Human-Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Showed Neuronal Differentiation, Neurite Extension, and Formation of Synaptic Structures in Rodent Ischemic Stroke Brains
Abstract
1. Introduction
2. Materials and Methods
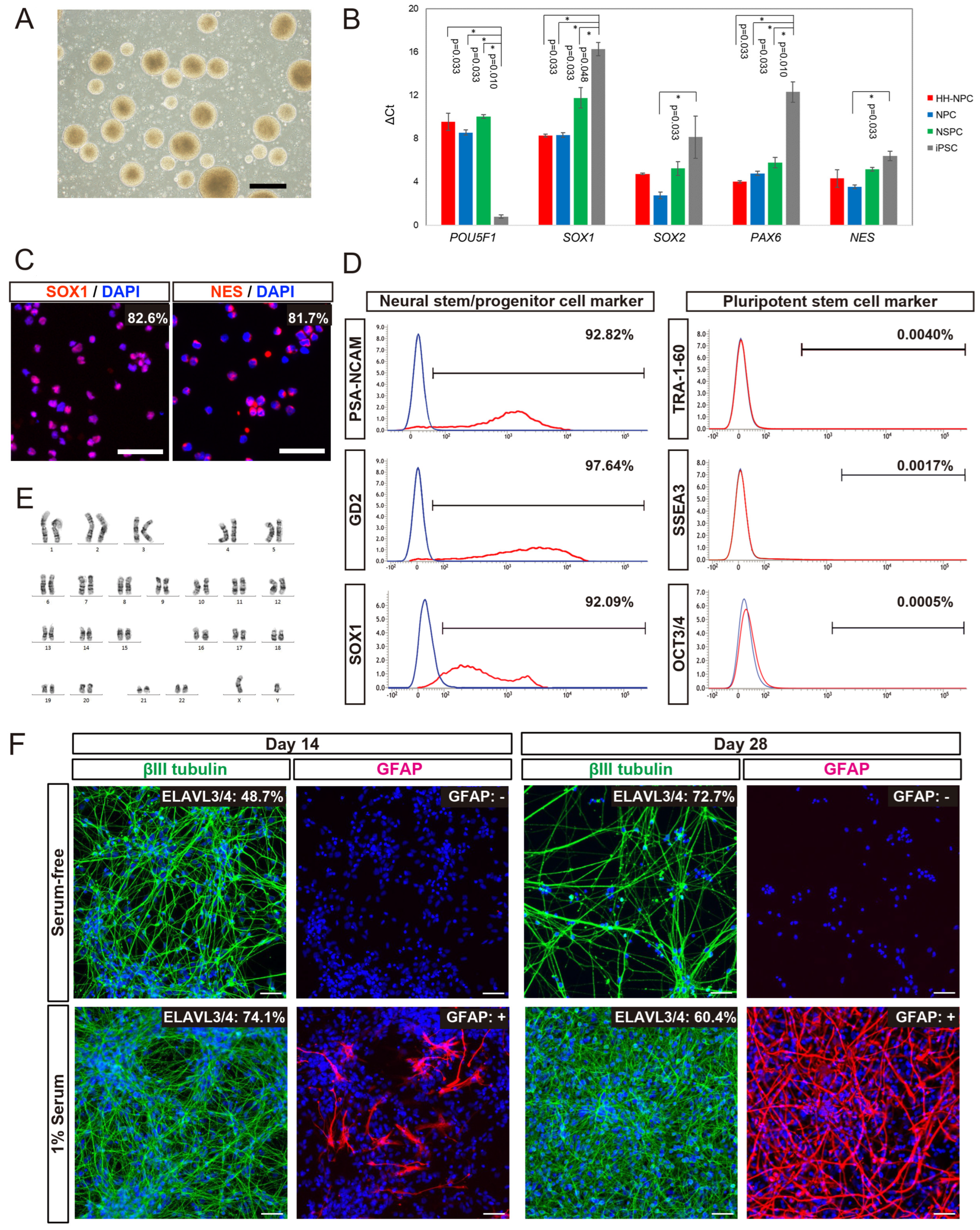
2.1. Neural Induction of hiPSCs
2.2. Transcript Analyses
2.3. Flow Cytometric (FCM) Analyses
2.4. Cell Proliferation Analysis
2.5. Karyotype Analysis
2.6. Copy Number Analysis
2.7. In Vitro Differentiation Assay
2.8. Immunocytochemical (ICC) Analysis
2.9. Animal Welfare
2.10. Rodent Middle Cerebral Artery Occlusion Model
2.11. Intracerebral Transplantation of hiPSC-NPCs
2.12. Neurological Functional Assessment
2.12.1. Neurological Deficit Score (NDS)
2.12.2. Step Test
2.12.3. Rotarod Test
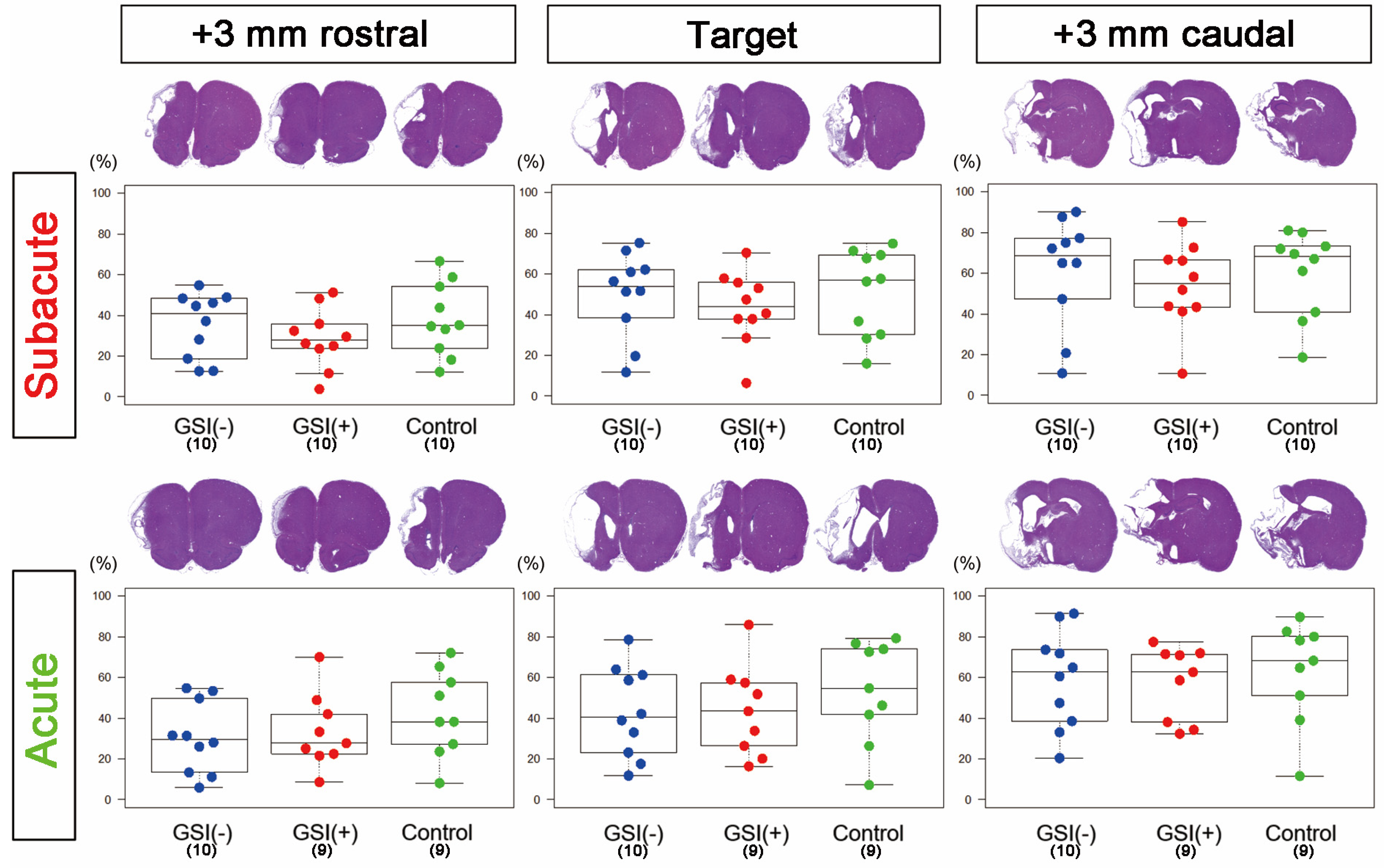
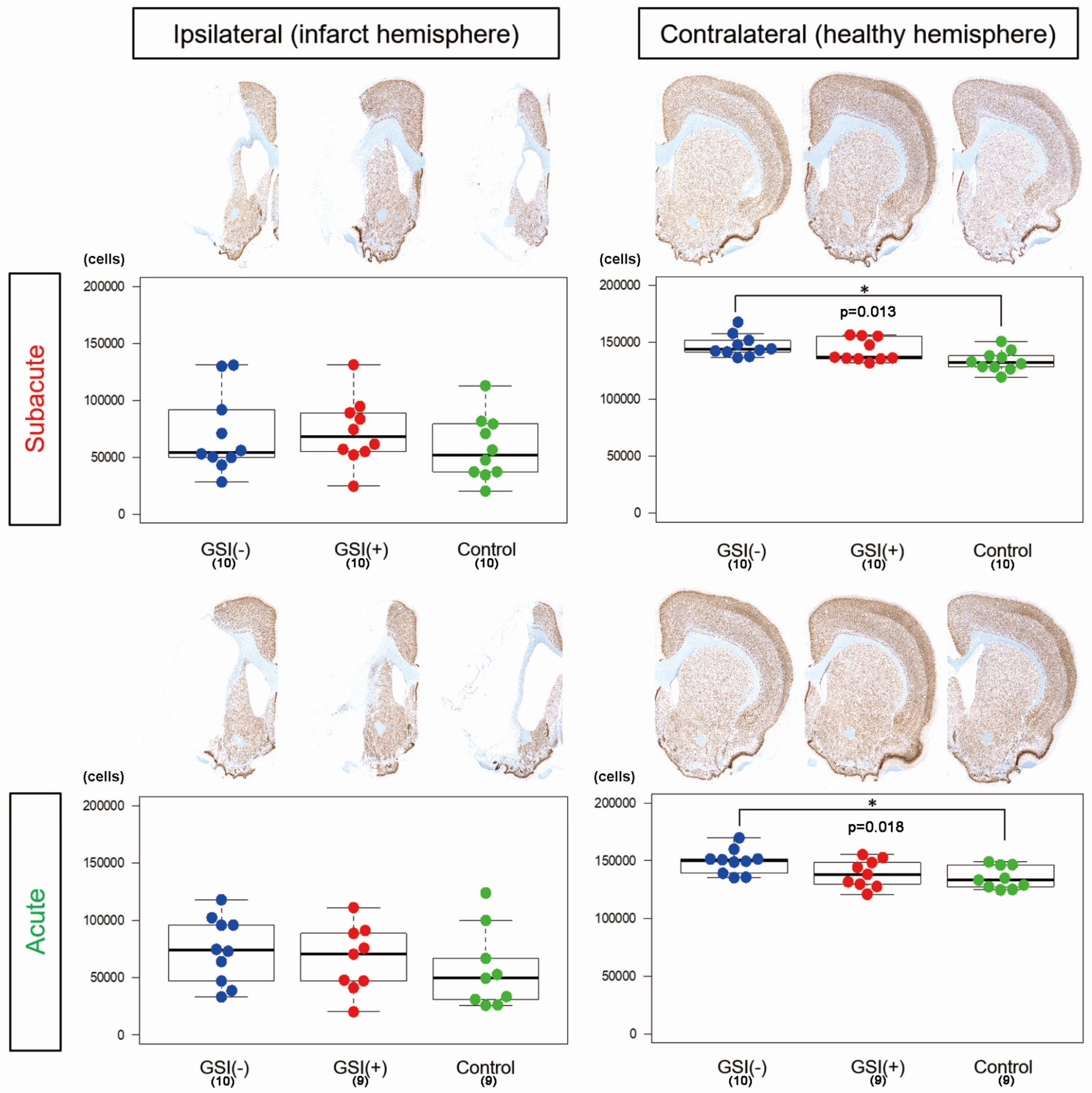
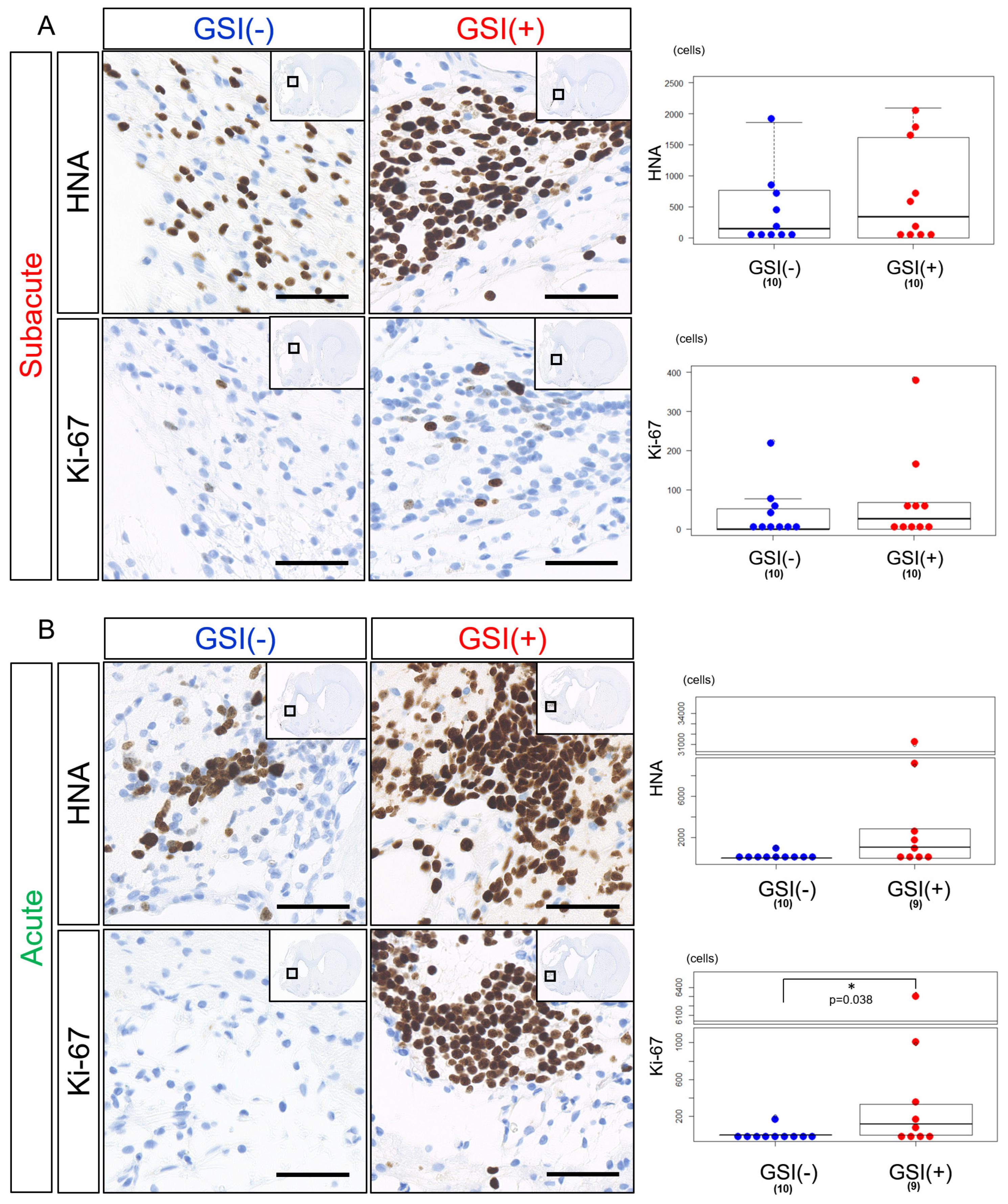
2.13. Histopathological Analysis
2.14. Imaging Analysis
2.15. Statistical Analysis
3. Results
3.1. Cellular Properties of hiPSC-NPCs Generated from HLA-Homo hiPSCs
3.2. Evaluations of Infarction Size and Numbers of Residual Neurons in Host Brains after Transplantation of HLA-Homo hiPSC-NPCs
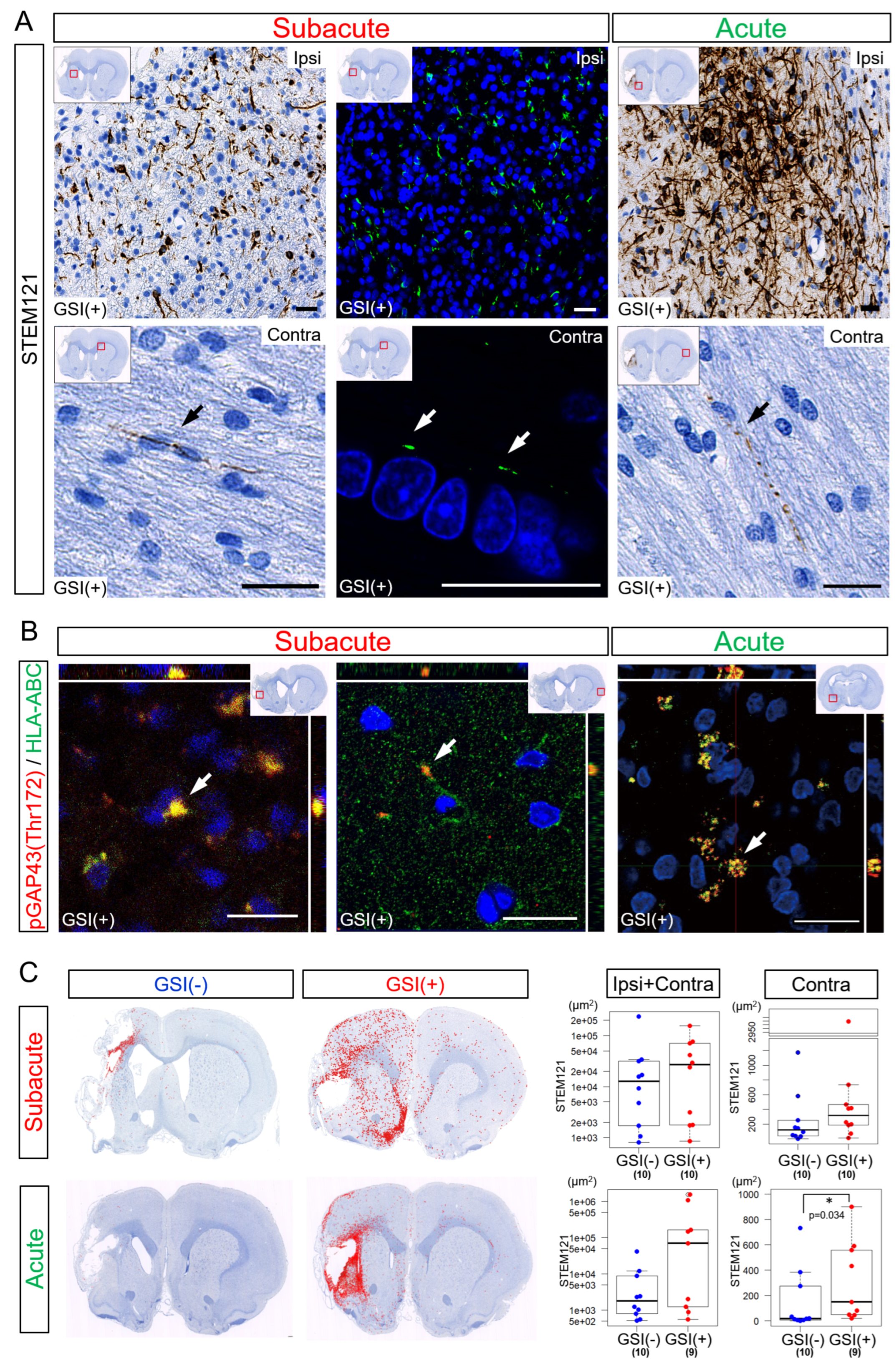
3.3. In Vivo Survival of the Transplanted HLA-Homo hiPSC-NPCs in the Ischemic Brain
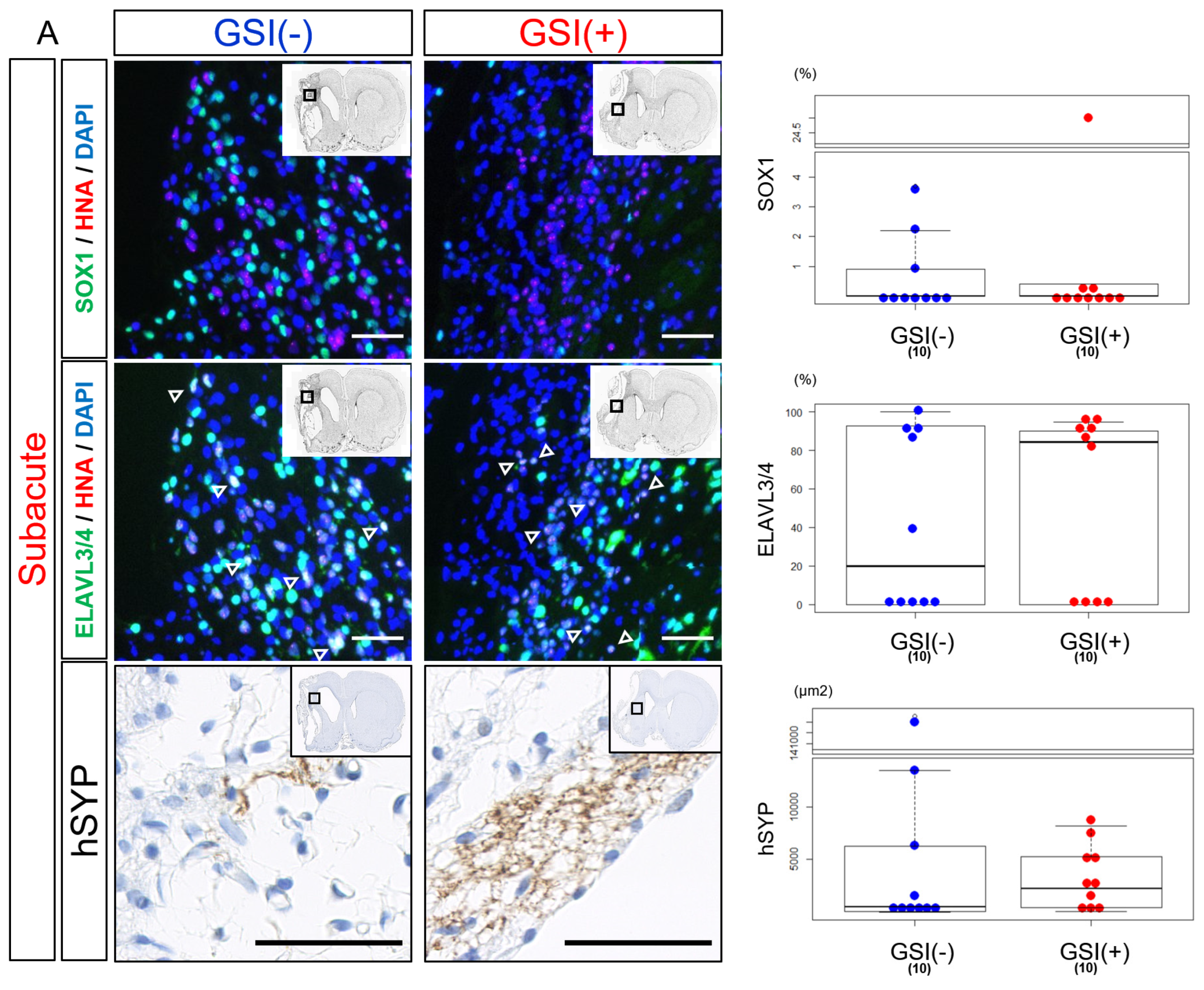
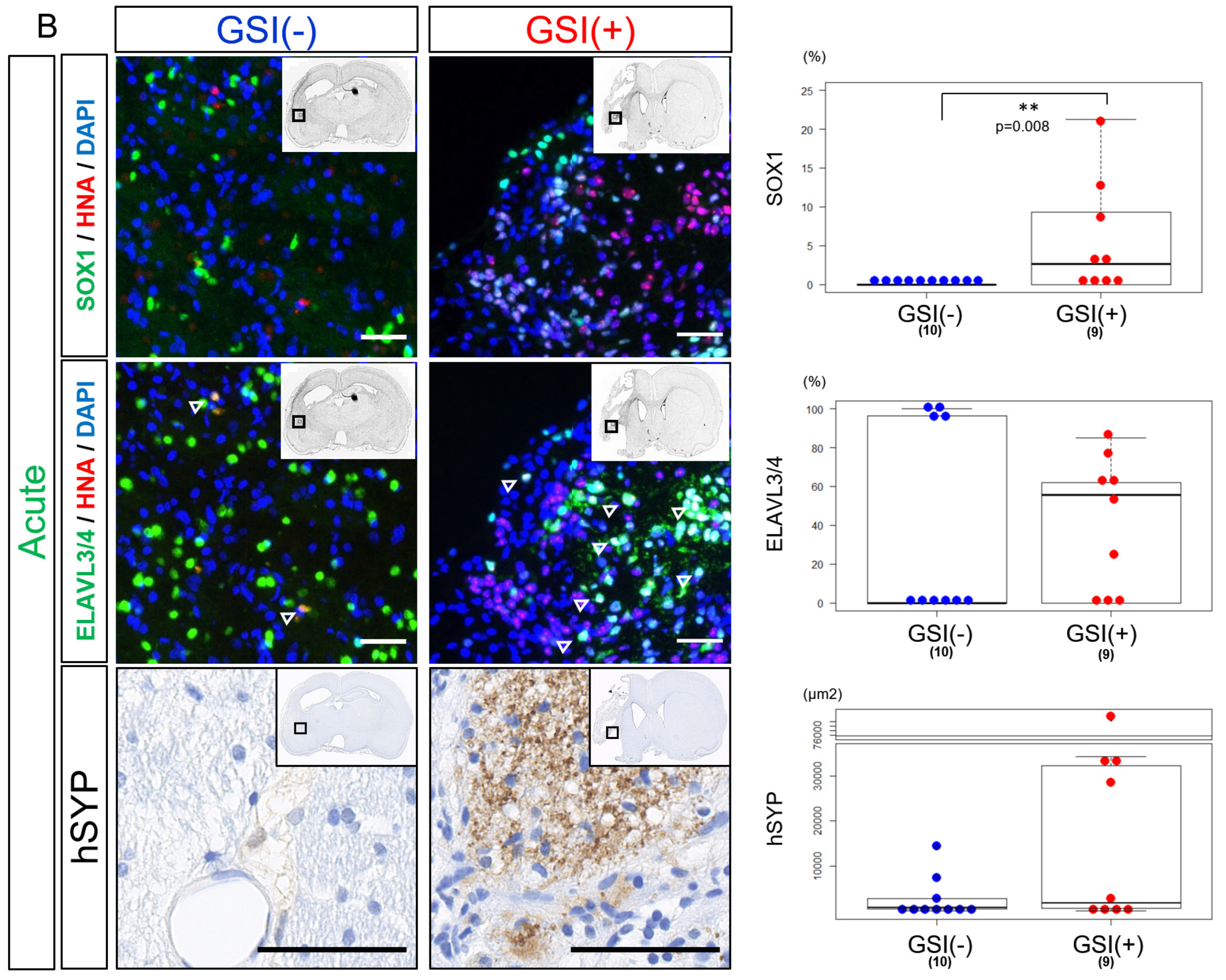
3.4. In Vivo Differentiation of the Transplanted HLA-Homo hiPSC-NPCs in the Ischemic Brain
3.5. Neurites Growth from the Transplanted HLA-Homo hiPSC-NPCs
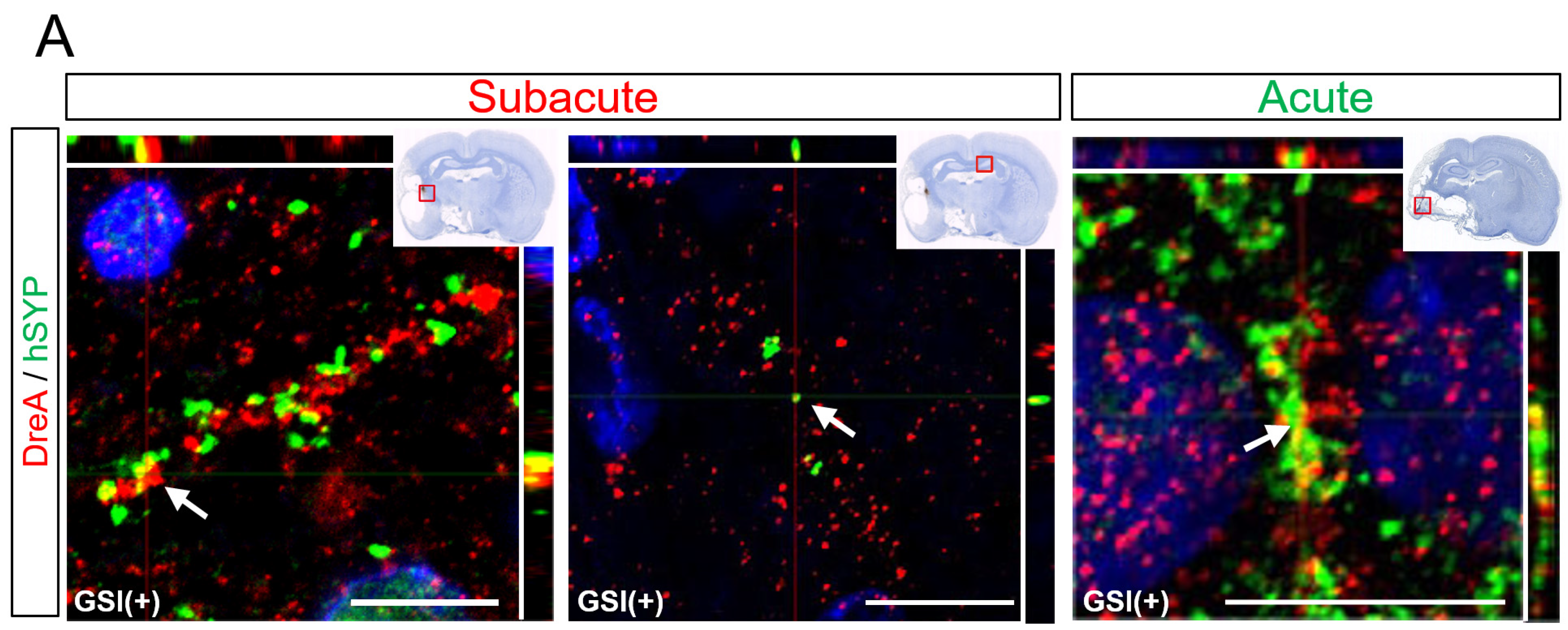
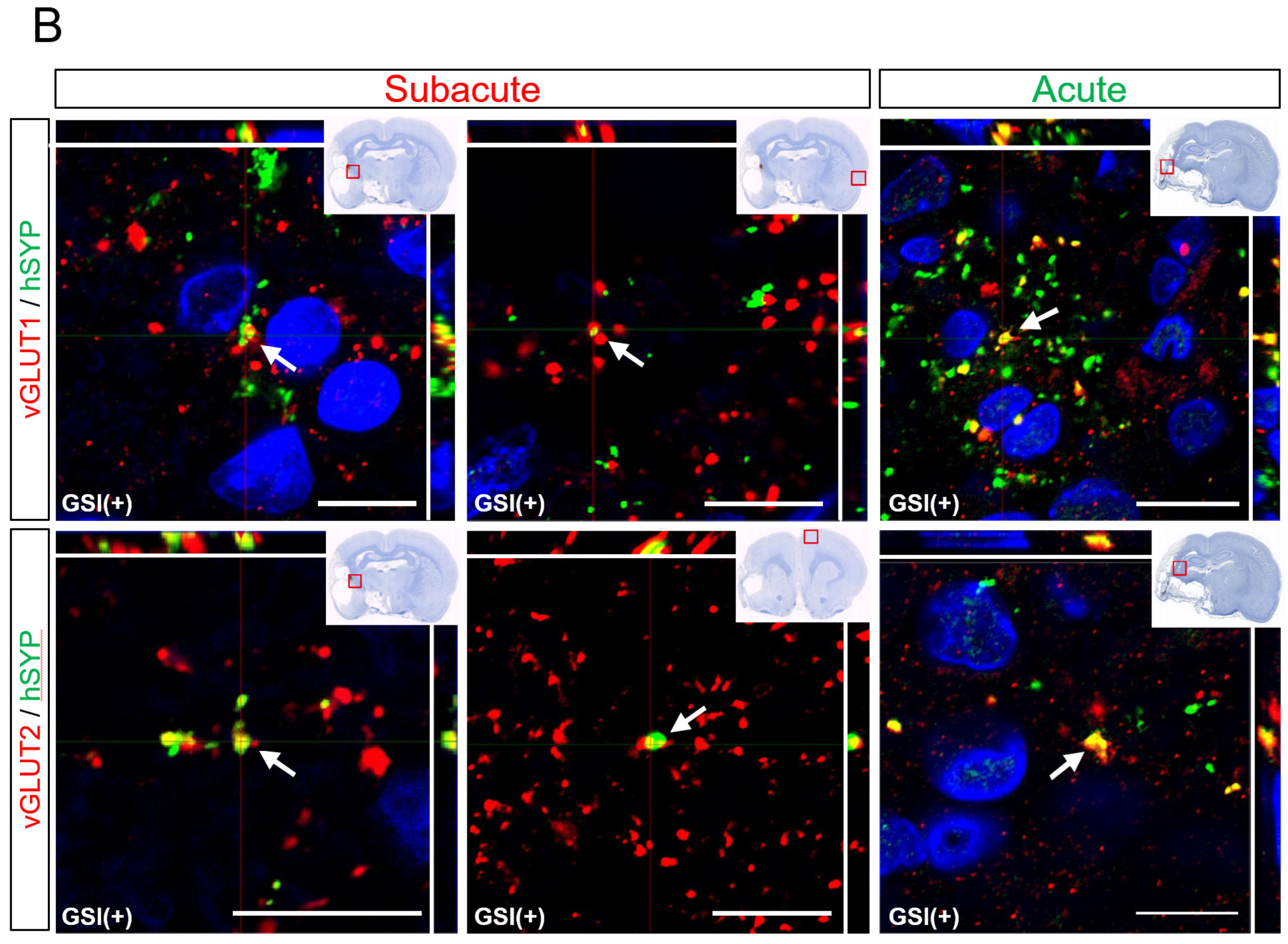
3.6. In Vivo Maturation of the Transplanted HLA-Homo hiPSC-NPCs in the Ischemic Brain
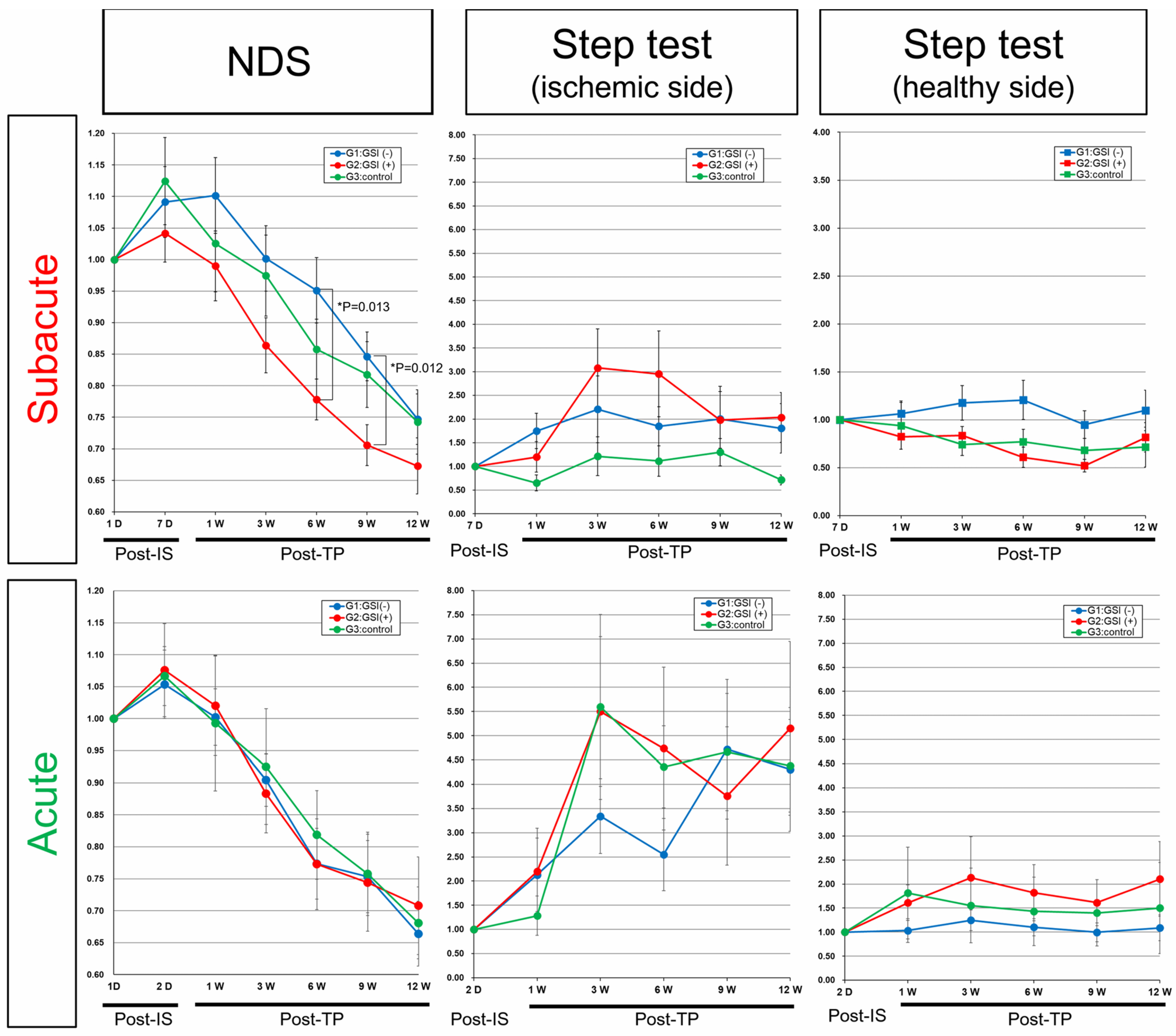
3.7. Neurological Functional Assessment after Intracerebral Transplantation of HLA-Homo hiPSC-NPCs
4. Discussion
4.1. Exploring Cell-Based Therapies in Ischemic Stroke: From Neuroprotection to Cell Replacement
4.2. Evaluating the Therapeutic Potential of Transplanted hiPSC-NPCs in Ischemic Stroke Recovery: Successes, Limitations, and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, Y.; Wakhloo, A.K.; Fisher, M. Advances in Acute Ischemic Stroke Therapy. Circ. Res. 2022, 130, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.F.; Rabinstein, A.A.; Middlebrooks, E.H.; Haranhalli, N.; Silliman, S.L.; Meschia, J.F.; Tawk, R.G. Diagnosis and Management of Acute Ischemic Stroke. Mayo Clin. Proc. 2018, 93, 523–538. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Lindvall, O.; Kokaia, Z. Stem cell research in stroke: How far from the clinic? Stroke 2011, 42, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Hasan, A.; Rizzi, R.; Althani, A.; Afifi, N.; Cenciarelli, C.; Caceci, T.; Shuaib, A. Potential of Stem Cell-Based Therapy for Ischemic Stroke. Front. Neurol. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl. Med. 2019, 8, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Kung, D.K.; Chen, H.I. Cell Therapy for Stroke: A Mechanistic Analysis. Neurosurgery 2021, 88, 733–745. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Hu, B.; Hong, Y.; Zhang, H.; Li, X.; Zhang, Y. Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Front. Cell. Neurosci. 2021, 15, 628940. [Google Scholar] [CrossRef]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef]
- Nishino, H.; Borlongan, C.V. Restoration of function by neural transplantation in the ischemic brain. Prog. Brain Res. 2000, 127, 461–476. [Google Scholar]
- Nishino, H.; Aihara, N.; Czurko, A.; Hashitani, T.; Isobe, Y.; Ichikawa, O.; Watari, H. Reconstruction of GABAergic transmission and behavior by striatal cell grafts in rats with ischemic infarcts in the middle cerebral artery. J. Neural Transplant. Plast. 1993, 4, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Aihara, N.; Mizukawa, K.; Koide, K.; Mabe, H.; Nishino, H. Striatal grafts in infarct striatopallidum increase GABA release, reorganize GABAA receptor and improve water-maze learning in the rat. Brain Res. Bull. 1994, 33, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Steinberg, G.K.; Wechsler, L.; Meltzer, C.C.; Elder, E.; Gebel, J.; Decesare, S.; Jovin, T.; Zafonte, R.; Lebowitz, J.; et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J. Neurosurg. 2005, 103, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Dinsmore, J.; Wu, J.; Henderson, G.V.; Stieg, P.; Caplan, L.R. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: A preliminary safety and feasibility study. Cerebrovasc. Dis. 2005, 20, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Pollock, K.; Stroemer, P.; Patel, S.; Stevanato, L.; Hope, A.; Miljan, E.; Dong, Z.; Hodges, H.; Price, J.; Sinden, J.D. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol. 2006, 199, 143–155. [Google Scholar] [CrossRef]
- Andres, R.H.; Horie, N.; Slikker, W.; Keren-Gill, H.; Zhan, K.; Sun, G.; Manley, N.C.; Pereira, M.P.; Sheikh, L.A.; McMillan, E.L.; et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011, 134, 1777–1789. [Google Scholar] [CrossRef]
- Smith, E.J.; Stroemer, R.P.; Gorenkova, N.; Nakajima, M.; Crum, W.R.; Tang, E.; Stevanato, L.; Sinden, J.D.; Modo, M. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells 2012, 30, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 2014, 5, 129. [Google Scholar] [CrossRef]
- Bühnemann, C.; Scholz, A.; Bernreuther, C.; Malik, C.Y.; Braun, H.; Schachner, M.; Reymann, K.G.; Dihné, M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain 2006, 129, 3238–3248. [Google Scholar] [CrossRef]
- Fujimoto, M.; Hayashi, H.; Takagi, Y.; Hayase, M.; Marumo, T.; Gomi, M.; Nishimura, M.; Kataoka, H.; Takahashi, J.; Hashimoto, N.; et al. Transplantation of telencephalic neural progenitors induced from embryonic stem cells into subacute phase of focal cerebral ischemia. Lab. Investig. 2012, 92, 522–531. [Google Scholar] [CrossRef][Green Version]
- Oki, K.; Tatarishvili, J.; Wood, J.; Koch, P.; Wattananit, S.; Mine, Y.; Monni, E.; Tornero, D.; Ahlenius, H.; Ladewig, J.; et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 2012, 30, 1120–1133. [Google Scholar] [CrossRef]
- Tornero, D.; Wattananit, S.; Grønning Madsen, M.; Koch, P.; Wood, J.; Tatarishvili, J.; Mine, Y.; Ge, R.; Monni, E.; Devaraju, K.; et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 2013, 136, 3561–3577. [Google Scholar] [CrossRef] [PubMed]
- Palma-Tortosa, S.; Tornero, D.; Grønning Hansen, M.; Monni, E.; Hajy, M.; Kartsivadze, S.; Aktay, S.; Tsupykov, O.; Parmar, M.; Deisseroth, K.; et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 9094–9100. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.E.; Oh, S.H.; Lee, S.; Kim, Y.H.; Park, H.J.; Ju, J.H.; Kim, H.S.; Huh, J.Y.; Song, J. Intracerebral transplantation of HLA-homozygous human iPSC-derived neural precursors ameliorates the behavioural and pathological deficits in a rodent model of ischaemic stroke. Cell Prolif. 2020, 53, e12884. [Google Scholar] [CrossRef]
- Gomi, M.; Takagi, Y.; Morizane, A.; Doi, D.; Nishimura, M.; Miyamoto, S.; Takahashi, J. Functional recovery of the murine brain ischemia model using human induced pluripotent stem cell-derived telencephalic progenitors. Brain Res. 2012, 1459, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Polentes, J.; Jendelova, P.; Cailleret, M.; Braun, H.; Romanyuk, N.; Tropel, P.; Brenot, M.; Itier, V.; Seminatore, C.; Baldauf, K.; et al. Human induced pluripotent stem cells improve stroke outcome and reduce secondary degeneration in the recipient brain. Cell Transplant. 2012, 21, 2587–2602. [Google Scholar] [CrossRef]
- Jensen, M.B.; Yan, H.; Krishnaney-Davison, R.; Al Sawaf, A.; Zhang, S.C. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J. Stroke Cerebrovasc. Dis. 2013, 22, 304–308. [Google Scholar] [CrossRef]
- Yuan, T.; Liao, W.; Feng, N.H.; Lou, Y.L.; Niu, X.; Zhang, A.J.; Wang, Y.; Deng, Z.F. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion. Stem Cell Res. Ther. 2013, 4, 73. [Google Scholar] [CrossRef]
- Mohamad, O.; Drury-Stewart, D.; Song, M.; Faulkner, B.; Chen, D.; Yu, S.P.; Wei, L. Vector-free and transgene-free human iPS cells differentiate into functional neurons and enhance functional recovery after ischemic stroke in mice. PLoS ONE 2013, 8, e64160. [Google Scholar] [CrossRef]
- Chau, M.J.; Deveau, T.C.; Song, M.; Gu, X.; Chen, D.; Wei, L. iPSC Transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells 2014, 32, 3075–3087. [Google Scholar] [CrossRef]
- Yu, S.P.; Tung, J.K.; Wei, Z.Z.; Chen, D.; Berglund, K.; Zhong, W.; Zhang, J.Y.; Gu, X.; Song, M.; Gross, R.E.; et al. Optochemogenetic Stimulation of Transplanted iPS-NPCs Enhances Neuronal Repair and Functional Recovery after Ischemic Stroke. J. Neurosci. 2019, 39, 6571–6594. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Jeong, Y.W.; Choi, W.; Noh, J.E.; Lee, S.; Kim, H.S.; Song, J. Multimodal Therapeutic Effects of Neural Precursor Cells Derived from Human-Induced Pluripotent Stem Cells through Episomal Plasmid-Based Reprogramming in a Rodent Model of Ischemic Stroke. Stem Cells Int. 2020, 2020, 4061516. [Google Scholar] [CrossRef] [PubMed]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, J.S.; Leem, J.W.; Huh, Y.J.; Kim, J.Y.; Kim, H.S.; Park, I.H.; Daley, G.Q.; Hwang, D.Y.; Kim, D.W. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. Rep. 2010, 6, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Sugai, K.; Fukuzawa, R.; Shofuda, T.; Fukusumi, H.; Kawabata, S.; Nishiyama, Y.; Higuchi, Y.; Kawai, K.; Isoda, M.; Kanematsu, D.; et al. Pathological classification of human iPSC-derived neural stem/progenitor cells towards safety assessment of transplantation therapy for CNS diseases. Mol. Brain 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kato, T.M.; Sato, Y.; Umekage, M.; Ichisaka, T.; Tsukahara, M.; Takasu, N.; Yamanaka, S. A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med 2023, 4, 51–66.e10. [Google Scholar] [CrossRef]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef]
- Okita, K.; Yamakawa, T.; Matsumura, Y.; Sato, Y.; Amano, N.; Watanabe, A.; Goshima, N.; Yamanaka, S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Nakagawa, M.; Taniguchi, Y.; Senda, S.; Takizawa, N.; Ichisaka, T.; Asano, K.; Morizane, A.; Doi, D.; Takahashi, J.; Nishizawa, M.; et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014, 4, 3594. [Google Scholar] [CrossRef]
- Kanemura, Y.; Mori, H.; Kobayashi, S.; Islam, O.; Kodama, E.; Yamamoto, A.; Nakanishi, Y.; Arita, N.; Yamasaki, M.; Okano, H.; et al. Evaluation of in vitro proliferative activity of human fetal neural stem/progenitor cells using indirect measurements of viable cells based on cellular metabolic activity. J. Neurosci. Res. 2002, 69, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- 11 Neoplasia. In ISCN 2020: An International System for Human Cytogenomic Nomenclature (2020); McGowan-Jordan, J., Hastings, R.J., Moore, S., Eds.; Karger: Basel, Switzerland, 2020; pp. 88–97. [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Koizumi, J.; Yoshida, Y.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema. Jpn. J. Stroke 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Leong, W.K.; Henshall, T.L.; Arthur, A.; Kremer, K.L.; Lewis, M.D.; Helps, S.C.; Field, J.; Hamilton-Bruce, M.A.; Warming, S.; Manavis, J.; et al. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl. Med. 2012, 1, 177–187. [Google Scholar] [CrossRef]
- Okubo, T.; Iwanami, A.; Kohyama, J.; Itakura, G.; Kawabata, S.; Nishiyama, Y.; Sugai, K.; Ozaki, M.; Iida, T.; Matsubayashi, K.; et al. Pretreatment with a γ-Secretase Inhibitor Prevents Tumor-like Overgrowth in Human iPSC-Derived Transplants for Spinal Cord Injury. Stem Cell Reports 2016, 7, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, H.; Tatsumi, M.; Nitta, S.; Ichise, R.; Muramatsu, K.; Iida, M.; Nishimura, S.; Umemura, K. Time courses of progress to the chronic stage of middle cerebral artery occlusion models in rats. Exp. Brain Res. 2002, 146, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Schallert, T.; De Ryck, M.; Whishaw, I.Q.; Ramirez, V.D.; Teitelbaum, P. Excessive bracing reactions and their control by atropine and L-DOPA in an animal analog of Parkinsonism. Exp. Neurol. 1979, 64, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Okada, M.; Kawagoe, Y.; Sato, Y.; Nozumi, M.; Ishikawa, Y.; Tamada, A.; Yamazaki, H.; Sekino, Y.; Kanemura, Y.; Shinmyo, Y.; et al. Phosphorylation of GAP-43 T172 is a molecular marker of growing axons in a wide range of mammals including primates. Mol. Brain 2021, 14, 66. [Google Scholar] [CrossRef]
- Shirao, T.; Hanamura, K.; Koganezawa, N.; Ishizuka, Y.; Yamazaki, H.; Sekino, Y. The role of drebrin in neurons. J. Neurochem. 2017, 141, 819–834. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanemura, Y.; Yamamoto, A.; Katsuma, A.; Fukusumi, H.; Shofuda, T.; Kanematsu, D.; Handa, Y.; Sumida, M.; Yoshioka, E.; Mine, Y.; et al. Human-Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Showed Neuronal Differentiation, Neurite Extension, and Formation of Synaptic Structures in Rodent Ischemic Stroke Brains. Cells 2024, 13, 671. https://doi.org/10.3390/cells13080671
Kanemura Y, Yamamoto A, Katsuma A, Fukusumi H, Shofuda T, Kanematsu D, Handa Y, Sumida M, Yoshioka E, Mine Y, et al. Human-Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Showed Neuronal Differentiation, Neurite Extension, and Formation of Synaptic Structures in Rodent Ischemic Stroke Brains. Cells. 2024; 13(8):671. https://doi.org/10.3390/cells13080671
Chicago/Turabian StyleKanemura, Yonehiro, Atsuyo Yamamoto, Asako Katsuma, Hayato Fukusumi, Tomoko Shofuda, Daisuke Kanematsu, Yukako Handa, Miho Sumida, Ema Yoshioka, Yutaka Mine, and et al. 2024. "Human-Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Showed Neuronal Differentiation, Neurite Extension, and Formation of Synaptic Structures in Rodent Ischemic Stroke Brains" Cells 13, no. 8: 671. https://doi.org/10.3390/cells13080671
APA StyleKanemura, Y., Yamamoto, A., Katsuma, A., Fukusumi, H., Shofuda, T., Kanematsu, D., Handa, Y., Sumida, M., Yoshioka, E., Mine, Y., Yamaguchi, R., Okada, M., Igarashi, M., Sekino, Y., Shirao, T., Nakamura, M., & Okano, H. (2024). Human-Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Showed Neuronal Differentiation, Neurite Extension, and Formation of Synaptic Structures in Rodent Ischemic Stroke Brains. Cells, 13(8), 671. https://doi.org/10.3390/cells13080671






