5-Hydroxymethylcytosine in Cell-Free DNA Predicts Immunotherapy Response in Lung Cancer
Abstract
1. Background
2. Methods
2.1. Patients and Sample Collection
2.2. Study Design
2.3. Treatment Response Assessments
2.4. Sample Processing and 5hmC Sequencing Analysis
2.5. Establishing 5hmC Predictive Signatures
2.6. Power and Statistical Analyses
3. Results
3.1. A 5hmC Predictive Signature Is Associated with PFS in ICI-Treated Patients
3.2. The 5hmC Predictive Signature Is Associated with Objective Response Rate
3.3. The 5hmC Predictive Signature Is Associated with Overall Survival in Patients Receiving ICIs
3.4. The 5hmC Predictive Signature Is Associated with Outcomes in Patients Receiving Single-Agent ICI Treatment
3.5. The 5hmC Predictive Signature Is Superior to Tumor PD-L1 Expression for ICI Response Prediction
3.6. The 5hmC Predictive Signature Is Specific to ICI Treatment Prediction
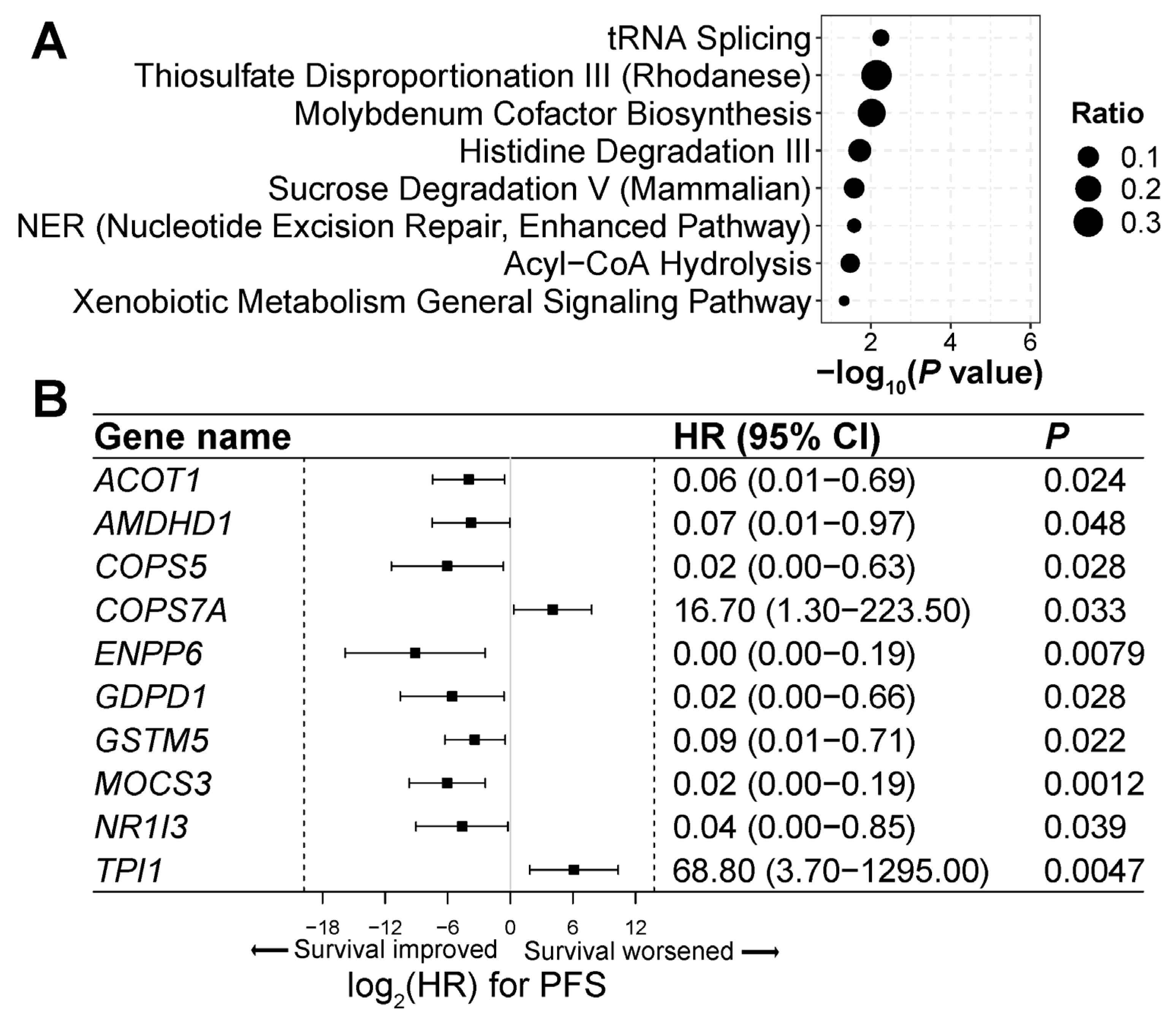
3.7. Genes and Pathways Associated with ICI Treatment Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, B.; Farooq, A.; Kanwal, S.; Asghar, K. Immunotherapy and targeted therapy for lung cancer: Current status and future perspectives. Front Pharmacol. 2022, 13, 1035171. [Google Scholar] [CrossRef] [PubMed]
- Mino-Kenudson, M.; Schalper, K.; Cooper, W.; Dacic, S.; Hirsch, F.R.; Jain, D.; Lopez-Rios, F.; Tsao, M.S.; Yatabe, Y.; Beasley, M.B.; et al. Predictive Biomarkers for Immunotherapy in Lung Cancer: Perspective From the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2022, 17, 1335–1354. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.; Chen, L.; Bissessur, A.S.; Chen, J.; Mao, M.; Ju, S.; Chen, L.; Chen, C.; Li, Z.; et al. Deoxyribonucleic Acid 5-Hydroxymethylation in Cell-Free Deoxyribonucleic Acid, a Novel Cancer Biomarker in the Era of Precision Medicine. Front. Cell Dev. Biol. 2021, 9, 744990. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Stroup, E.K.; Zhang, Z.; Chiu, B.C.; Zhang, W. Towards precision medicine: Advances in 5-hydroxymethylcytosine cancer biomarker discovery in liquid biopsy. Cancer Commun. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Applebaum, M.A.; Barr, E.K.; Karpus, J.; Nie, J.; Zhang, Z.; Armstrong, A.E.; Uppal, S.; Sukhanova, M.; Zhang, W. 5-Hydroxymethylcytosine Profiles Are Prognostic of Outcome in Neuroblastoma and Reveal Transcriptional Networks That Correlate With Tumor Phenotype. JCO Precis Oncol. 2019, 18, 00402. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Gao, S.; Qi, W.; Shi, C.; Qiu, M.; Yang, F.; Bai, S.; Li, H.; Wang, Z.; Sun, Z.; et al. 5-Hydroxymethylcytosine as a potential epigenetic biomarker in papillary thyroid carcinoma. Oncol Lett. 2019, 18, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Bosio, M.; Salvaterra, E.; Datturi, F.; Morbini, P.; Zorzetto, M.; Inghilleri, S.; Tomaselli, S.; Mangiarotti, P.; Meloni, F.; Cerveri, I.; et al. 5-hydroxymethylcytosine but not MTAP methylation status can stratify malignant pleural mesothelioma based on the lineage of origin. Multidiscip. Respir. Med. 2018, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.W.; Getchell, C.R.; McCarthy, E.T.; Ohman, A.W.; Sasamoto, N.; Xu, S.; Ko, J.Y.; Gupta, M.; Shafrir, A.; Medina, J.E.; et al. Epigenetic Reprogramming Strategies to Reverse Global Loss of 5-Hydroxymethylcytosine, a Prognostic Factor for Poor Survival in High-grade Serous Ovarian Cancer. Clin. Cancer Res. 2018, 24, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, M.; Yuan, Q.; Guo, Y.; Hutchinson, J.N.; Su, L.; Zheng, Y.; Wang, J.; Mucci, L.A.; Lin, X.; et al. Epigenomic analysis of 5-hydroxymethylcytosine (5hmC) reveals novel DNA methylation markers for lung cancers. Neoplasia 2020, 22, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Q.; Liu, T.; Zhang, W.; Zeng, X.T.; Guo, Z. Prognostic value of downregulated 5-hydroxymethylcytosine expression in renal cell carcinoma: A 10 year follow-up retrospective study. J. Cancer 2020, 11, 1212–1222. [Google Scholar] [CrossRef]
- Liao, Y.; Gu, J.; Wu, Y.; Long, X.; Ge, D.I.; Xu, J.; Ding, J. Low level of 5-Hydroxymethylcytosine predicts poor prognosis in non-small cell lung cancer. Oncol. Lett. 2016, 11, 3753–3760. [Google Scholar] [CrossRef]
- Ghoneim, H.E.; Zamora, A.E.; Thomas, P.G.; Youngblood, B.A. Cell-Intrinsic Barriers of T Cell-Based Immunotherapy. Trends Mol. Med. 2016, 22, 1000–1011. [Google Scholar] [CrossRef]
- Xiao, Q.; Nobre, A.; Pineiro, P.; Berciano-Guerrero, M.A.; Alba, E.; Cobo, M.; Lauschke, V.M.; Barragan, I. Genetic and Epigenetic Biomarkers of Immune Checkpoint Blockade Response. J. Clin. Med. 2020, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Tsagaratou, A.; Äijö, T.; Lio, C.W.; Yue, X.; Huang, Y.; Jacobsen, S.E.; Lähdesmäki, H.; Rao, A. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, E3306–E3315. [Google Scholar] [CrossRef] [PubMed]
- Tsiouplis, N.J.; Bailey, D.W.; Chiou, L.F.; Wissink, F.J.; Tsagaratou, A. TET-Mediated Epigenetic Regulation in Immune Cell Development and Disease. Front. Cell Dev. Biol. 2021, 8, 623948. [Google Scholar] [CrossRef]
- Asgarova, A.; Asgarov, K.; Godet, Y.; Peixoto, P.; Nadaradjane, A.; Boyer-Guittaut, M.; Galaine, J.; Guenat, D.; Mougey, V.; Perrard, J.; et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology 2018, 7, e1423170. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.C.; Konkel, J.E.; Prendergast, C.T.; Thomson, J.P.; Ottaviano, R.; Leech, M.D.; Kay, O.; Zandee, S.E.; Sweenie, C.H.; Wraith, D.C.; et al. Epigenetic modification of the PD-1 (Pdcd1) promoter in effector CD4+ T cells tolerized by peptide immunotherapy. Elife 2014, 3, e03416. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.X.; Chen, Y.X.; Wang, Z.X.; Zhao, Q.; He, M.M.; Wang, Y.N.; Wang, F.; Xu, R.H. Alteration in TET1 as potential biomarker for immune checkpoint blockade in multiple cancers. J. Immunother. Cancer 2019, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Mambetsariev, I.; Li, H.; Chen, C.; Fricke, J.; Fann, P.; Kulkarni, P.; Xing, Y.; Lee, P.P.; Bild, A.; et al. Association of molecular characteristics with survival in advanced non-small cell lung cancer patients treated with checkpoint inhibitors. Lung Cancer 2020, 146, 174–181. [Google Scholar] [CrossRef]
- Guler, G.D.; Ning, Y.; Coruh, C.; Mognol, G.P.; Phillips, T.; Nabiyouni, M.; Hazen, K.; Scott, A.; Volkmuth, W.; Levy, S. Plasma cell-free DNA hydroxymethylation profiling reveals anti-PD-1 treatment response and resistance biology in non-small cell lung cancer. J. Immunother. Cancer 2024, 12, e008028. [Google Scholar] [CrossRef]
- Han, D.; Lu, X.; Shih, A.H.; Nie, J.; You, Q.; Xu, M.M.; Melnick, A.M.; Levine, R.L.; He, C. A Highly Sensitive and Robust Method for Genome-wide 5hmC Profiling of Rare Cell Populations. Mol. Cell 2016, 63, 711–719. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Lu, X.; You, L.; Song, Y.; Luo, Z.; Zhang, J.; Nie, J.; Zheng, W.; Xu, D.; et al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers. Cell Res. 2017, 27, 1243–1257. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Gao, C.; Xing, Y.; Qi, Z.; Liu, R.; Wang, Y.; Zhang, X.; Yang, Y.G.; Li, X.; et al. 5-Hydroxymethylome in Circulating Cell-free DNA as A Potential Biomarker for Non-small-cell Lung Cancer. Genom. Proteom. Bioinform. 2018, 16, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Guler, G.D.; Ning, Y.; Ku, C.J.; Phillips, T.; McCarthy, E.; Ellison, C.K.; Bergamaschi, A.; Collin, F.; Lloyd, P.; Scott, A.; et al. Detection of early stage pancreatic cancer using 5-hydroxymethylcytosine signatures in circulating cell free DNA. Nat. Commun. 2020, 11, 5270. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wu, W.; Wu, C.; Li, M.; Sun, F.; Zheng, L.; Liu, G.; Li, X.; Yun, Z.; Tang, J.; et al. 5-Hydroxymethylcytosine signature in circulating cell-free DNA as a potential diagnostic factor for early-stage colorectal cancer and precancerous adenoma. Mol. Oncol. 2020, 15, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, L.; Zhang, Z.; Zhang, X.; Lu, X.; Liu, W.; Shi, G.; Ge, Y.; Gao, P.; Yang, Y.; et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut 2019, 68, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sun, B.; Chen, C.; Gao, C.; Zhang, J.; Lu, X.; Wang, L.; Li, X.; Xing, Y.; Liu, R.; et al. Circulating tumor DNA 5-hydroxymethylcytosine as a novel diagnostic biomarker for esophageal cancer. Cell Res. 2018, 28, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.C.; Zhang, Z.; You, Q.; Zeng, C.; Stepniak, E.; Bracci, P.M.; Yu, K.; Venkataraman, G.; Smith, S.M.; He, C.; et al. Prognostic implications of 5-hydroxymethylcytosines from circulating cell-free DNA in diffuse large B-cell lymphoma. Blood Adv. 2019, 3, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Luo, K.; Shi, H.; Yan, X.; Huang, R.; Zhao, B.; Zhang, J.; Xie, D.; Zhang, W. Integrated 5-hydroxymethylcytosine and fragmentation signatures as enhanced biomarkers in lung cancer. Clin. Epigenet. 2022, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, S.; West-Szymanski, D.; Karpus, J.; Shah, S.; Ganguly, S.; Smith, J.; Zu, Y.; He, C.; Li, Z. Cell-free DNA 5-hydroxymethylcytosine is an emerging marker of acute myeloid leukemia. Sci. Rep. 2022, 12, 12410. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Bernicker, E.; He, C.; Li, Z. Cell-free DNA 5-hydroxymethylcytosine as a marker for common cancer detection. Clin. Transl. Discov. 2022, 2, e136. [Google Scholar] [CrossRef]
- Shao, J.; Shah, S.; Ganguly, S.; Zu, Y.; He, C.; Li, Z. Classification of Acute Myeloid Leukemia by Cell-Free DNA 5-Hydroxymethylcytosine. Genes 2023, 14, 1180. [Google Scholar] [CrossRef]
- Shao, J.; Shah, S.; Ganguly, S.; Zu, Y.; He, C.; Li, Z. Cell-free DNA 5-hydroxymethylcytosine is highly sensitive for MRD assessment in acute myeloid leukemia. Clin. Epigenet. 2023, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Olsen, J.O.; Kasparian, S.; He, C.; Bernicker, E.; Li, Z. Cell-Free DNA 5-Hydroxymethylcytosine Signatures for Lung Cancer Prognosis. Cells 2024, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Blanche, P.; Dartigues, J.F.; Jacqmin-Gadda, H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat. Med. 2013, 32, 5381–5397. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Miskovic, V.; Zanitti, M.; Trovo, F.; Genova, C.; Viscardi, G.; Rebuzzi, S.E.; Mazzeo, L.; Provenzano, L.; Kosta, S.; et al. Artificial intelligence for predictive biomarker discovery in immuno-oncology: A systematic review. Ann. Oncol. 2024, 35, 29–65. [Google Scholar] [CrossRef] [PubMed]
- Duruisseaux, M.; Martinez-Cardus, A.; Calleja-Cervantes, M.E.; Moran, S.; Castro de Moura, M.; Davalos, V.; Pineyro, D.; Sanchez-Cespedes, M.; Girard, N.; Brevet, M.; et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: A multicentre, retrospective analysis. Lancet Respir. Med. 2018, 6, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Li, X.; Gao, Y.; Guo, S.; Sun, D.; Zhou, H.; Sun, Y.; Wang, P.; Zhi, H.; Bai, J.; et al. MeImmS: Predict Clinical Benefit of Anti-PD-1/PD-L1 Treatments Based on DNA Methylation in Non-small Cell Lung Cancer. Front. Genet. 2021, 12, 676449. [Google Scholar] [CrossRef]
- Liu, G.; Claret, F.X.; Zhou, F.; Pan, Y. Jab1/COPS5 as a Novel Biomarker for Diagnosis, Prognosis, Therapy Prediction and Therapeutic Tools for Human Cancer. Front. Pharmacol. 2018, 9, 135. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Yang, G.; Liu, G.; Pan, Y. Pan-cancer analyses of Jab1/COPS5 reveal oncogenic role and clinical outcome in human cancer. Heliyon 2022, 8, e12553. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zou, J.; Lai, C.T.; Zeng, T.; Peng, J.; Zou, W.D.; Cao, B.; Liu, D.; Zhu, L.Y.; et al. Comprehensive analysis of the glutathione S-transferase Mu (GSTM) gene family in ovarian cancer identifies prognostic and expression significance. Front. Oncol. 2022, 12, 968547. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhang, J.; Chen, G.; Cao, W.; Chen, H.; Chen, S. Aberrant expression of GSTM5 in lung adenocarcinoma is associated with DNA hypermethylation and poor prognosis. BMC Cancer 2022, 22, 685. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Wang, J.; Liu, J.; Wang, Z.; Mao, Y.; Liu, S.; Liao, X.; Chen, J. Identification and verification of the prognostic value of the glutathione S-transferase Mu genes in gastric cancer. Oncol. Lett. 2020, 20, 100. [Google Scholar] [CrossRef]
- Li, J.D.; Chen, Y.; Jing, S.W.; Wang, L.T.; Zhou, Y.H.; Liu, Z.S.; Song, C.; Li, D.Z.; Wang, H.Q.; Huang, Z.G.; et al. Triosephosphate isomerase 1 may be a risk predictor in laryngeal squamous cell carcinoma: A multi-centered study integrating bulk RNA, single-cell RNA, and protein immunohistochemistry. Eur. J. Med. Res. 2023, 28, 591. [Google Scholar] [CrossRef]
- Yang, X.; Ye, C.; Zheng, H.; Dai, C.; Zhu, Y. Systemic Analyses of the Expression of TPI1 and Its Associations with Tumor Microenvironment in Lung Adenocarcinoma and Squamous Cell Carcinoma. Dis. Markers 2022, 2022, 6258268. [Google Scholar] [CrossRef]
- Jin, X.; Wang, D.; Lei, M.; Guo, Y.; Cui, Y.; Chen, F.; Sun, W.; Chen, X. TPI1 activates the PI3K/AKT/mTOR signaling pathway to induce breast cancer progression by stabilizing CDCA5. J. Transl. Med. 2022, 20, 191. [Google Scholar] [CrossRef]
- Jiang, J.; Zhan, X.; Xu, G.; Liang, T.; Yu, C.; Liao, S.; Chen, L.; Huang, S.; Sun, X.; Yi, M.; et al. Glycolysis- and immune-related novel prognostic biomarkers of Ewing’s sarcoma: Glucuronic acid epimerase and triosephosphate isomerase 1. Aging 2021, 13, 17516–17535. [Google Scholar] [CrossRef]
- Yu, W.L.; Yu, G.; Dong, H.; Chen, K.; Xie, J.; Yu, H.; Ji, Y.; Yang, G.S.; Li, A.J.; Cong, W.M.; et al. Proteomics analysis identified TPI1 as a novel biomarker for predicting recurrence of intrahepatic cholangiocarcinoma. J. Gastroenterol. 2020, 55, 1171–1182. [Google Scholar] [CrossRef]
- Kublbeck, J.; Niskanen, J.; Honkakoski, P. Metabolism-Disrupting Chemicals and the Constitutive Androstane Receptor CAR. Cells 2020, 9, 2306. [Google Scholar] [CrossRef]
- Klepsch, V.; Moschen, A.R.; Tilg, H.; Baier, G.; Hermann-Kleiter, N. Nuclear Receptors Regulate Intestinal Inflammation in the Context of IBD. Front. Immunol. 2019, 10, 1070. [Google Scholar] [CrossRef]

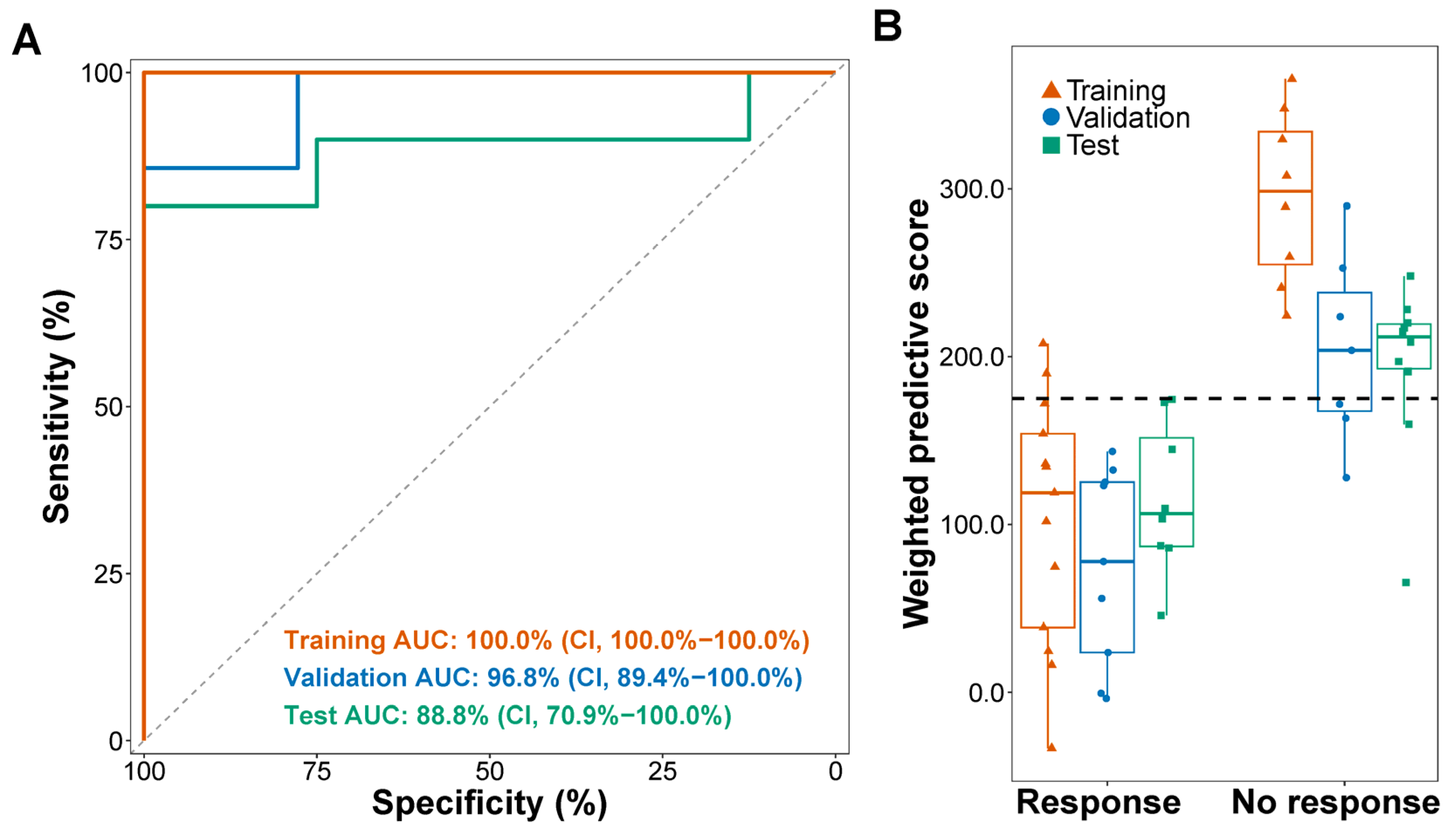

| Responders (No.) | Non-Responders (No.) | Objective Response Rate (95% CI) | ||
|---|---|---|---|---|
| Training | Low wp-score | 11 | 0 | 100.0% (71.5–100.0%) |
| High wp-score | 2 | 8 | 20.0% (2.5–55.6%) | |
| Validation | Low wp-score | 9 | 3 | 75.0% (42.8–94.5%) |
| High wp-score | 0 | 4 | 0.0% (0.0–60.2%) | |
| Test | Low wp-score | 8 | 2 | 80.0% (44.4–97.5%) |
| High wp-score | 0 | 8 | 0.0% (0.0–36.9%) | |
| Single agent | Low wp-score | 7 | 1 | 87.5% (47.4–99.7%) |
| High wp-score | 0 | 7 | 0.0% (0.0–41.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Xu, Y.; Olsen, R.J.; Kasparian, S.; Sun, K.; Mathur, S.; Zhang, J.; He, C.; Chen, S.-H.; Bernicker, E.H.; et al. 5-Hydroxymethylcytosine in Cell-Free DNA Predicts Immunotherapy Response in Lung Cancer. Cells 2024, 13, 715. https://doi.org/10.3390/cells13080715
Shao J, Xu Y, Olsen RJ, Kasparian S, Sun K, Mathur S, Zhang J, He C, Chen S-H, Bernicker EH, et al. 5-Hydroxymethylcytosine in Cell-Free DNA Predicts Immunotherapy Response in Lung Cancer. Cells. 2024; 13(8):715. https://doi.org/10.3390/cells13080715
Chicago/Turabian StyleShao, Jianming, Yitian Xu, Randall J. Olsen, Saro Kasparian, Kai Sun, Sunil Mathur, Jun Zhang, Chuan He, Shu-Hsia Chen, Eric H. Bernicker, and et al. 2024. "5-Hydroxymethylcytosine in Cell-Free DNA Predicts Immunotherapy Response in Lung Cancer" Cells 13, no. 8: 715. https://doi.org/10.3390/cells13080715
APA StyleShao, J., Xu, Y., Olsen, R. J., Kasparian, S., Sun, K., Mathur, S., Zhang, J., He, C., Chen, S.-H., Bernicker, E. H., & Li, Z. (2024). 5-Hydroxymethylcytosine in Cell-Free DNA Predicts Immunotherapy Response in Lung Cancer. Cells, 13(8), 715. https://doi.org/10.3390/cells13080715






