Evolution of Blood Innate Immune Cell Phenotypes Following SARS-CoV-2 Infection in Hospitalized Patients with COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. The Description of the Study and Participants
2.2. Blood Collection and Cell Enumeration
2.3. Flow Cytometry
2.4. Luminex
2.5. Statistics
3. Results
3.1. Participants
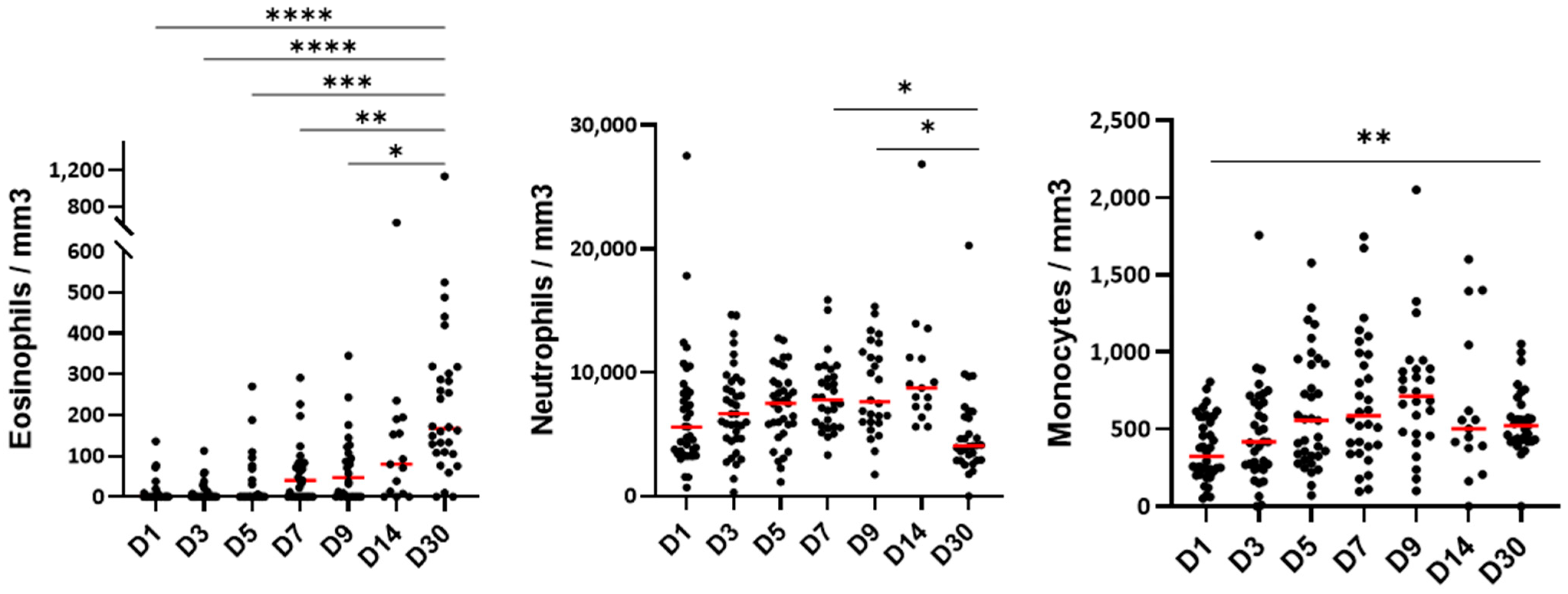
3.2. The Blood Cell Count During the Hospitalization
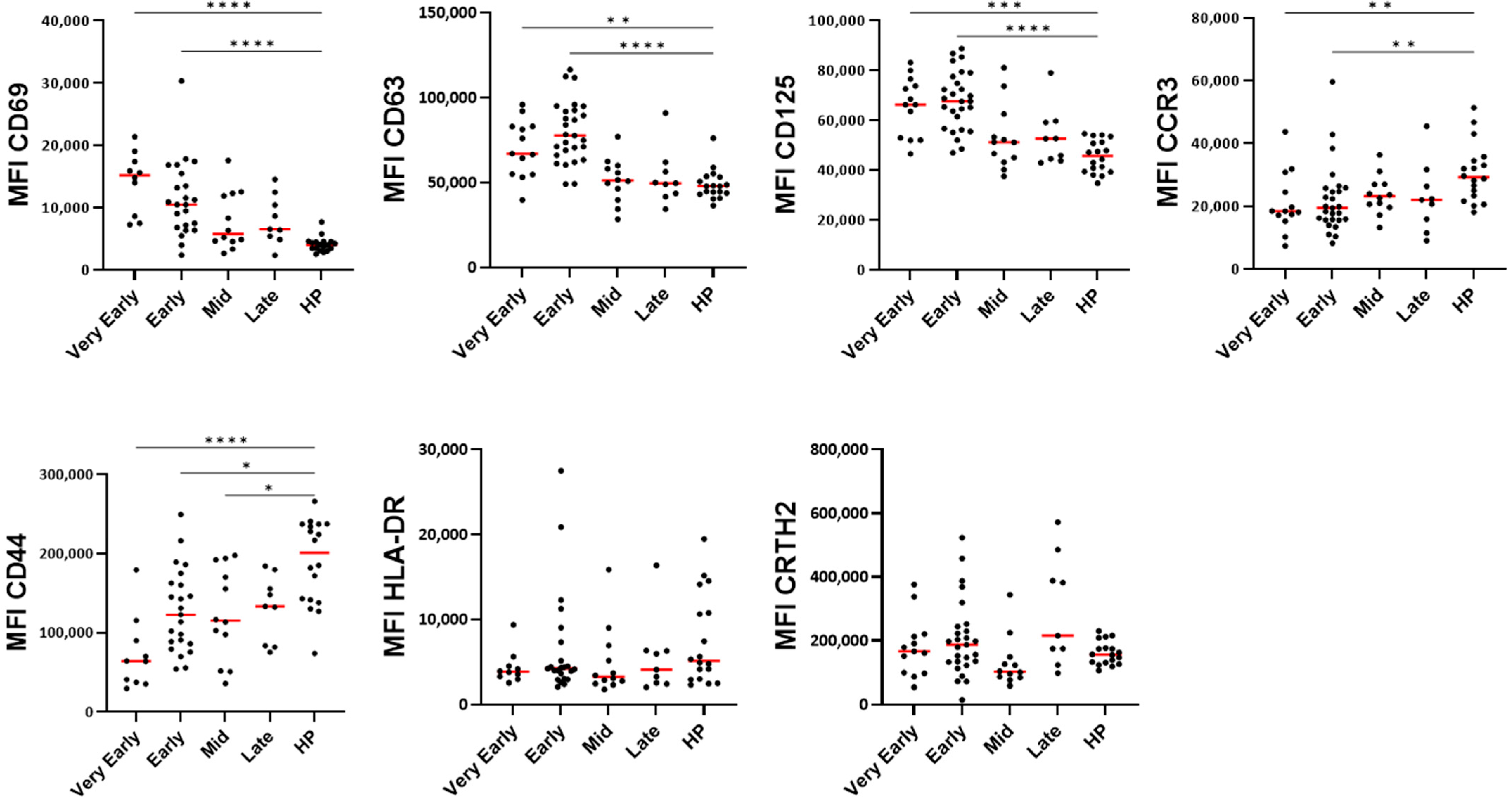
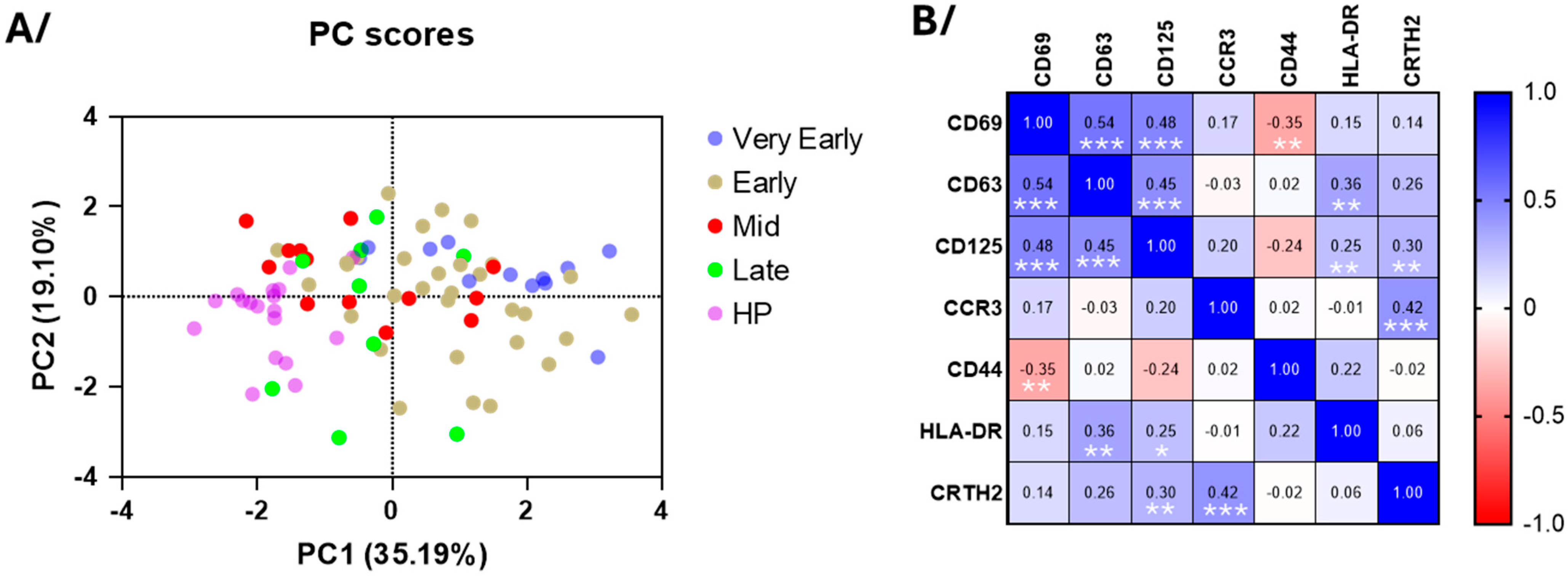
3.3. Blood Eosinophil Phenotype
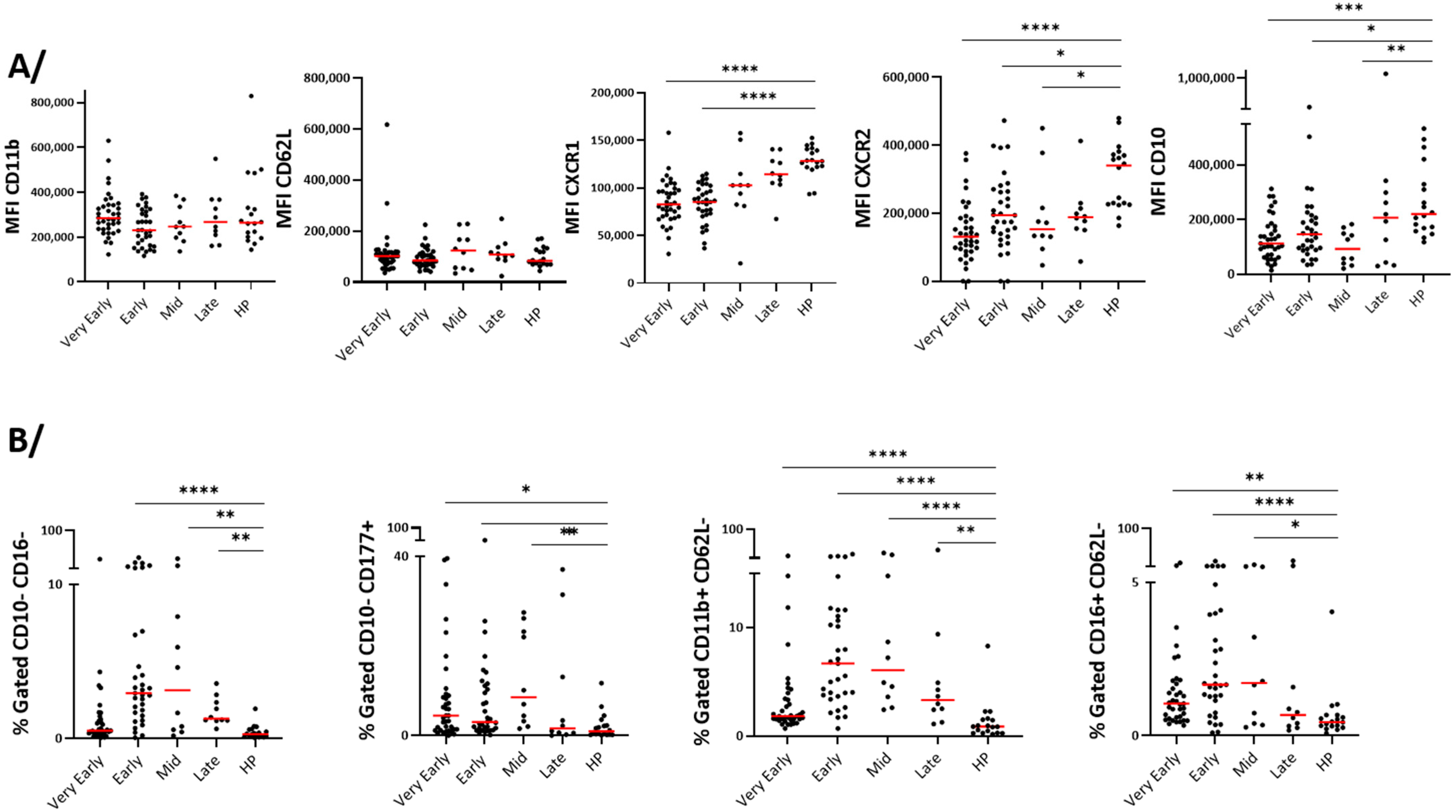
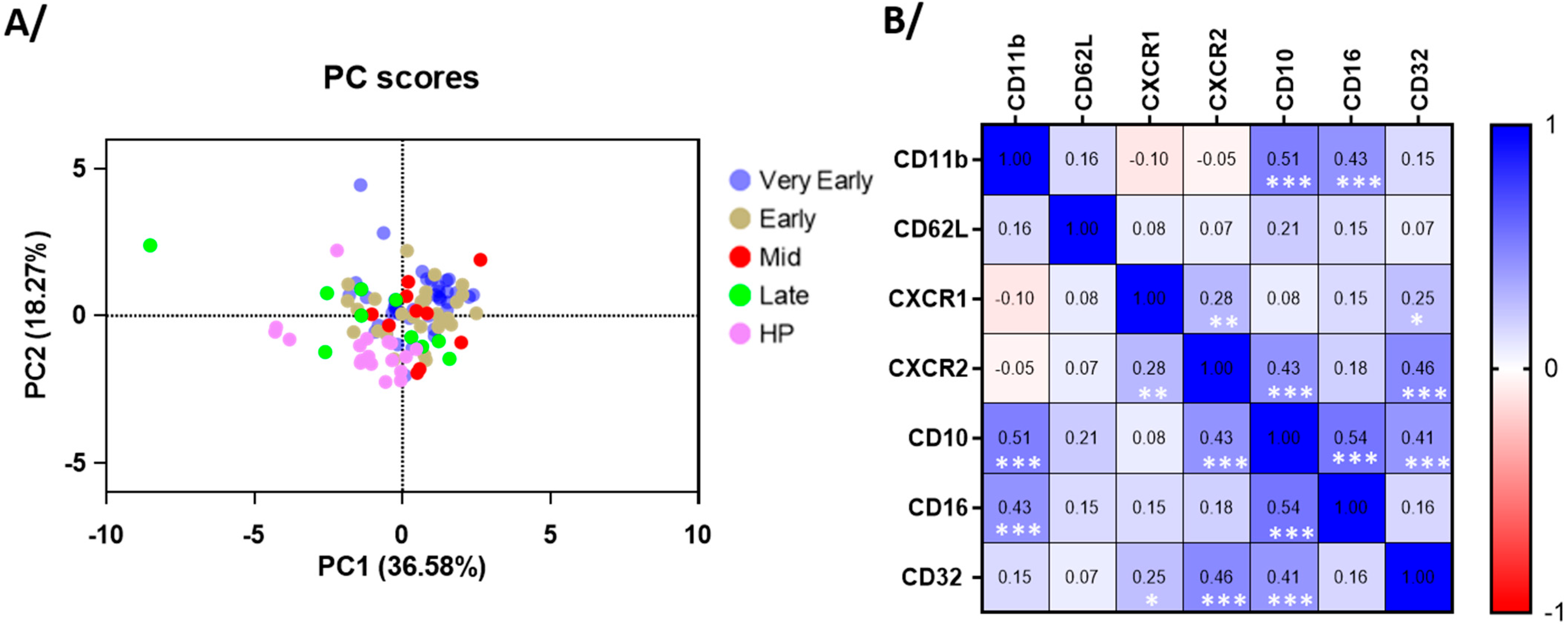
3.4. Blood Neutrophil Phenotype
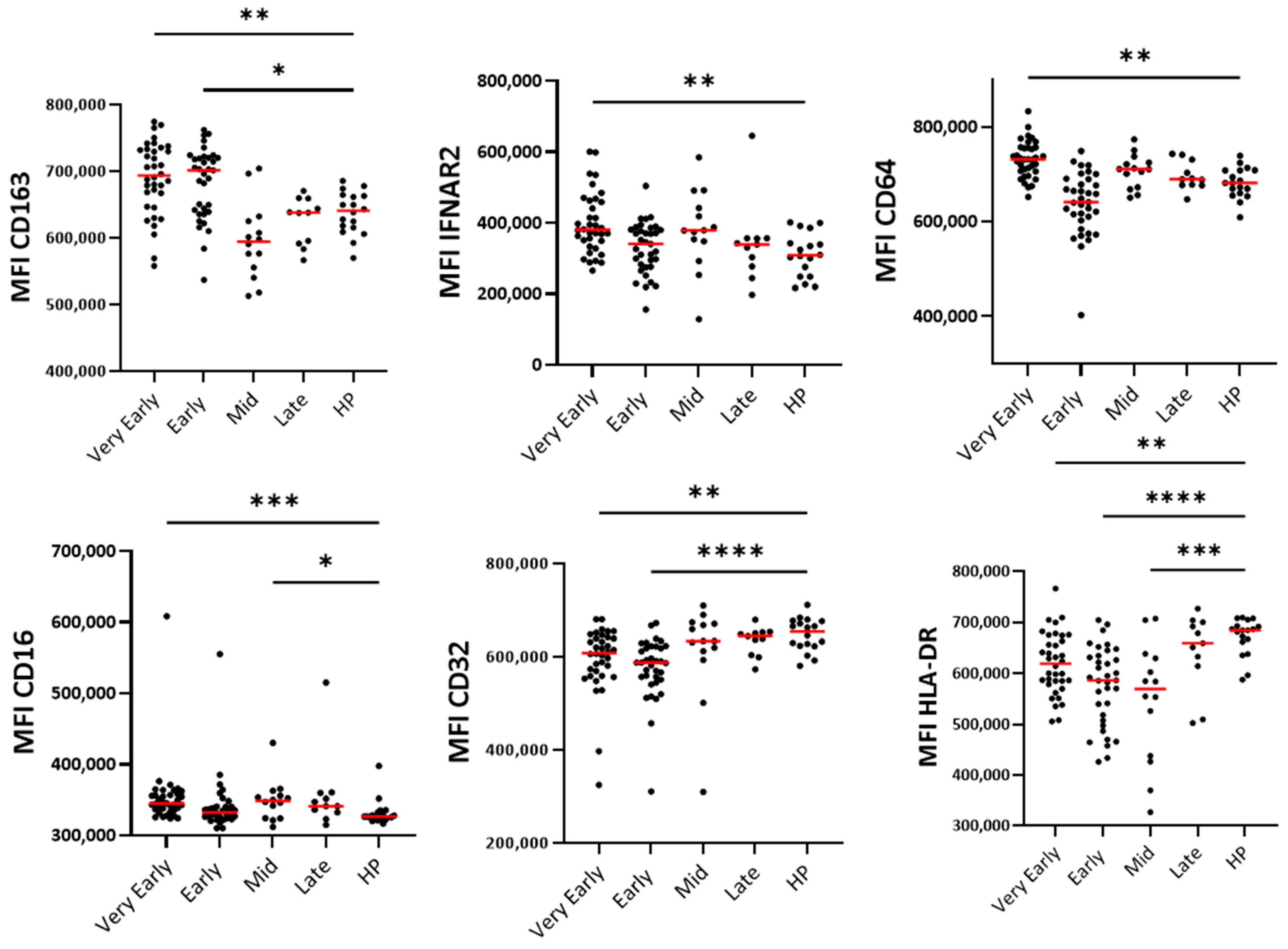
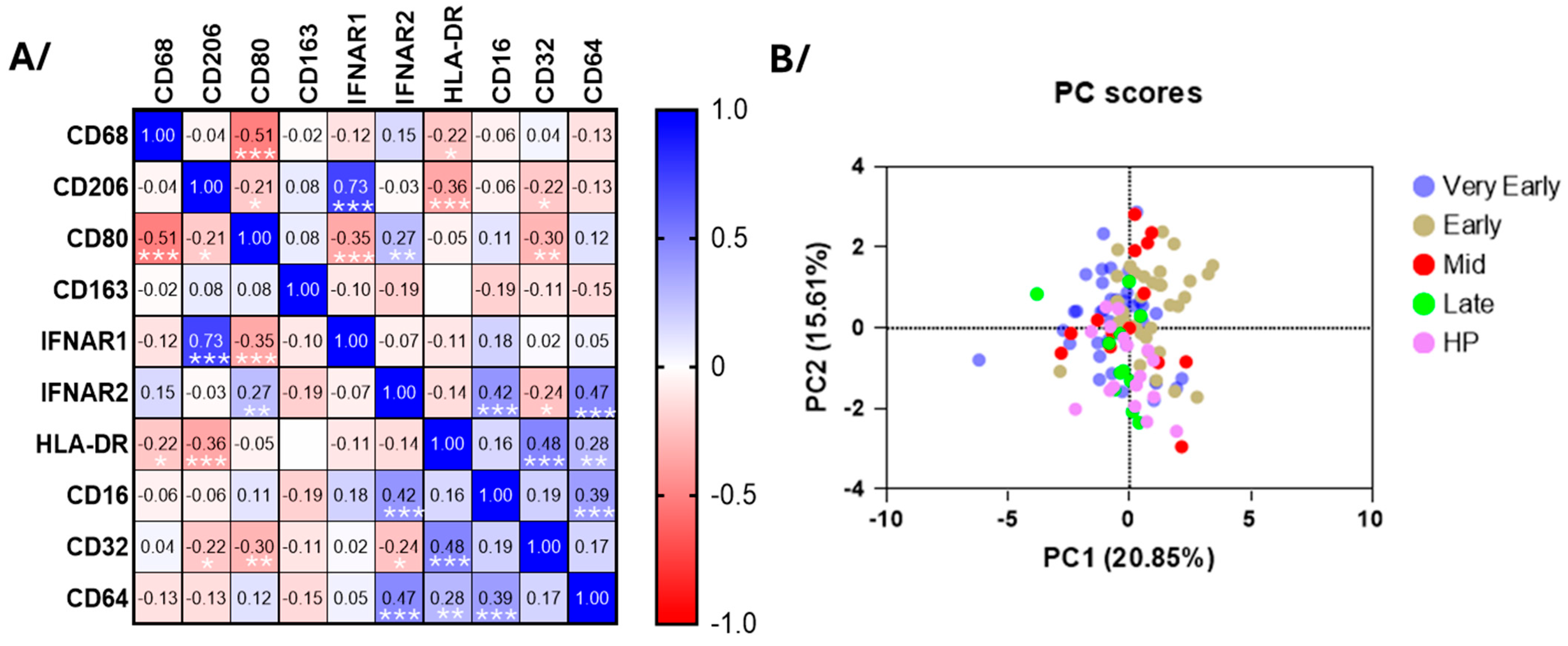
3.5. Blood Monocyte Phenotype
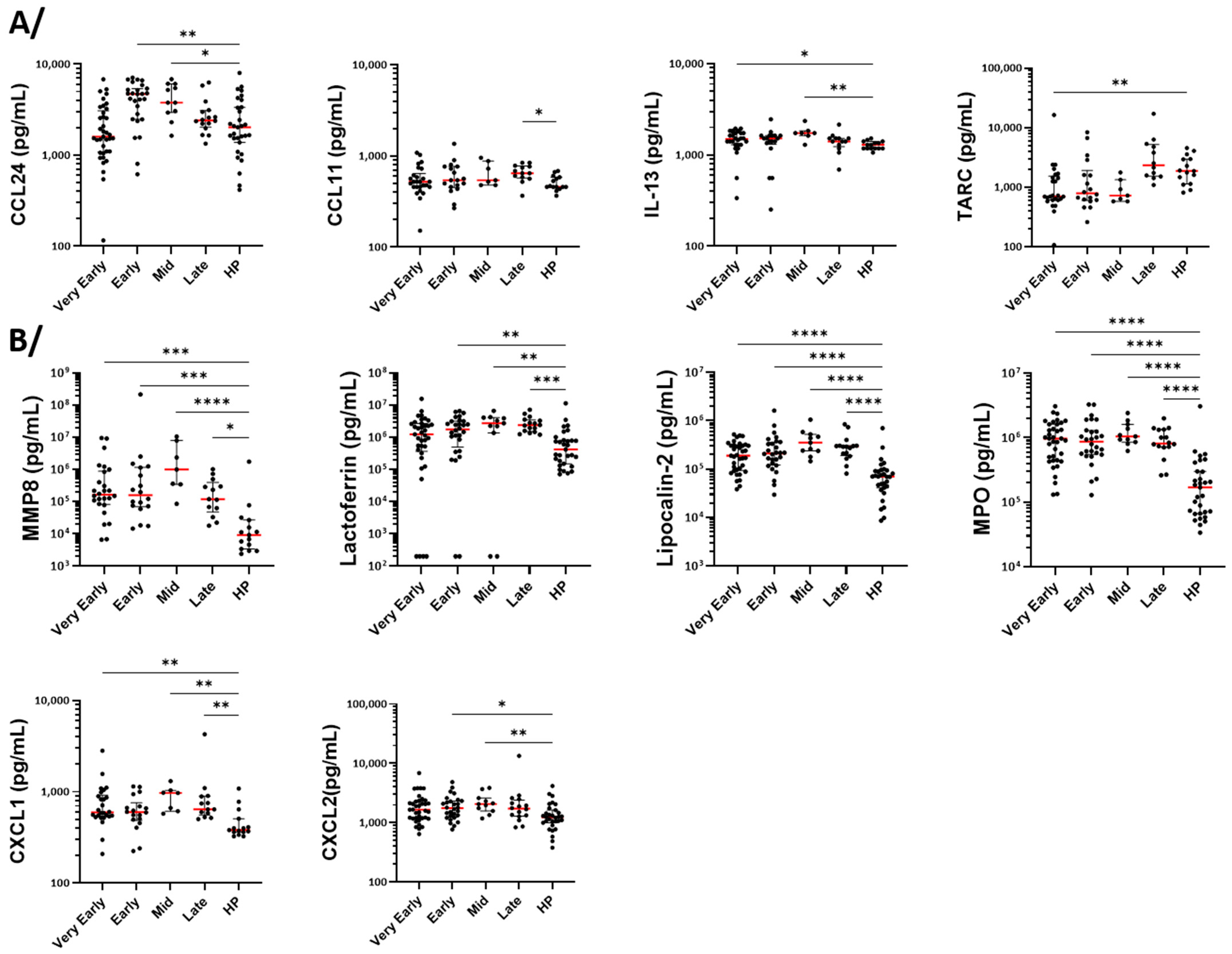
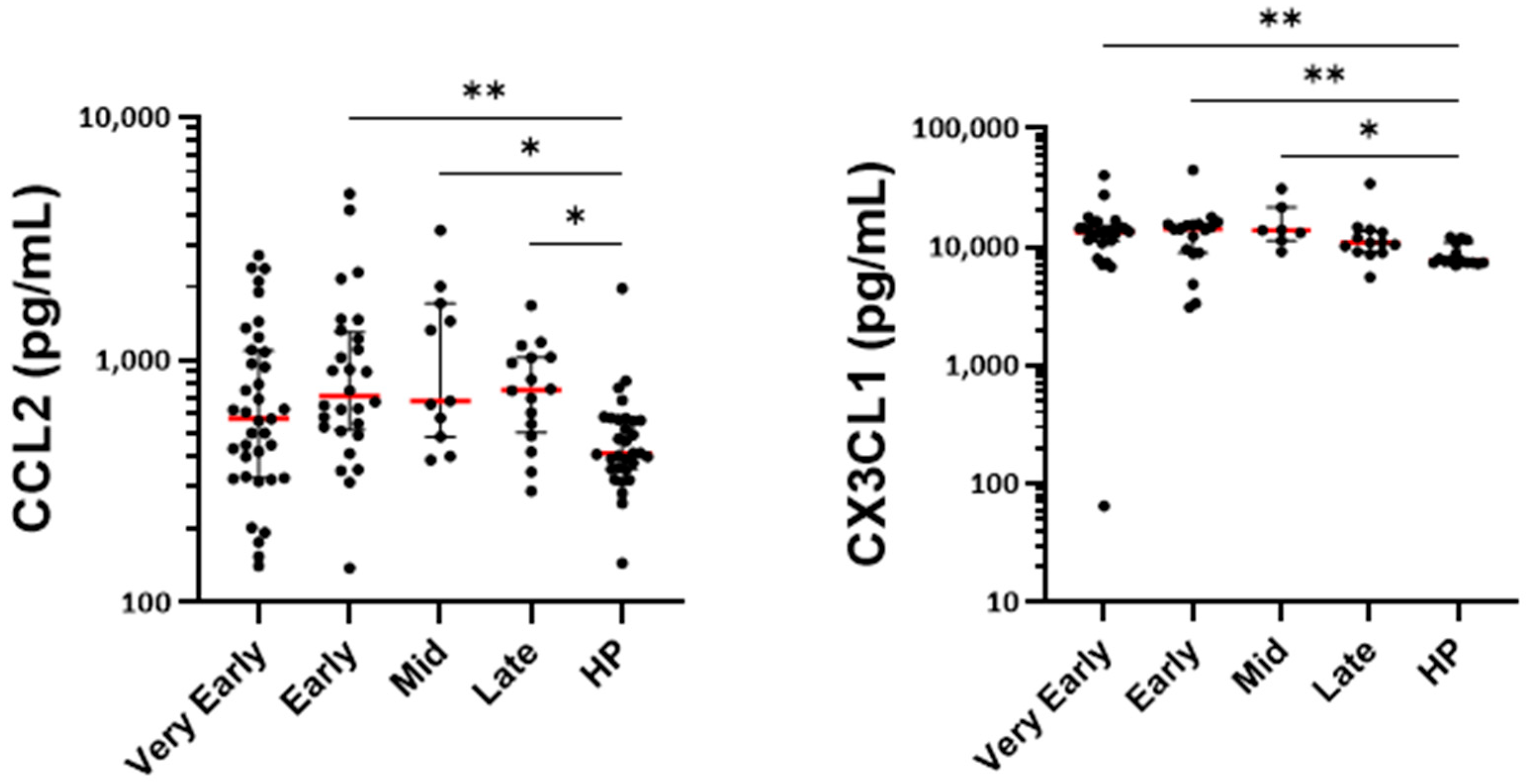
3.6. Measurement of Cytokines, Growth Factors, and Markers of Cell Activation in Serum
4. Discussion
4.1. Eosinopenia and Change in Phenotype
4.2. Neutrophilia and Change in Phenotype
4.3. Monocytes
4.4. General Roles of the Innate Cells in Viral Infections and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| D1 | day 1 |
| MFI | median fluorescence intensity |
| NETosis | neutrophil extracellular traps formation |
| PCA | principal component analysis |
| SARS | severe acute respiratory syndrome |
| T2 | type-2 |
References
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J.; Diallo, A.B.; Boumaza, A.; Lopez, A.; Michel, M.; Allardet-Servent, J.; Mezouar, S.; Sereme, Y.; Busnel, J.M.; Miloud, T.; et al. A Granulocytic Signature Identifies COVID-19 and Its Severity. J. Infect. Dis. 2020, 222, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Lourda, M.; Dzidic, M.; Hertwig, L.; Bergsten, H.; Palma Medina, L.M.; Sinha, I.; Kvedaraite, E.; Chen, P.; Muvva, J.R.; Gorin, J.B.; et al. High-dimensional profiling reveals phenotypic heterogeneity and disease-specific alterations of granulocytes in COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2109123118. [Google Scholar] [CrossRef]
- Jukema, B.N.; Smit, K.; Hopman, M.T.E.; Bongers, C.; Pelgrim, T.C.; Rijk, M.H.; Platteel, T.N.; Venekamp, R.P.; Zwart, D.L.M.; Rutten, F.H.; et al. Neutrophil and Eosinophil Responses Remain Abnormal for Several Months in Primary Care Patients With COVID-19 Disease. Front. Allergy 2022, 3, 942699. [Google Scholar] [CrossRef] [PubMed]
- Koenderman, L.; Siemers, M.J.; van Aalst, C.; Bongers, S.H.; Spijkerman, R.; Bindels, B.J.J.; Giustarini, G.; van Goor, H.M.R.; Kaasjager, K.A.H.; Vrisekoop, N. The Systemic Immune Response in COVID-19 Is Associated with a Shift to Formyl-Peptide Unresponsive Eosinophils. Cells 2021, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cerrillo, I.; Landete, P.; Aldave, B.; Sanchez-Alonso, S.; Sanchez-Azofra, A.; Marcos-Jimenez, A.; Avalos, E.; Alcaraz-Serna, A.; de Los Santos, I.; Mateu-Albero, T.; et al. COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Investig. 2020, 130, 6290–6300. [Google Scholar] [CrossRef]
- Lindsley, A.W.; Schwartz, J.T.; Rothenberg, M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McKenna, E.; Wubben, R.; Isaza-Correa, J.M.; Melo, A.M.; Mhaonaigh, A.U.; Conlon, N.; O’Donnell, J.S.; Ni Cheallaigh, C.; Hurley, T.; Stevenson, N.J.; et al. Neutrophils in COVID-19: Not Innocent Bystanders. Front. Immunol. 2022, 13, 864387. [Google Scholar] [CrossRef]
- Carissimo, G.; Xu, W.; Kwok, I.; Abdad, M.Y.; Chan, Y.H.; Fong, S.W.; Puan, K.J.; Lee, C.Y.; Yeo, N.K.; Amrun, S.N.; et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat. Commun. 2020, 11, 5243. [Google Scholar] [CrossRef]
- Millrud, C.R.; Kagedal, A.; Kumlien Georen, S.; Winqvist, O.; Uddman, R.; Razavi, R.; Munck-Wikland, E.; Cardell, L.O. NET-producing CD16(high) CD62L(dim) neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int. J. Cancer 2017, 140, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Avila, J.A.; Adrover, J.M.; Hidalgo, A. Neutrophils in Homeostasis, Immunity, and Cancer. Immunity 2017, 46, 15–28. [Google Scholar] [CrossRef]
- Spijkerman, R.; Jorritsma, N.K.N.; Bongers, S.H.; Bindels, B.J.J.; Jukema, B.N.; Hesselink, L.; Hietbrink, F.; Leenen, L.P.H.; van Goor, H.M.R.; Vrisekoop, N.; et al. An increase in CD62L(dim) neutrophils precedes the development of pulmonary embolisms in COVID-19 patients. Scand. J. Immunol. 2021, 93, e13023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Jin, Y.; Jin, Y.; Shen, Y.; Qu, Q.; Chen, C. The imbalance of circulating PD-L1-expressing non-classical/ classical monocytes is involved in immunocompromised host related pulmonary opportunistic infection. Innate Immun. 2025, 31, 17534259251316152. [Google Scholar] [CrossRef] [PubMed]
- Drake, K.A.; Talantov, D.; Tong, G.J.; Lin, J.T.; Verheijden, S.; Katz, S.; Leung, J.M.; Yuen, B.; Krishna, V.; Wu, M.J.; et al. Multi-omic profiling reveals early immunological indicators for identifying COVID-19 Progressors. Clin. Immunol. 2023, 256, 109808. [Google Scholar] [CrossRef]
- Martinez-Diz, S.; Marin-Benesiu, F.; Lopez-Torres, G.; Santiago, O.; Diaz-Cuellar, J.F.; Martin-Esteban, S.; Cortes-Valverde, A.I.; Arenas-Rodriguez, V.; Cuenca-Lopez, S.; Porras-Quesada, P.; et al. Relevance of TMPRSS2, CD163/CD206, and CD33 in clinical severity stratification of COVID-19. Front. Immunol. 2022, 13, 1094644. [Google Scholar] [CrossRef]
- Rubio-Rivas, M.; Mora-Lujan, J.M.; Formiga, F.; Arevalo-Canas, C.; Lebron Ramos, J.M.; Villalba Garcia, M.V.; Fonseca Aizpuru, E.M.; Diez-Manglano, J.; Arnalich Fernandez, F.; Romero Cabrera, J.L.; et al. WHO Ordinal Scale and Inflammation Risk Categories in COVID-19. Comparative Study of the Severity Scales. J. Gen. Intern. Med. 2022, 37, 1980–1987. [Google Scholar] [CrossRef]
- Dezoteux, F.; Marcant, P.; Dendooven, A.; Delaunay, E.; Esnault, S.; Trauet, J.; Lefevre, G.; Staumont-Salle, D. Enhanced Siglec-8 and HLA-DR and Reduced CRTH2 Surface Expression, Highlight a Distinct Phenotypic Signature of Circulating Eosinophils in Atopic Dermatitis. J. Leukoc. Biol. 2025, 117, qiaf023. [Google Scholar] [CrossRef]
- Carmo, L.A.; Bonjour, K.; Ueki, S.; Neves, J.S.; Liu, L.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F.; Melo, R.C.N. CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils. J. Leukoc. Biol. 2016, 100, 391–401. [Google Scholar] [CrossRef]
- Zimmermann, N.; Hershey, G.K.; Foster, P.S.; Rothenberg, M.E. Chemokines in asthma: Cooperative interaction between chemokines and IL-13. J. Allergy Clin. Immunol. 2003, 111, 227–242. [Google Scholar]
- Stockfelt, M.; Christenson, K.; Andersson, A.; Bjorkman, L.; Padra, M.; Brundin, B.; Ganguly, K.; Asgeirsdottir, H.; Lindén, S.; Qvarfordt, I.; et al. Increased CD11b and Decreased CD62L in Blood and Airway Neutrophils from Long-Term Smokers with and without COPD. J. Innate Immun. 2020, 12, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.H.; Xu, L.; Rahimpour, R.; Mitchell, G.; DeVries, M.E.; Pickering, J.G.; Singhal, S.K.; Feldman, R.D.; Kelvin, D.J. CXCR1 and CXCR2 are rapidly down-modulated by bacterial endotoxin through a unique agonist-independent, tyrosine kinase-dependent mechanism. J. Immunol. 1998, 161, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Demaret, J.; Lefevre, G.; Vuotto, F.; Trauet, J.; Duhamel, A.; Labreuche, J.; Varlet, P.; Dendooven, A.; Stabler, S.; Gachet, B.; et al. Severe SARS-CoV-2 patients develop a higher specific T-cell response. Clin. Transl. Immunol. 2020, 9, e1217. [Google Scholar] [CrossRef]
- Zaid, Y.; Dore, E.; Dubuc, I.; Archambault, A.S.; Flamand, O.; Laviolette, M.; Flamand, N.; Boilard, E.; Flamand, L. Chemokines and eicosanoids fuel the hyperinflammation within the lungs of patients with severe COVID-19. J. Allergy Clin. Immunol. 2021, 148, 368–380.e3. [Google Scholar] [CrossRef]
- Gebremeskel, S.; Schanin, J.; Coyle, K.M.; Butuci, M.; Luu, T.; Brock, E.C.; Xu, A.; Wong, A.; Leung, J.; Korver, W.; et al. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021, 12, 650331. [Google Scholar] [CrossRef] [PubMed]
- Esnault, S.; Hebert, A.S.; Jarjour, N.N.; Coon, J.J.; Mosher, D.F. Proteomic and Phosphoproteomic Changes Induced by Prolonged Activation of Human Eosinophils with IL-3. J. Proteome Res. 2018, 17, 2102–2111. [Google Scholar] [CrossRef]
- Esnault, S.; Khosravi, M.; Kelly, E.A.; Liu, L.Y.; Bochkov, Y.A.; Tattersall, M.C.; Jarjour, N.N. Increased IL-6 and Potential IL-6 trans-signalling in the airways after an allergen challenge. Clin. Exp. Allergy 2021, 51, 564–573. [Google Scholar] [CrossRef]
- Kelly, E.A.; Esnault, S.; Liu, L.Y.; Evans, M.D.; Johansson, M.W.; Mathur, S.; Mosher, D.F.; Denlinger, L.C.; Jarjour, N.N. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype, in asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 1385–1395. [Google Scholar] [CrossRef]
- Alfaro, C.; Teijeira, A.; Onate, C.; Perez, G.; Sanmamed, M.F.; Andueza, M.P.; Alignani, D.; Labiano, S.; Azpilikueta, A.; Rodriguez-Paulete, A.; et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 3924–3936. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Meidaninikjeh, S.; Sabouni, N.; Marzouni, H.Z.; Bengar, S.; Khalili, A.; Jafari, R. Monocytes and macrophages in COVID-19: Friends and foes. Life Sci. 2021, 269, 119010. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Morales-Nebreda, L.; Markov, N.S.; Swaminathan, S.; Querrey, M.; Guzman, E.R.; Abbott, D.A.; Donnelly, H.K.; Donayre, A.; Goldberg, I.A.; et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 2021, 590, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, A.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef]
- Lee, A.J.; Chen, B.; Chew, M.V.; Barra, N.G.; Shenouda, M.M.; Nham, T.; van Rooijen, N.; Jordana, M.; Mossman, K.L.; Schreiber, R.D.; et al. Inflammatory monocytes require type I interferon receptor signaling to activate NK cells via IL-18 during a mucosal viral infection. J. Exp. Med. 2017, 214, 1153–1167. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Meizlish, M.L.; Pine, A.B.; Bishai, J.D.; Goshua, G.; Nadelmann, E.R.; Simonov, M.; Chang, C.H.; Zhang, H.; Shallow, M.; Bahel, P.; et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021, 5, 1164–1177. [Google Scholar] [CrossRef]
- Drake, M.G.; Bivins-Smith, E.R.; Proskocil, B.J.; Nie, Z.; Scott, G.D.; Lee, J.J.; Lee, N.A.; Fryer, A.D.; Jacoby, D.B. Human and Mouse Eosinophils Have Antiviral Activity against Parainfluenza Virus. Am. J. Respir. Cell Mol. Biol. 2016, 55, 387–394. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Rosenberg, H.F. Respiratory syncytial virus infection: Immune response, immunopathogenesis, and treatment. Clin. Microbiol. Rev. 1999, 12, 298–309. [Google Scholar] [CrossRef]
- Handzel, Z.T.; Busse, W.W.; Sedgwick, J.B.; Vrtis, R.; Lee, W.M.; Kelly, E.A.; Gern, J.E. Eosinophils bind rhinovirus and activate virus specific T cells. J. Immunol. 1998, 160, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli-Guimaraes, P.H.; Jones, S.M.; Voehringer, D.; Mayer-Barber, K.D.; Samarasinghe, A.E. Eosinophils as modulators of host defense during parasitic, fungal, bacterial, and viral infections. J. Leukoc. Biol. 2024, 116, 1301–1323. [Google Scholar] [CrossRef] [PubMed]
| Patients with SARS-CoV-2 Infection | Healthy Participants | ||
|---|---|---|---|
| Number of enrolled participants (n) | 40 | 18 | |
| Age; median (years) [Min–Max] | 63 [41–99] | 71 [53–89] | |
| Sex; Males/Females (n) [% Males] | 28/9 [75%] | 10/8 [55%] | |
| Deceased (n) | 5 | ||
| Severity status (WHO scale); median [Min–Max] | 5 [4–8] | ||
| Symptoms | Fever | 24 [60%] | |
| Cough | 24 [60%] | ||
| Dyspnea | 32 [80%] | ||
| Treatments | Antibiotics | 26 [65%] | |
| Antivirals | 12 [30%] | ||
| Corticosteroids | 30 [75%] | ||
| Participants | Eosinophils (Flow Cytometry) | Neutrophils (Flow Cytometry) | Monocytes (Flow Cytometry) | Mediators in Sera (Luminex) |
|---|---|---|---|---|
| Patients COVID-19 | ||||
| Very early phase (D1 to D3) | 13 (10 for antibody panel 2) | 37 | 36 | 37 |
| Early phase (D5 to D9) | 27 (23 for antibody panel 2) | 33 | 35 | 28 |
| Mid-phase (D14) | 13 | 10 | 14 | 11 |
| Late Phase (D30) | 10 | 10 | 11 | 16 |
| Healthy Particpants | 18 | 18 | 18 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dendooven, A.; Esnault, S.; Jacob, M.; Trauet, J.; Delaunay, E.; Guerrier, T.; Samarasinghe, A.E.; Mirgot, F.; Vuotto, F.; Faure, K.; et al. Evolution of Blood Innate Immune Cell Phenotypes Following SARS-CoV-2 Infection in Hospitalized Patients with COVID-19. Cells 2025, 14, 1093. https://doi.org/10.3390/cells14141093
Dendooven A, Esnault S, Jacob M, Trauet J, Delaunay E, Guerrier T, Samarasinghe AE, Mirgot F, Vuotto F, Faure K, et al. Evolution of Blood Innate Immune Cell Phenotypes Following SARS-CoV-2 Infection in Hospitalized Patients with COVID-19. Cells. 2025; 14(14):1093. https://doi.org/10.3390/cells14141093
Chicago/Turabian StyleDendooven, Arnaud, Stephane Esnault, Marie Jacob, Jacques Trauet, Emeline Delaunay, Thomas Guerrier, Amali E. Samarasinghe, Floriane Mirgot, Fanny Vuotto, Karine Faure, and et al. 2025. "Evolution of Blood Innate Immune Cell Phenotypes Following SARS-CoV-2 Infection in Hospitalized Patients with COVID-19" Cells 14, no. 14: 1093. https://doi.org/10.3390/cells14141093
APA StyleDendooven, A., Esnault, S., Jacob, M., Trauet, J., Delaunay, E., Guerrier, T., Samarasinghe, A. E., Mirgot, F., Vuotto, F., Faure, K., Poissy, J., Lambert, M., Labalette, M., Lefèvre, G., & Demaret, J. (2025). Evolution of Blood Innate Immune Cell Phenotypes Following SARS-CoV-2 Infection in Hospitalized Patients with COVID-19. Cells, 14(14), 1093. https://doi.org/10.3390/cells14141093









