovary through an interplay with FOXL2. Nat. Commun. 2019, 10, 5116. [Google Scholar] [CrossRef]
- Mork, L.; Maatouk, D.M.; McMahon, J.A.; Guo, J.J.; Zhang, P.; McMahon, A.P.; Capel, B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol. Reprod. 2012, 86, 37. [Google Scholar] [CrossRef]
- Ng, A.; Tan, S.; Singh, G.; Rizk, P.; Swathi, Y.; Tan, T.Z.; Huang, R.Y.; Leushacke, M.; Barker, N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat. Cell Biol. 2014, 16, 745–757. [Google Scholar] [CrossRef]
- Rastetter, R.H.; Bernard, P.; Palmer, J.S.; Chassot, A.A.; Chen, H.; Western, P.S.; Ramsay, R.G.; Chaboissier, M.C.; Wilhelm, D. Marker genes identify three somatic cell types in the fetal mouse ovary. Dev. Biol. 2014, 394, 242–252. [Google Scholar] [CrossRef]
- Fowler, P.A.; Anderson, R.A.; Saunders, P.T.; Kinnell, H.; Mason, J.I.; Evans, D.B.; Bhattacharya, S.; Flannigan, S.; Franks, S.; Monteiro, A. Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J. Clin. Endocrinol. Metab. 2011, 96, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
| 1 A. Top 10 Downregulated Genes | ||||||
| Name | Chrom | ENSEMBL | Region | Max TPM | Fold Change | FDR p-Value |
| ENSRNOG 00000065112 | 1 | ENSRNOG00000065112 | 167210874..167213149 | 7.4 | −902.74 | 0.00 |
| Lce1m | 2 | ENSRNOG00000009581 | complement (178637495..178638697) | 6.26 | −280.11 | 0.00 |
| ENSRNOG 00000065518 | 2 | ENSRNOG00000065518 | complement (178666387..178668404) | 6.7 | −149.27 | 0.00 |
| Abcb1b | 4 | ENSRNOG00000066042 | complement (25242798..25325199) | 24.2 | −28.71 | 0.00 |
| Snrpc-ps3 | 10 | ENSRNOG00000017586 | complement (14191662..14192401) | 6.69 | −16.78 | 0.00 |
| Ihh | 9 | ENSRNOG00000018059 | complement (76504315..76510532) | 118.52 | −15.20 | 0.00 |
| Fam111a | 1 | ENSRNOG00000012067 | 209640953..209656547 | 156.28 | −8.39 | 0.00 |
| Hhip | 19 | ENSRNOG00000018268 | 27863213..27952528 | 39.48 | −7.87 | 0.00 |
| Gal | 1 | ENSRNOG00000015156 | complement (200650439..200654959) | 9.89 | −6.63 | 0.00 |
| Kcns2 | 7 | ENSRNOG00000066111 | 66022352..66028422 | 9.77 | −6.52 | 0.00 |
| 1 B. Top 10 Upregulated Genes | ||||||
| Name | Chrom | ENSEMBL | Region | Max TPM | Fold Change | FDR p-Value |
| LOC685716 | 11 | ENSRNOG00000054404 | 55280418..55303827 | 10.84 | 315.03 | 0.00 |
| LOC100360791 | 1 | ENSRNOG00000033517 | 19107386..19108232 | 470.98 | 17.11 | 0.00 |
| Slc27a5 | 1 | ENSRNOG00000019626 | complement (73616564..73627172) | 5.13 | 13.92 | 0.00 |
| Aldh1a7 | 1 | ENSRNOG00000017878 | complement (218201472..218248906) | 9.85 | 11.66 | 0.00 |
| ENSRNOG 00000066616 | 3 | ENSRNOG00000066616 | 149220737..149228279 | 5.05 | 9.67 | 0.00 |
| Actc1 | 3 | ENSRNOG00000008536 | complement (100811987..100817523) | 7.04 | 9.55 | 0.00 |
| Adcyap1r1 | 4 | ENSRNOG00000012098 | 84593892..84642700 | 25.86 | 9.29 | 0.00 |
| Adh6_1 | 2 | ENSRNOG00000012436 | 226797303..226808892 | 13.71 | 6.90 | 0.00 |
| Gabrr3 | 11 | ENSRNOG00000001679 | complement (40902812..40955263) | 10.62 | 6.76 | 0.00 |
| Cpa2 | 4 | ENSRNOG00000028092 | 59160357..59183674 | 250.63 | 6.74 | 0.00 |
| Name | Chrom | ENSEMBL | Region | Max TPM | Fold Change | FDR p-Value |
|---|---|---|---|---|---|---|
| Rbpjl | 3 | ENSRNOG00000026295 | 153134140..153146513 | 10.90 | 5.79 | 0.00 |
| Dbx2 | 7 | ENSRNOG00000006885 | complement (126772227..126802564) | 5.18 | 5.36 | 0.00 |
| Dmrt1 | 1 | ENSRNOG00000016075 | 223142859..223241333 | 6.22 | 5.13 | 0.00 |
| Npas2 | 9 | ENSRNOG00000013408 | 41463830..41642320 | 36.43 | 4.07 | 0.00 |
| Pou5f1 | 20 | ENSRNOG00000046487 | complement (3223129..3227891) | 20.60 | 2.59 | 0.00 |
| Setbp1 | 18 | ENSRNOG00000016208 | complement (72191035..72552556) | 5.24 | 2.18 | 0.00 |
| Hoxc6 | 7 | ENSRNOG00000063956 | 134135502..134148392 | 6.50 | 2.07 | 0.00 |
| Scx | 7 | ENSRNOG00000021812 | 108176608..108178626 | 13.02 | 2.04 | 0.00 |
| Pparg | 4 | ENSRNOG00000008839 | 148423194..148548468 | 14.06 | −2.04 | 0.00 |
| Mycn | 6 | ENSRNOG00000051372 | complement (35717764..35723590) | 58.93 | −2.05 | 0.00 |
| Fosl2 | 6 | ENSRNOG00000068412 | complement (24300956..24320034) | 36.83 | −2.10 | 0.00 |
| Egr1 | 18 | ENSRNOG00000019422 | 26462981..26466766 | 67.29 | −2.17 | 0.00 |
| Osr2 | 7 | ENSRNOG00000011136 | 66487839..66495224 | 92.84 | −2.26 | 0.00 |
| Nr5a1 | 3 | ENSRNOG00000012682 | complement (22465502..22486328) | 173.03 | −2.35 | 0.00 |
| Foxo1 | 2 | ENSRNOG00000013397 | 136312168..136387790 | 97.01 | −2.38 | 0.00 |
| Fos | 6 | ENSRNOG00000008015 | 105121170..105124036 | 23.72 | −2.52 | 0.00 |
| Myc | 7 | ENSRNOG00000004500 | 93593705..93598630 | 47.62 | −2.60 | 0.00 |
| Nr4a1 | 7 | ENSRNOG00000007607 | 132374840..132389297 | 87.54 | −4.97 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakravarthi, V.P.; Dilower, I.; Ghosh, S.; Borosha, S.; Mohamadi, R.; Dahiya, V.; Vo, K.; Lee, E.B.; Ratri, A.; Kumar, V.; et al. ERβ Regulation of Indian Hedgehog Expression in the First Wave of Ovarian Follicles. Cells 2024, 13, 644. https://doi.org/10.3390/cells13070644
Chakravarthi VP, Dilower I, Ghosh S, Borosha S, Mohamadi R, Dahiya V, Vo K, Lee EB, Ratri A, Kumar V, et al. ERβ Regulation of Indian Hedgehog Expression in the First Wave of Ovarian Follicles. Cells. 2024; 13(7):644. https://doi.org/10.3390/cells13070644
Chicago/Turabian StyleChakravarthi, V. Praveen, Iman Dilower, Subhra Ghosh, Shaon Borosha, Ryan Mohamadi, Vinesh Dahiya, Kevin Vo, Eun B. Lee, Anamika Ratri, Vishnu Kumar, and et al. 2024. "ERβ Regulation of Indian Hedgehog Expression in the First Wave of Ovarian Follicles" Cells 13, no. 7: 644. https://doi.org/10.3390/cells13070644





