Somatic Mutations in Surgically Treated Colorectal Liver Metastases: An Overview
Abstract
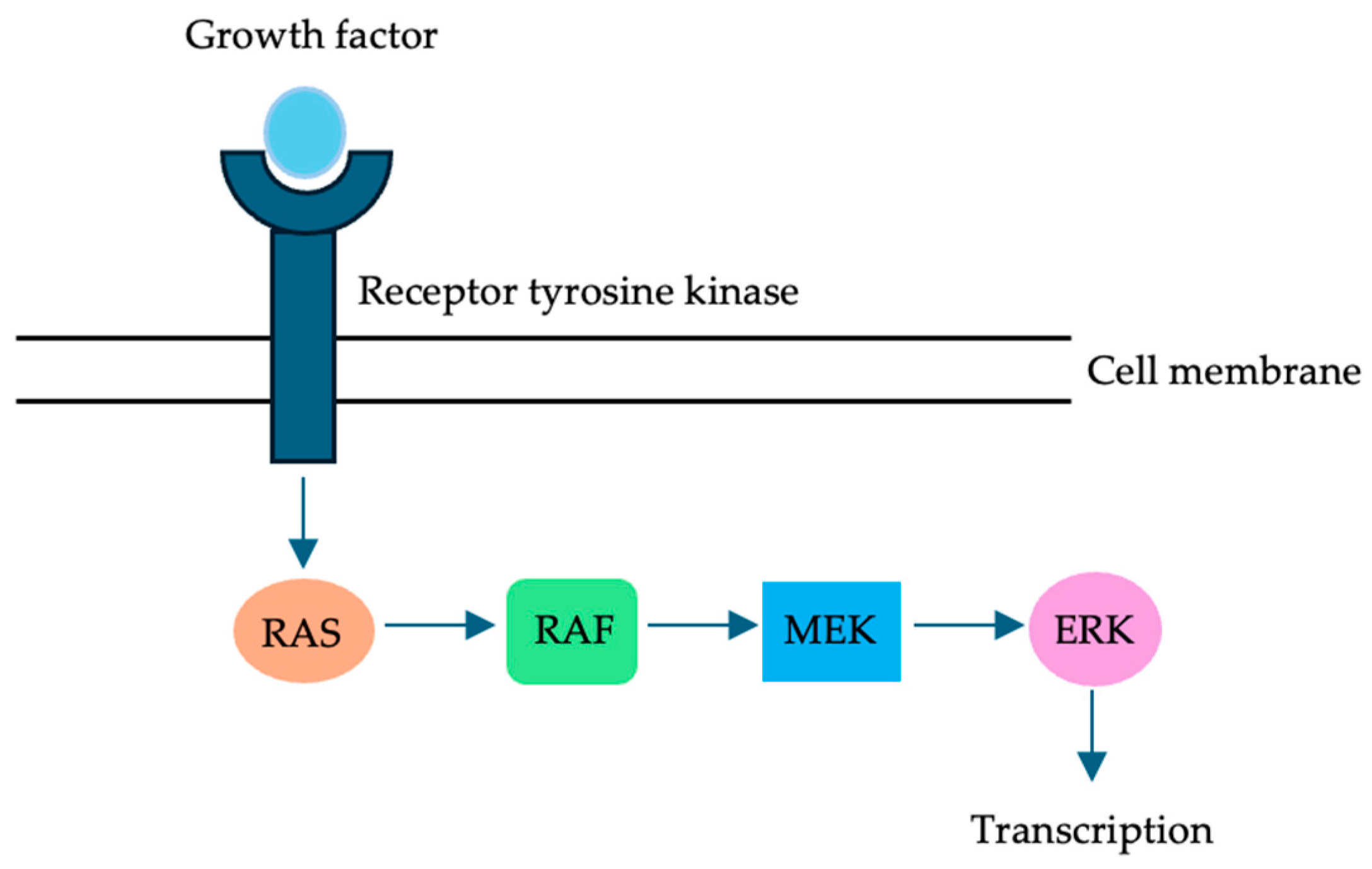
:1. Introduction
2. RAS in CRLM
2.1. The Prognostic Role of RAS Mutations
2.2. The Prognostic Role of RAS Mutations on a Granular Level
2.3. RAS Mutations Do Not Function in a Vacuum: Context Matters
2.4. RAS Mutations Can Inform Treatment: Optimal Surgical Margin Width
2.5. RAS Mutations Can Inform Treatment: Optimal Surgical Technique
2.6. RAS Mutations Can Inform Treatment: Response to Chemotherapy
2.7. RAS Mutations Can Inform Post-Hepatectomy Surveillance
3. BRAF in CRLM
3.1. The Prognostic Role of BRAF
3.2. The Prognostic Role of BRAF on a Granular Level
3.3. BRAF vs. KRAS/BRAF Co-Mutation
3.4. Is BRAF a “Biologic” Contraindication to Surgery?
3.5. BRAF and Disease Recurrence
4. MMR Genes in CRLM
5. TP53 and SMAD4 in CRLM
5.1. The Prognostic Role of TP53
5.2. The Prognostic Role of SMAD4
5.3. The Impact of TP53 and SMAD4 Co-Mutations
6. Other Somatic Mutations in CRLM
7. Discussion
8. Recommendations for Future Research
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.K.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Pawlik, T.M. Molecular Mechanisms of Colorectal Liver Metastases. Cells. 2023, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Kanas, G.P.; Taylor, A.; Primrose, J.N.; Langeberg, W.J.; Kelsh, M.A.; Mowat, F.S.; Alexander, D.D.; Choti, M.A.; Poston, G. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin Epidemiol. 2012, 4, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Berardi, G.; Chou, J.; Gonen, M.; Balachandran, V.P.; Drebin, J.; Jarnagin, W.R.; Kingham, T.P.; Soares, K.C.; Wei, A.; D’Angelica, M. A Model to Predict Treatment Failure in Patients Undergoing Upfront Surgery for Resectable Colorectal Liver Metastases. Ann. Surg. Oncol. 2023, 30, 2820–2827. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Turtoi, A.; Blomme, A.; Debois, D.; Somja, J.; Delvaux, D.; Patsos, G.; Di Valentin, E.; Peulen, O.; Mutijima, E.N.; De Pauw, E.; et al. Organized proteomic heterogeneity in colorectal cancer liver metastases and implications for therapies. Hepatology 2014, 59, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.; Tekkis, P.P.; Welsh, F.K.; O’Rourke, T.; John, T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann. Surg. 2008, 247, 125–135. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318; discussion 318–321. [Google Scholar] [CrossRef]
- Chen, F.L.; Wang, Y.Y.; Liu, W.; Xing, B.C. Prognostic factors in colorectal liver metastases patients with various tumor numbers treated by liver resection: A single-center, retrospective study. World J. Surg. Oncol. 2022, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Bagante, F.; Moris, D.; Cloyd, J.; Spartalis, E.; Pawlik, T.M. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: A systematic review of the current evidence. Surg. Oncol. 2018, 27, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, P.; Utria, A.F.; Beck, A.C.; Chun, Y.S.; Howe, J.R.; Weigel, R.J.; Vauthey, J.N.; Hassan, I. The Prognostic Impact of KRAS Mutation in Patients Having Curative Resection of Synchronous Colorectal Liver Metastases. J. Gastrointest. Surg. 2019, 23, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Schirripa, M.; Bergamo, F.; Cremolini, C.; Casagrande, M.; Lonardi, S.; Aprile, G.; Yang, D.; Marmorino, F.; Pasquini, G.; Sensi, E.; et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br. J. Cancer 2015, 112, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Pikoulis, E.; Papaconstantinou, D.; Pikouli, A.; Wang, J.; Theodoridis, C.; Margonis, G.A. Reevaluating the prognostic value of RAS mutation status in patients with resected liver metastases from colorectal cancer: A systematic review and meta-analysis. J. Hepatobiliary Pancreat. Sci. 2021, 28, 637–647. [Google Scholar] [CrossRef]
- Olthof, P.B.; Buettner, S.; Andreatos, N.; Wang, J.; Løes, I.M.; Wagner, D.; Sasaki, K.; Macher-Beer, A.; Kamphues, C.; Pozios, I.; et al. KRAS alterations in colorectal liver metastases: Shifting to exon, codon, and point mutations. Br. J. Surg. 2022, 109, 804–807. [Google Scholar] [CrossRef]
- Passot, G.; Denbo, J.W.; Yamashita, S.; Kopetz, S.E.; Chun, Y.S.; Maru, D.; Overman, M.J.; Brudvik, K.W.; Conrad, C.; Aloia, T.A.; et al. Is hepatectomy justified for patients with RAS mutant colorectal liver metastases? An analysis of 524 patients undergoing curative liver resection. Surgery 2017, 161, 332–340. [Google Scholar] [CrossRef]
- Margonis, G.A.; Kim, Y.; Spolverato, G.; Ejaz, A.; Gupta, R.; Cosgrove, D.; Anders, R.; Karagkounis, G.; Choti, M.A.; Pawlik, T.M. Association Between Specific Mutations in KRAS Codon 12 and Colorectal Liver Metastasis. JAMA Surg. 2015, 150, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, R.; Wang, Z.; Geng, Y.; Lin, J.; Ma, K.; Zuo, J.L.; Lu, L.; Zhang, J.B.; Zhu, W.W.; et al. Meta-analysis of the association between primary tumour location and prognosis after surgical resection of colorectal liver metastases. Br. J. Surg. 2019, 106, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Saltz, L.B.; Balachandran, V.P.; Peter Kingham, T.; DeMatteo, R.P.; et al. The Impact of Primary Tumor Location on Long-Term Survival in Patients Undergoing Hepatic Resection for Metastatic Colon Cancer. Ann. Surg. Oncol. 2018, 25, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Belias, M.; Sasaki, K.; Wang, J.; Andreatos, N.; Kamphues, C.; Kyriakos, G.; Seeliger, H.; Beyer, K.; Kreis, M.E.; Margonis, G.A. Is Laterality Prognostic in Resected KRAS-Mutated Colorectal Liver Metastases? A Systematic Review and Meta-Analysis. Cancers 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Kim, Y.; Sasaki, K.; Samaha, M.; Buettner, S.; Amini, N.; Pawlik, T.M. Activating KRAS mutation is prognostic only among patients who receive preoperative chemotherapy before resection of colorectal liver metastases. J. Surg. Oncol. 2016, 114, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Kopetz, S.; Maru, D.M.; Chen, S.S.; Zimmitti, G.; Brouquet, A.; Shindoh, J.; Curley, S.A.; Garrett, C.; Overman, M.J.; et al. Adjuvant chemotherapy with FOLFOX for primary colorectal cancer is associated with increased somatic gene mutations and inferior survival in patients undergoing hepatectomy for metachronous liver metastases. Ann. Surg. 2012, 256, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Furukawa, K.; Takayashiki, T.; Kuboki, S.; Takano, S.; Ohtsuka, M. Differential effects of KRAS mutational status on long-term survival according to the timing of colorectal liver metastases. BMC Cancer 2021, 21, 412. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Mise, Y.; Chung, M.H.; Chun, Y.S.; Kopetz, S.E.; Passot, G.; Conrad, C.; Maru, D.M.; Aloia, T.A.; Vauthey, J.N. RAS Mutation Predicts Positive Resection Margins and Narrower Resection Margins in Patients Undergoing Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2016, 23, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Sasaki, K.; Andreatos, N.; Kim, Y.; Merath, K.; Wagner, D.; Wilson, A.; Buettner, S.; Amini, N.; Antoniou, E.; et al. KRAS Mutation Status Dictates Optimal Surgical Margin Width in Patients Undergoing Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2017, 24, 264–271. [Google Scholar] [CrossRef]
- Bertsimas, D.; Margonis, G.A.; Sujichantararat, S.; Boerner, T.; Ma, Y.; Wang, J.; Kamphues, C.; Sasaki, K.; Tang, S.; Gagniere, J.; et al. Using Artificial Intelligence to Find the Optimal Margin Width in Hepatectomy for Colorectal Cancer Liver Metastases. JAMA Surg. 2022, 157, e221819. [Google Scholar] [CrossRef]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Sasaki, K.; Ijzermans, J.N.M.; van Vugt, J.L.A.; Pawlik, T.M.; Choti, M.A.; Cameron, J.L.; He, J.; et al. Anatomical Resections Improve Disease-free Survival in Patients with KRAS-mutated Colorectal Liver Metastases. Ann. Surg. 2017, 266, 641–649. [Google Scholar] [CrossRef]
- Joechle, K.; Vreeland, T.J.; Vega, E.A.; Okuno, M.; Newhook, T.E.; Panettieri, E.; Chun, Y.S.; Tzeng, C.D.; Aloia, T.A.; Lee, J.E.; et al. Anatomic Resection Is Not Required for Colorectal Liver Metastases with RAS Mutation. J. Gastrointest. Surg. 2020, 24, 1033–1039. [Google Scholar] [CrossRef]
- Pikoulis, E.; Papaconstantinou, D.; Pikouli, A.; Pararas, N.; Buettner, S.; Wang, J.; Stasinos, G.; Belias, M.; Dellaportas, D.; Pozios, I.; et al. Is Precision Surgery Applicable to Colorectal Liver Metastases? A Systematic Review and Meta-analysis of Studies that Investigate the Association of Surgical Technique with Outcomes in the Context of Distinct Tumor Biology. Ann. Surg. Oncol. 2024, 31, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Zimmitti, G.; Shindoh, J.; Mise, Y.; Kopetz, S.; Loyer, E.M.; Andreou, A.; Cooper, A.B.; Kaur, H.; Aloia, T.A.; Maru, D.M.; et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann. Surg. Oncol. 2015, 22, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, N.E.; Chou, J.F.; Capanu, M.; Gewirtz, A.N.; Cercek, A.; Kingham, T.P.; Jarnagin, W.R.; Fong, Y.C.; DeMatteo, R.P.; Allen, P.J.; et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 2014, 120, 3965–3971. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kopetz, S.; Lillemoe, H.A.; Hwang, H.; Wang, X.; Tzeng, C.D.; Chun, Y.S.; Aloia, T.A.; Vauthey, J.N. A New Surveillance Algorithm After Resection of Colorectal Liver Metastases Based on Changes in Recurrence Risk and RAS Mutation Status. J. Natl. Compr. Cancer Netw. 2020, 18, 1500–1508. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Strickler, J.H.; Bekaii-Saab, T.S.; Yaeger, R. BRAF-Mutated Advanced Colorectal Cancer: A Rapidly Changing Therapeutic Landscape. J. Clin. Oncol. 2022, 40, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Gau, L.; Ribeiro, M.; Pereira, B.; Poirot, K.; Dupré, A.; Pezet, D.; Gagnière, J. Impact of BRAF mutations on clinical outcomes following liver surgery for colorectal liver metastases: An updated meta-analysis. Eur. J. Surg. Oncol. 2021, 47, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Bachet, J.B.; Moreno-Lopez, N.; Vigano, L.; Marchese, U.; Gelli, M.; Raoux, L.; Truant, S.; Laurent, C.; Herrero, A.; Le Roy, B.; et al. BRAF mutation is not associated with an increased risk of recurrence in patients undergoing resection of colorectal liver metastases. Br. J. Surg. 2019, 106, 1237–1247. [Google Scholar] [CrossRef]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Kim, Y.; Wagner, D.; Sasaki, K.; Beer, A.; Schwarz, C.; Løes, I.M.; Smolle, M.; et al. Association of BRAF Mutations with Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg. 2018, 153, e180996. [Google Scholar] [CrossRef]
- Margonis, G.A.; Boerner, T.; Bachet, J.B.; Buettner, S.; Moretto, R.; Andreatos, N.; Sartore-Bianchi, A.; Wang, J.; Kamphues, C.; Gagniere, J.; et al. Demystifying BRAF Mutation Status in Colorectal Liver Metastases: A Multi-institutional, Collaborative Approach to 6 Open Clinical Questions. Ann. Surg. 2023, 278, e540–e548. [Google Scholar] [CrossRef] [PubMed]
- Gagnière, J.; Dupré, A.; Gholami, S.S.; Pezet, D.; Boerner, T.; Gönen, M.; Kingham, T.P.; Allen, P.J.; Balachandran, V.P.; De Matteo, R.P.; et al. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Ann. Surg. 2020, 271, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Takahashi, S.; Nomura, S.; Kojima, M.; Kudo, M.; Sugimoto, M.; Konishi, M.; Gotohda, N.; Taniguchi, H.; Yoshino, T. BRAF V600E potentially determines “Oncological Resectability” for “Technically Resectable” colorectal liver metastases. Cancer Med. 2021, 10, 6998–7011. [Google Scholar] [CrossRef]
- Li, G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef]
- Ionov, Y.; Peinado, M.A.; Malkhosyan, S.; Shibata, D.; Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef]
- Turner, K.M.; Delman, A.M.; Wima, K.; Quillin, R.C.; Shah, S.A.; Ahmad, S.A.; Patel, S.H.; Wilson, G.C. Microsatellite instability is associated with worse overall survival in resectable colorectal liver metastases. Am. J. Surg. 2023, 225, 322–327. [Google Scholar] [CrossRef]
- Matteo, B.; Gaetano, P.; Delfina, T.; Riccardo, M.; Roberto, S.; Guglielmo, P.; Claudia, C.; Carmelo, L.; Carla, C.; Daris, F.; et al. Immunohistochemical evaluation of microsatellite instability in resected colorectal liver metastases: A preliminary experience. Med. Oncol. 2020, 37, 63. [Google Scholar] [CrossRef]
- Xourafas, D.; Mizuno, T.; Cloyd, J.M. The impact of somatic SMAD4 mutations in colorectal liver metastases. Chin. Clin. Oncol. 2019, 8, 52. [Google Scholar] [CrossRef]
- Chun, Y.S.; Passot, G.; Yamashita, S.; Nusrat, M.; Katsonis, P.; Loree, J.M.; Conrad, C.; Tzeng, C.D.; Xiao, L.; Aloia, T.A.; et al. Deleterious Effect of RAS and Evolutionary High-risk TP53 Double Mutation in Colorectal Liver Metastases. Ann. Surg. 2019, 269, 917–923. [Google Scholar] [CrossRef]
- Frankel, T.L.; Vakiani, E.; Nathan, H.; DeMatteo, R.P.; Kingham, T.P.; Allen, P.J.; Jarnagin, W.R.; Kemeny, N.E.; Solit, D.B.; D’Angelica, M.I. Mutation location on the RAS oncogene affects pathologic features and survival after resection of colorectal liver metastases. Cancer 2017, 123, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Kim, B.; Ooh, S.J.; Choi, M.K.; Lee, D.W.; Han, S.; Kim, S.H.; Park, S.J.; Back, J.Y.; Park, S.C.; et al. The prognostic impact of RAS and TP53 mutation according to primary tumor location in colorectal liver metastases. JCO 2021, 39, 3582. [Google Scholar] [CrossRef]
- Molleví, D.G.; Serrano, T.; Ginestà, M.M.; Valls, J.; Torras, J.; Navarro, M.; Ramos, E.; Germà, J.R.; Jaurrieta, E.; Moreno, V.; et al. Mutations in TP53 are a prognostic factor in colorectal hepatic metastases undergoing surgical resection. Carcinogenesis 2007, 28, 1241–1246. [Google Scholar] [CrossRef]
- Russo, A.; Bazan, V.; Iacopetta, B.; Kerr, D.; Soussi, T.; Gebbia, N.; TP53-CRC Collaborative Study Group. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: Influence of tumor site, type of mutation, and adjuvant treatment. J. Clin. Oncol. 2005, 23, 7518–7528. [Google Scholar] [CrossRef]
- Lillemoe, H.A.; Passot, G.; Kawaguchi, Y.; DeBellis, M.; Glehen, O.; Chun, Y.S.; Tzeng, C.D.; Aloia, T.A.; Lopez, J.; Vauthey, J.N. RAS/TP53 Co-mutation is Associated with Worse Survival After Concurrent Resection of Colorectal Liver Metastases and Extrahepatic Disease. Ann. Surg. 2022, 276, 357–362. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, M.; Moreno-Rubio, J.; Suárez-García, I.; Cejas, P.; Madero, R.; Casado, E.; Jiménez, A.; Sereno, M.; Gómez-Raposo, C.; Zambrana, F.; et al. SMAD4 and TS expression might predict the risk of recurrence after resection of colorectal liver metastases. Clin. Transl. Oncol. 2015, 17, 133–138. [Google Scholar] [CrossRef]
- Mizuno, T.; Cloyd, J.M.; Vicente, D.; Omichi, K.; Chun, Y.S.; Kopetz, S.E.; Maru, D.; Conrad, C.; Tzeng, C.D.; Wei, S.H.; et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2018, 44, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Liang, T.; Wang, Y.; Wu, H.; Liu, S.; Xie, L.; Liang, J.; Wang, C.; Tan, Y. Prognostic role and clinicopathological features of SMAD4 gene mutation in colorectal cancer: A systematic review and meta-analysis. BMC Gastroenterol. 2021, 21, 297. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kopetz, S.; Newhook, T.E.; De Bellis, M.; Chun, Y.S.; Tzeng, C.D.; Aloia, T.A.; Vauthey, J.N. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin Cancer Res. 2019, 25, 5843–5851. [Google Scholar] [CrossRef]
- Datta, J.; Smith, J.J.; Chatila, W.K.; McAuliffe, J.C.; Kandoth, C.; Vakiani, E.; Frankel, T.L.; Ganesh, K.; Wasserman, I.; Lipsyc-Sharf, M.; et al. Coaltered Ras/B-raf and TP53 Is Associated with Extremes of Survivorship and Distinct Patterns of Metastasis in Patients with Metastatic Colorectal Cancer. Clin. Cancer Res. 2020, 26, 1077–1085. [Google Scholar] [CrossRef]
- Lang, H.; Baumgart, J.; Heinrich, S.; Tripke, V.; Passalaqua, M.; Maderer, A.; Galle, P.R.; Roth, W.; Kloth, M.; Moehler, M. Extended Molecular Profiling Improves Stratification and Prediction of Survival After Resection of Colorectal Liver Metastases. Ann. Surg. 2019, 270, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Chun, Y.S.; Kopetz, S.E.; Maru, D.; Conrad, C.; Aloia, T.A.; Vauthey, J.N. APC and PIK3CA Mutational Cooperativity Predicts Pathologic Response and Survival in Patients Undergoing Resection for Colorectal Liver Metastases. Ann. Surg. 2020, 272, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Sawai, H.; Yasuda, A.; Ochi, N.; Ma, J.; Matsuo, Y.; Wakasugi, T.; Takahashi, H.; Funahashi, H.; Sato, M.; Takeyama, H. Loss of PTEN expression is associated with colorectal cancer liver metastasis and poor patient survival. BMC Gastroenterol. 2008, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, Y.; Inamura, K.; Yamamoto, N.; Mise, Y.; Saiura, A.; Ishikawa, Y.; Takahashi, S.; Kanda, H. Impact of CDX2 expression status on the survival of patients after curative resection for colorectal cancer liver metastasis. BMC Cancer 2018, 18, 980. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Jones, R.P.; Giuliante, F.; Shindoh, J.; Passot, G.; Chung, M.H.; Song, J.; Li, L.; Dagenborg, V.J.; Fretland, Å.A.; et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann. Surg. 2019, 269, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.B.; Fischer, J.P.; Baer, B.R.; Blake, J.F.; Bouhana, K.; Briere, D.M.; Brown, K.D.; Burgess, L.E.; Burns, A.C.; Burkard, M.R.; et al. Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of Cancer. J. Med. Chem. 2020, 63, 6679–6693. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Xiong, Y.; Safaee, N.; Nowak, R.P.; Donovan, K.A.; Yuan, C.J.; Nabet, B.; Gero, T.W.; Feru, F.; Li, L.; et al. Exploring Targeted Degradation Strategy for Oncogenic KRASG12C. Cell Chem. Biol. 2020, 27, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.J.; Chu, L.; Nalawansha, D.A.; Li, K.; Crews, C.M. Targeted Degradation of Oncogenic KRASG12C by VHL-Recruiting PROTACs. ACS Cent. Sci. 2020, 6, 1367–1375. [Google Scholar] [CrossRef]
- Sakamoto, K.; Masutani, T.; Hirokawa, T. Generation of KS-58 as the first K-Ras(G12D)-inhibitory peptide presenting anti-cancer activity in vivo. Sci. Rep. 2020, 10, 21671. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kamada, Y.; Sameshima, T.; Yaguchi, M.; Niida, A.; Sasaki, S.; Miwa, M.; Ohkubo, S.; Sakamoto, J.I.; Kamaura, M.; et al. K-Ras(G12D)-selective inhibitory peptides generated by random peptide T7 phage display technology. Biochem. Biophys. Res. Commun. 2017, 484, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.W.; Tsai, S.T.; Hattori, T.; Fedele, C.; Koide, A.; Yang, C.; Hou, X.; Zhang, Y.; Neel, B.G.; O’Bryan, J.P.; et al. Selective and noncovalent targeting of RAS mutants for inhibition and degradation. Nat. Commun. 2021, 12, 2656. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Patgiri, A.; Yadav, K.K.; Arora, P.S.; Bar-Sagi, D. An orthosteric inhibitor of the Ras-Sos interaction. Nat. Chem. Biol. 2011, 7, 585–587. [Google Scholar] [CrossRef] [PubMed]



| KRAS |
| BRAF |
|
| KRAS |
|
|
| BRAF |
|
|
| Other Mutations |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Botvinov, J.; Bhatt, A.J.; Beyer, K.; Kreis, M.E.; Adam, M.; Alseidi, A.; Margonis, G.A. Somatic Mutations in Surgically Treated Colorectal Liver Metastases: An Overview. Cells 2024, 13, 679. https://doi.org/10.3390/cells13080679
Wang J, Botvinov J, Bhatt AJ, Beyer K, Kreis ME, Adam M, Alseidi A, Margonis GA. Somatic Mutations in Surgically Treated Colorectal Liver Metastases: An Overview. Cells. 2024; 13(8):679. https://doi.org/10.3390/cells13080679
Chicago/Turabian StyleWang, Jane, Julia Botvinov, Aarshvi Jahnvi Bhatt, Katharina Beyer, Martin E. Kreis, Mohamed Adam, Adnan Alseidi, and Georgios Antonios Margonis. 2024. "Somatic Mutations in Surgically Treated Colorectal Liver Metastases: An Overview" Cells 13, no. 8: 679. https://doi.org/10.3390/cells13080679
APA StyleWang, J., Botvinov, J., Bhatt, A. J., Beyer, K., Kreis, M. E., Adam, M., Alseidi, A., & Margonis, G. A. (2024). Somatic Mutations in Surgically Treated Colorectal Liver Metastases: An Overview. Cells, 13(8), 679. https://doi.org/10.3390/cells13080679







