4-Oxo-2-Nonenal- and Agitation-Induced Aggregates of α-Synuclein and Phosphorylated α-Synuclein with Distinct Biophysical Properties and Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cloning, Expression, and Purification of Recombinant Human α-Syn
2.2. Preparation of p-α-Syn
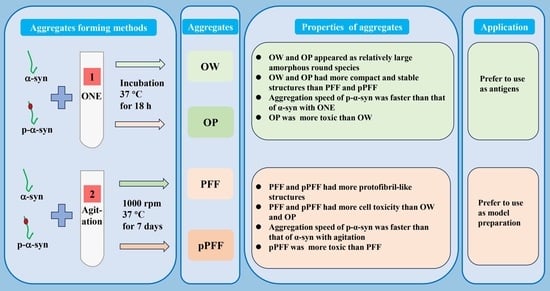
2.3. Preparation of ONE-Induced Wild-Type (WT) α-Syn Aggregates (OW), ONE-Induced p-α-Syn Aggregates (OP), Agitation-Induced α-Syn PFF, and p-α-Syn Preformed Fibrils (pPFF)
2.4. Thioflavin T (ThT) Staining
2.5. Transmission Electron Microscopy (TEM)
2.6. Digestion with Proteinase K (PK)
2.7. WB Analysis
2.8. Cell Culture, Immunofluorescence, and Confocal Microscopy
2.9. Cell Viability Assay
2.10. Mice
2.11. Open Field Test
2.12. Rotarod Test
2.13. Aggregates as Calibrators in Enzyme-Linked Immunosorbent Assay (ELISA)
2.14. Statistical Analysis
3. Results
3.1. ONE- and Agitation-Induced Formation of α-Syn and p-α-Syn Aggregates with Distinct Morphologies and Structures
3.2. ONE- and Agitation-Induced Formation of α-Syn and p-α-Syn Aggregates at Distinct Speeds
3.3. Stability and Compactness of ONE- and Agitation-Induced α-Syn and p-α-Syn Aggregates
3.4. ONE- and Agitation-Induced Aggregates Reduced Cell Viability and Formed p-α-Syn Inclusions to Different Degrees
3.5. ONE- and Agitation-Induced Aggregates Could Be Used as Standard Antigens for the Sandwich ELISA in Different OD Values
3.6. The Detection of Aggregates α-Syn and p-α-Syn in the Plasma of Transgenic Mice Using OW- and OP -Special Sandwich Elisa Assay Demonstrated Concordance with the Observed Motor Dysfunction in These Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, J.M.; Lin, Y.; Heo, Y.; Lee, Y.H. Polymorphism in alpha-synuclein oligomers and its implications in toxicity under disease conditions. Front. Mol. Biosci. 2022, 9, 959425. [Google Scholar] [CrossRef]
- Delenclos, M.; Burgess, J.D.; Lamprokostopoulou, A.; Outeiro, T.F.; Vekrellis, K.; McLean, P.J. Cellular models of alpha-synuclein toxicity and aggregation. J. Neurochem. 2019, 150, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.H.; et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, 6307. [Google Scholar] [CrossRef] [PubMed]
- Gribaudo, S.; Tixador, P.; Bousset, L.; Fenyi, A.; Lino, P.; Melki, R.; Peyrin, J.M.; Perrier, A.L. Propagation of alpha-Synuclein Strains within Human Reconstructed Neuronal Network. Stem Cell Rep. 2019, 12, 230–244. [Google Scholar] [CrossRef]
- Oueslati, A. Implication of Alpha-Synuclein Phosphorylation at S129 in Synucleinopathies: What Have We Learned in the Last Decade. J. Park. Dis. 2016, 6, 39–51. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; McLean, P.J.; Lozano, A.M.; Lang, A.E. alpha-Synuclein oligomers and clinical implications for Parkinson disease. Ann. Neurol. 2013, 73, 155–169. [Google Scholar] [CrossRef]
- Cremades, N.; Chen, S.W.; Dobson, C.M. Structural Characteristics of alpha-Synuclein Oligomers. Int. Rev. Cell Mol. Biol. 2017, 329, 79–143. [Google Scholar] [CrossRef]
- O’Hara, D.M.; Kalia, S.K.; Kalia, L.V. Methods for detecting toxic alpha-synuclein species as a biomarker for Parkinson’s disease. Crit. Rev. Clin. Lab. Sci. 2020, 57, 291–307. [Google Scholar] [CrossRef]
- Nasstrom, T.; Wahlberg, T.; Karlsson, M.; Nikolajeff, F.; Lannfelt, L.; Ingelsson, M.; Bergstrom, J. The lipid peroxidation metabolite 4-oxo-2-nonenal cross-links alpha-synuclein causing rapid formation of stable oligomers. Biochem. Biophys. Res. Commun. 2009, 378, 872–876. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Blair, I.A. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 2000, 13, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.; Gronnemose, A.L.; Pedersen, J.N.; Nowak, J.S.; Christiansen, G.; Nielsen, J.; Mulder, F.A.A.; Otzen, D.E.; Jorgensen, T.J.D. Lipid Peroxidation Products HNE and ONE Promote and Stabilize Alpha-Synuclein Oligomers by Chemical Modifications. Biochemistry 2021, 60, 3644–3658. [Google Scholar] [CrossRef]
- Nasstrom, T.; Fagerqvist, T.; Barbu, M.; Karlsson, M.; Nikolajeff, F.; Kasrayan, A.; Ekberg, M.; Lannfelt, L.; Ingelsson, M.; Bergstrom, J. The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of alpha-synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Radic. Biol. Med. 2011, 50, 428–437. [Google Scholar] [CrossRef]
- Polinski, N.K.; Volpicelli-Daley, L.A.; Sortwell, C.E.; Luk, K.C.; Cremades, N.; Gottler, L.M.; Froula, J.; Duffy, M.F.; Lee, V.M.Y.; Martinez, T.N.; et al. Best Practices for Generating and Using Alpha-Synuclein Pre-Formed Fibrils to Model Parkinson’s Disease in Rodents. J. Park. Dis. 2018, 8, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Luk, K.C.; Lee, V.M. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 2014, 9, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Song, C.; O’Brien, P.; Stieber, A.; Branch, J.R.; Brunden, K.R.; Trojanowski, J.Q.; Lee, V.M. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20051–20056. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Marotta, N.P.; Ara, J.; Meymand, E.S.; Zhang, B.; Kameda, H.; Koike, M.; Luk, K.C.; Trojanowski, J.Q.; Lee, V.M. alpha-Synuclein aggregates amplified from patient-derived Lewy bodies recapitulate Lewy body diseases in mice. Nat. Commun. 2023, 14, 6892. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Q.; Xing, H.; Gao, G.; Liu, J.; Huang, Y.; Yang, H. Characterization of a Novel Monoclonal Antibody for Serine-129 Phosphorylated alpha-Synuclein: A Potential Application for Clinical and Basic Research. Front. Neurol. 2022, 13, 821792. [Google Scholar] [CrossRef]
- Mbefo, M.K.; Paleologou, K.E.; Boucharaba, A.; Oueslati, A.; Schell, H.; Fournier, M.; Olschewski, D.; Yin, G.; Zweckstetter, M.; Masliah, E.; et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J. Biol. Chem. 2010, 285, 2807–2822. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, W.; Lu, Y.; Tian, H.; Duan, C.; Lu, L.; Gao, G.; Wu, X.; Wang, X.; Yang, H. Piperlongumine restores the balance of autophagy and apoptosis by increasing BCL2 phosphorylation in rotenone-induced Parkinson disease models. Autophagy 2018, 14, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lu, Y.; Zhang, Q.; Liu, W.; Yang, R.; Jiao, J.; Liu, J.; Gao, G.; Yang, H. Piperine promotes autophagy flux by P2RX4 activation in SNCA/alpha-synuclein-induced Parkinson disease model. Autophagy 2022, 18, 559–575. [Google Scholar] [CrossRef]
- Li, W.; Hu, J.; Li, X.; Lu, Z.; Li, X.; Wang, C.; Yu, S. Receptor-Dependent Endocytosis Mediates alpha-Synuclein Oligomer Transport into Red Blood Cells. Front. Aging Neurosci. 2022, 14, 899892. [Google Scholar] [CrossRef] [PubMed]
- Fagerqvist, T.; Nasstrom, T.; Ihse, E.; Lindstrom, V.; Sahlin, C.; Tucker, S.M.; Kasaryan, A.; Karlsson, M.; Nikolajeff, F.; Schell, H.; et al. Off-pathway alpha-synuclein oligomers seem to alter alpha-synuclein turnover in a cell model but lack seeding capability in vivo. Amyloid 2013, 20, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Villar-Pique, A.; Lopes da Fonseca, T.; Outeiro, T.F. Structure, function and toxicity of alpha-synuclein: The Bermuda triangle in synucleinopathies. J. Neurochem. 2016, 139 (Suppl. S1), 240–255. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Nakajo, S.; Tu, P.H.; Tomita, T.; Nakaya, K.; Lee, V.M.; Trojanowski, J.Q.; Iwatsubo, T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998, 152, 879–884. [Google Scholar] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Cairns, N.J.; Lantos, P.L.; Goedert, M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 1998, 251, 205–208. [Google Scholar] [CrossRef]
- Majbour, N.K.; Vaikath, N.N.; Van Dijk, K.D.; Ardah, M.T.; Varghese, S.; Vesterager, L.B.; Montezinho, L.P.; Poole, S.; Safieh-Garabedian, B.; Tokuda, T.; et al. Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson’s disease. Mol. Neurodegener. 2016, 11, 7. [Google Scholar] [CrossRef]
- El-Agnaf, O.M.; Salem, S.A.; Paleologou, K.E.; Curran, M.D.; Gibson, M.J.; Court, J.A.; Schlossmacher, M.G.; Allsop, D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006, 20, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, T.; Qureshi, M.M.; Ardah, M.T.; Varghese, S.; Shehab, S.A.; Kasai, T.; Ishigami, N.; Tamaoka, A.; Nakagawa, M.; El-Agnaf, O.M. Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 2010, 75, 1766–1772. [Google Scholar] [CrossRef]
- Park, M.J.; Cheon, S.M.; Bae, H.R.; Kim, S.H.; Kim, J.W. Elevated levels of alpha-synuclein oligomer in the cerebrospinal fluid of drug-naive patients with Parkinson’s disease. J. Clin. Neurol. 2011, 7, 215–222. [Google Scholar] [CrossRef]
- Paleologou, K.E.; Kragh, C.L.; Mann, D.M.; Salem, S.A.; Al-Shami, R.; Allsop, D.; Hassan, A.H.; Jensen, P.H.; El-Agnaf, O.M. Detection of elevated levels of soluble alpha-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain 2009, 132 Pt 4, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Polinski, N.K. A Summary of Phenotypes Observed in the In Vivo Rodent Alpha-Synuclein Preformed Fibril Model. J. Park. Dis. 2021, 11, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Almandoz-Gil, L.; Welander, H.; Ihse, E.; Khoonsari, P.E.; Musunuri, S.; Lendel, C.; Sigvardson, J.; Karlsson, M.; Ingelsson, M.; Kultima, K.; et al. Low molar excess of 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote oligomerization of alpha-synuclein through different pathways. Free Radic. Biol. Med. 2017, 110, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Lee, H.G.; Liu, Q.; Perry, G.; Smith, M.A.; Sayre, L.M. 4-Oxo-2-nonenal is both more neurotoxic and more protein reactive than 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005, 18, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- May-Zhang, L.S.; Yermalitsky, V.; Melchior, J.T.; Morris, J.; Tallman, K.A.; Borja, M.S.; Pleasant, T.; Amarnath, V.; Song, W.; Yancey, P.G.; et al. Modified sites and functional consequences of 4-oxo-2-nonenal adducts in HDL that are elevated in familial hypercholesterolemia. J. Biol. Chem. 2019, 294, 19022–19033. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.J.; Ho, D.H.; Park, E.; Jung, J.W.; Cho, K.; Hong, J.H.; Lee, H.J.; Kim, K.P.; Lee, S.J. Lipid peroxidation product 4-hydroxy-2-nonenal promotes seeding-capable oligomer formation and cell-to-cell transfer of alpha-synuclein. Antioxid. Redox Signal. 2013, 18, 770–783. [Google Scholar] [CrossRef]
- Cruz, M.; Tusell, J.M.; Grillo-Bosch, D.; Albericio, F.; Serratosa, J.; Rabanal, F.; Giralt, E. Inhibition of beta-amyloid toxicity by short peptides containing N-methyl amino acids. J. Pept. Res. 2004, 63, 324–328. [Google Scholar] [CrossRef]
- Alam, P.; Bousset, L.; Melki, R.; Otzen, D.E. alpha-synuclein oligomers and fibrils: A spectrum of species; a spectrum of toxicities. J. Neurochem. 2019, 150, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef]
- Dryanovski, D.I.; Guzman, J.N.; Xie, Z.; Galteri, D.J.; Volpicelli-Daley, L.A.; Lee, V.M.; Miller, R.J.; Schumacker, P.T.; Surmeier, D.J. Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J. Neurosci. 2013, 33, 10154–10164. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Watanabe, Y.; Tsujimura, A.; Tatebe, H.; Miyata, S.; Tokuda, T.; Mizuno, T.; Tanaka, M. Differential expression of alpha-synuclein in hippocampal neurons. PLoS ONE 2014, 9, e89327. [Google Scholar] [CrossRef] [PubMed]
- Campenot, R.B.; Lund, K.; Mok, S.A. Production of compartmented cultures of rat sympathetic neurons. Nat. Protoc. 2009, 4, 1869–1887. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Singh, P.K.; Sahay, S.; Jha, N.N.; Jacob, R.S.; Sen, S.; Kumar, A.; Riek, R.; Maji, S.K. Structure based aggregation studies reveal the presence of helix-rich intermediate during alpha-Synuclein aggregation. Sci. Rep. 2015, 5, 9228. [Google Scholar] [CrossRef] [PubMed]
- Dalfo, E.; Ferrer, I. Early alpha-synuclein lipoxidation in neocortex in Lewy body diseases. Neurobiol. Aging 2008, 29, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Arad, E.; Green, H.; Jelinek, R.; Rapaport, H. Revisiting thioflavin T (ThT) fluorescence as a marker of protein fibrillation—The prominent role of electrostatic interactions. J. Colloid Interface Sci. 2020, 573, 87–95. [Google Scholar] [CrossRef]
- LeVine, H., III. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999, 309, 274–284. [Google Scholar]
- Ma, M.R.; Hu, Z.W.; Zhao, Y.F.; Chen, Y.X.; Li, Y.M. Phosphorylation induces distinct alpha-synuclein strain formation. Sci. Rep. 2016, 6, 37130. [Google Scholar] [CrossRef] [PubMed]
- Paleologou, K.E.; Schmid, A.W.; Rospigliosi, C.C.; Kim, H.Y.; Lamberto, G.R.; Fredenburg, R.A.; Lansbury, P.T., Jr.; Fernandez, C.O.; Eliezer, D.; Zweckstetter, M.; et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J. Biol. Chem. 2008, 283, 16895–16905. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Feng, R.; Chen, F.; Wu, Z.; Li, D.; Qiu, X. Rapid FRET Assay for the Early Detection of Alpha-Synuclein Aggregation in Parkinson’s Disease. ACS Chem. Neurosci. 2024, 15, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Jethva, P.N.; Kardani, J.R.; Roy, I. Modulation of alpha-synuclein aggregation by dopamine in the presence of MPTP and its metabolite. FEBS J. 2011, 278, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Weinreb, P.H.; Lansbury, P.T., Jr. The core Alzheimer’s peptide NAC forms amyloid fibrils which seed and are seeded by beta-amyloid: Is NAC a common trigger or target in neurodegenerative disease? Chem. Biol. 1995, 2, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Murray, I.V.; Trojanowski, J.Q.; Lee, V.M. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J. Biol. Chem. 2001, 276, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Bockmann, A.; Meier, B.H.; et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013, 4, 2575. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Kafle, D.; Singh, R.K.; Hung, Y.C. Effects of 4-Oxo-2-nonenal on biochemical properties of bovine heart mitochondria. Food Sci. Nutr. 2022, 10, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef]
- Chen, W.R.; Chen, J.C.; Chang, S.Y.; Chao, C.T.; Wu, Y.R.; Chen, C.M.; Chou, C. Phosphorylated alpha-synuclein in diluted human serum as a biomarker for Parkinson’s disease. Biomed. J. 2022, 45, 914–922. [Google Scholar] [CrossRef]
- Andersen, C.B.; Lausdahl, A.K.; Nielsen, J.; Clausen, M.P.; Mulder, F.A.A.; Otzen, D.E.; Arnspang, E.C. 4-Oxo-2-nonenal-Induced alpha-Synuclein Oligomers Interact with Membranes in the Cell, Leading to Mitochondrial Fragmentation. Biochemistry 2023, 62, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.D.; Zacharopoulou, M.; Moons, R.; Fusco, G.; Seetaloo, N.; Chiki, A.; Woodhams, P.J.; Mela, I.; Lashuel, H.A.; Phillips, J.J.; et al. Extent of N-terminus exposure of monomeric alpha-synuclein determines its aggregation propensity. Nat. Commun. 2020, 11, 2820. [Google Scholar] [CrossRef] [PubMed]
- Semenyuk, P.I. Alpha-synuclein phosphorylation induces amyloid conversion via enhanced electrostatic bridging: Insights from molecular modeling of the full-length protein. Biophys. Chem. 2024, 307, 107196. [Google Scholar] [CrossRef] [PubMed]
- Samuel, F.; Flavin, W.P.; Iqbal, S.; Pacelli, C.; Renganathan, S.D.S.; Trudeau, L.E.; Campbell, E.M.; Fraser, P.E.; Tandon, A. Effects of Serine 129 Phosphorylation on alpha-Synuclein Aggregation, Membrane Association, and Internalization. J. Biol. Chem. 2016, 291, 4374–4385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, R.; Pan, B.; Xu, H.; Olufemi, M.F.; Gathagan, R.J.; Li, Y.; Zhang, L.; Zhang, J.; Xiang, W.; et al. Post-translational modifications of soluble alpha-synuclein regulate the amplification of pathological alpha-synuclein. Nat. Neurosci. 2023, 26, 213–225. [Google Scholar] [CrossRef] [PubMed]








| Species | OW | OP | PFF | pPFF |
|---|---|---|---|---|
| Incubation times | 18 h | 18 h | 7 days | 7 days |
| Fibrils width | 5.828 ± 2.368 nm | 7.597± 3.241 nm (*) | 4.017± 2.205 nm (#) | 5.851 ± 2.128 nm (*,$) |
| ThT (ratio to control) | 6.762 ± 0.9715 | 18.47 ± 0.4895 (****) | 35.78 ± 2.35 (####) | 46.76 ± 1.568 (****,$$$$) |
| p-α-syn aggregate speed (ratio to α-syn) | 1 | 1.409 ± 0.016 (18 h) (***) | 1 | 1.497 ±0.111 (7Days) (***) |
| Density (ratio to OW) | 1 | 1.119 ± 0.1126 | 0.733 ± 0.0469 (#) | 0.8981 ± 0.09846 ($) |
| SDS-Stability of aggregates (ratio to monomeric protein) | 13.13 ± 2.239 | 7.143 ± 2.284 | 2.335 ± 0.3494 (###) | 3.201 ± 1.068 ($) |
| PK Resistance | ++ | +++ | + | ++ |
| Cell toxic sensibility times | 6 h | 3 h | 1 h | 1 h |
| Prefer application | Antigens for ELISA | Antigens for ELISA | Seeds for model | Seeds for model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Liu, W.; Zhang, Q.; Jiao, J.; Wang, Z.; Gao, G.; Yang, H. 4-Oxo-2-Nonenal- and Agitation-Induced Aggregates of α-Synuclein and Phosphorylated α-Synuclein with Distinct Biophysical Properties and Biomedical Applications. Cells 2024, 13, 739. https://doi.org/10.3390/cells13090739
Wang T, Liu W, Zhang Q, Jiao J, Wang Z, Gao G, Yang H. 4-Oxo-2-Nonenal- and Agitation-Induced Aggregates of α-Synuclein and Phosphorylated α-Synuclein with Distinct Biophysical Properties and Biomedical Applications. Cells. 2024; 13(9):739. https://doi.org/10.3390/cells13090739
Chicago/Turabian StyleWang, Tie, Weijin Liu, Qidi Zhang, Jie Jiao, Zihao Wang, Ge Gao, and Hui Yang. 2024. "4-Oxo-2-Nonenal- and Agitation-Induced Aggregates of α-Synuclein and Phosphorylated α-Synuclein with Distinct Biophysical Properties and Biomedical Applications" Cells 13, no. 9: 739. https://doi.org/10.3390/cells13090739
APA StyleWang, T., Liu, W., Zhang, Q., Jiao, J., Wang, Z., Gao, G., & Yang, H. (2024). 4-Oxo-2-Nonenal- and Agitation-Induced Aggregates of α-Synuclein and Phosphorylated α-Synuclein with Distinct Biophysical Properties and Biomedical Applications. Cells, 13(9), 739. https://doi.org/10.3390/cells13090739