Phosphodiesterase Type 5 Inhibitors in Male Reproduction: Molecular Mechanisms and Clinical Implications for Fertility Management
Abstract
1. Introduction
2. Mechanisms of Action of Phosphodiesterases in Cyclic Nucleotide Regulation
2.1. Regulatory Mechanisms of PDEs
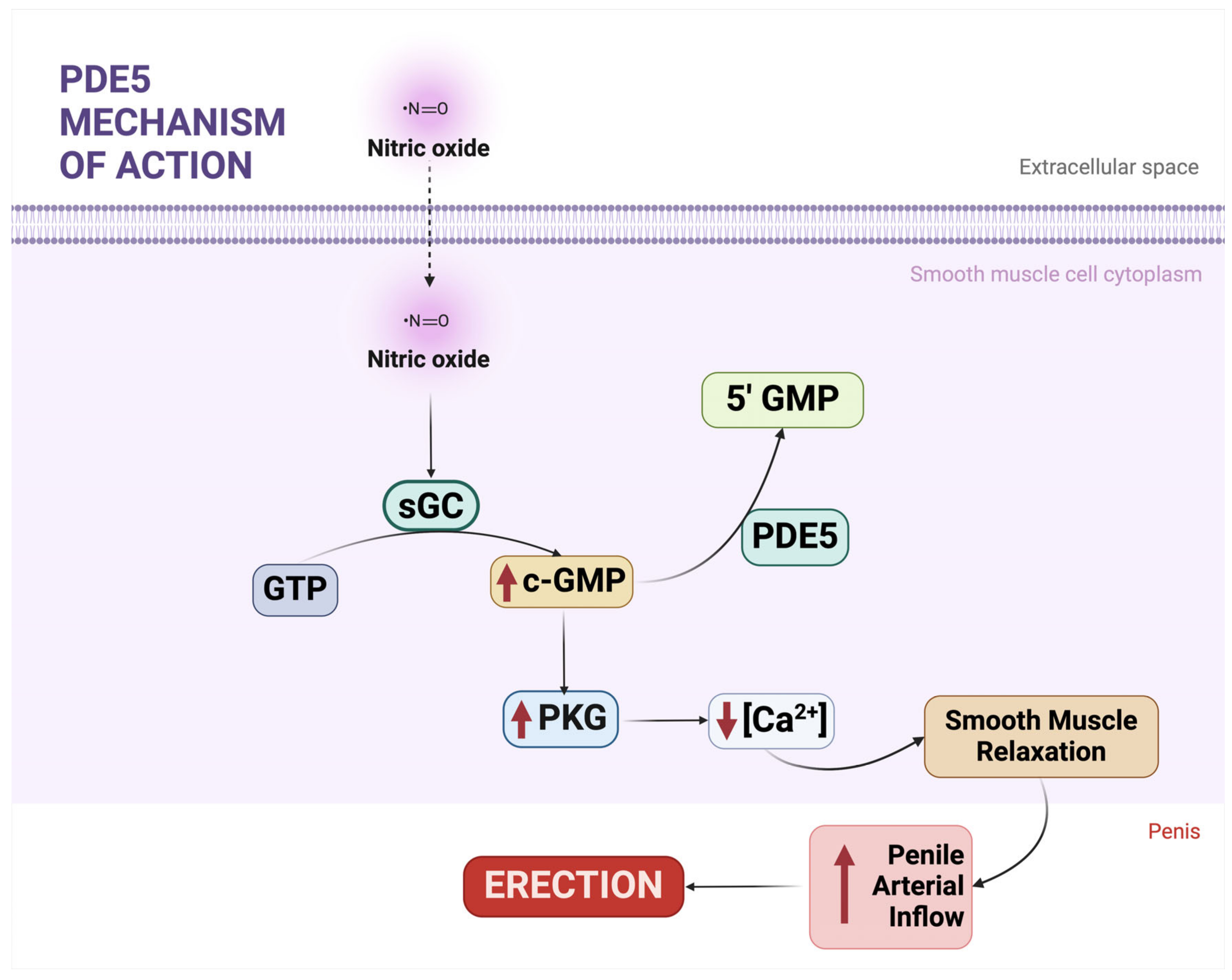
2.2. The NO-cGMP-PKG Pathway
2.3. PDE5 and Its Biological and Therapeutic Significance
2.4. Systemic Roles of PDE5
2.5. PDEs in Reproductive Tissues
3. Phosphodiesterases in the Male Reproductive System
3.1. Phosphodiesterases and cGMP Regulation in the Testis
3.1.1. PDEs in Myoid Cells and the Peritubular Lamina Propria
3.1.2. PDEs in Leydig Cells
3.1.3. PDEs in Sertoli Cells
3.1.4. PDEs in Germ Cells
3.2. Phosphodiesterase Inhibitors and cGMP Signaling in Male Accessory Genital Glands
3.2.1. The Epididymis
3.2.2. The Vas Deferens
3.2.3. The Seminal Vesicles
3.2.4. The Prostate
4. PDE5 Inhibitors and Semen Quality
4.1. Mechanisms of PDE5 Inhibitor Action Regarding Sperm Function
4.2. In Vivo Evidence
4.3. In Vitro Evidence
4.4. PDE5 Inhibition in Sperm Capacitation and Functional Assays
5. Limitations, Research Gaps, and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.C.; Scott, M.; Eisenberg, M.L. Male Fertility as a Proxy for Health. J. Clin. Med. 2024, 13, 5559. [Google Scholar] [CrossRef]
- Kaltsas, A.; Dimitriadis, F.; Zachariou, D.; Zikopoulos, A.; Symeonidis, E.N.; Markou, E.; Tien, D.M.B.; Takenaka, A.; Sofikitis, N.; Zachariou, A. From Diagnosis to Treatment: Comprehensive Care by Reproductive Urologists in Assisted Reproductive Technology. Medicina 2023, 59, 1835. [Google Scholar] [CrossRef] [PubMed]
- Paronetto, M.P.; Crescioli, C. Rethinking of phosphodiesterase 5 inhibition: The old, the new and the perspective in human health. Front. Endocrinol. 2024, 15, 1461642. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Kaltsas, A.; Zachariou, A.; Mamoulakis, C.; Tsiampali, C.; Giannakis, I.; Paschopoulos, M.; Papatsoris, A.; Loutradis, D.; Tsounapi, P.; et al. PDE5 inhibitors and male reproduction: Is there a place for PDE5 inhibitors in infertility clinics or andrology laboratories? Int. J. Urol. 2022, 29, 1405–1418. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Giannakis, D.; Pardalidis, N.; Zikopoulos, K.; Paraskevaidis, E.; Giotitsas, N.; Kalaboki, V.; Tsounapi, P.; Baltogiannis, D.; Georgiou, I.; et al. Effects of phosphodiesterase-5 inhibitors on sperm parameters and fertilizing capacity. Asian J. Androl. 2008, 10, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, T.; Alghobary, M.; Hanafy, N.S.; Abosief, A. Oral phosphodiesterase type 5 inhibitors and male reproductive potential: An overview. Sex. Med. Rev. 2023, 11, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Murata, T.; Shimizu, K.; Degerman, E.; Maurice, D.; Manganiello, V. Cyclic nucleotide phosphodiesterases: Important signaling modulators and therapeutic targets. Oral Dis. 2015, 21, e25–e50. [Google Scholar] [CrossRef]
- Lin, C.S.; Lin, G.; Xin, Z.C.; Lue, T.F. Expression, distribution and regulation of phosphodiesterase 5. Curr. Pharm. Des. 2006, 12, 3439–3457. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef]
- Lomas, O.; Zaccolo, M. Phosphodiesterases maintain signaling fidelity via compartmentalization of cyclic nucleotides. Physiology 2014, 29, 141–149. [Google Scholar] [CrossRef]
- Brown, K.M.; Lee, L.C.; Findlay, J.E.; Day, J.P.; Baillie, G.S. Cyclic AMP-specific phosphodiesterase, PDE8A1, is activated by protein kinase A-mediated phosphorylation. FEBS Lett. 2012, 586, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Houslay, M.D.; Baillie, G.S. The role of ERK2 docking and phosphorylation of PDE4 cAMP phosphodiesterase isoforms in mediating cross-talk between the cAMP and ERK signalling pathways. Biochem. Soc. Trans. 2003, 31, 1186–1190. [Google Scholar] [CrossRef]
- Jager, R.; Russwurm, C.; Schwede, F.; Genieser, H.G.; Koesling, D.; Russwurm, M. Activation of PDE10 and PDE11 phosphodiesterases. J. Biol. Chem. 2012, 287, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the ·NO/cGMP signaling pathway. Biochim. Biophys. Acta 1999, 1411, 334–350. [Google Scholar] [CrossRef]
- Murad, F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Zikopoulos, A.; Dimitriadis, F.; Sheshi, D.; Politis, M.; Moustakli, E.; Symeonidis, E.N.; Chrisofos, M.; Sofikitis, N.; Zachariou, A. Oxidative Stress and Erectile Dysfunction: Pathophysiology, Impacts, and Potential Treatments. Curr. Issues Mol. Biol. 2024, 46, 8807–8834. [Google Scholar] [CrossRef]
- Schlossmann, J.; Ammendola, A.; Ashman, K.; Zong, X.; Huber, A.; Neubauer, G.; Wang, G.X.; Allescher, H.D.; Korth, M.; Wilm, M.; et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature 2000, 404, 197–201. [Google Scholar] [CrossRef]
- Hofmann, F.; Ammendola, A.; Schlossmann, J. Rising behind NO: cGMP-dependent protein kinases. J. Cell Sci. 2000, 113 Pt 10, 1671–1676. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, Y.; Basang, W.; Wang, X.; Li, C.; Zhou, X. Roles of Nitric Oxide in the Regulation of Reproduction: A Review. Front. Endocrinol. 2021, 12, 752410. [Google Scholar] [CrossRef] [PubMed]
- Tzoumas, N.; Farrah, T.E.; Dhaun, N.; Webb, D.J. Established and emerging therapeutic uses of PDE type 5 inhibitors in cardiovascular disease. Br. J. Pharmacol. 2020, 177, 5467–5488. [Google Scholar] [CrossRef] [PubMed]
- Drobnis, E.Z.; Nangia, A.K. Phosphodiesterase Inhibitors (PDE Inhibitors) and Male Reproduction. Adv. Exp. Med. Biol. 2017, 1034, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Lue, T.F.; Padma-Nathan, H.; Rosen, R.C.; Steers, W.D.; Wicker, P.A. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N. Engl. J. Med. 1998, 338, 1397–1404. [Google Scholar] [CrossRef]
- Rondina, M.T.; Weyrich, A.S. Targeting phosphodiesterases in anti-platelet therapy. Handb. Exp. Pharmacol. 2012, 210, 225–238. [Google Scholar] [CrossRef]
- Coskuner, E.R.; Ozkan, B. Reno-protective effects of Phosphodiesterase 5 inhibitors. Clin. Exp. Nephrol. 2021, 25, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.; Brown, Z.; Burns, A.; Williams, T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst. Rev. 2019, 1, CD012621. [Google Scholar] [CrossRef]
- Kukreja, R.C.; Salloum, F.N.; Das, A. Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. J. Am. Coll. Cardiol. 2012, 59, 1921–1927. [Google Scholar] [CrossRef]
- Crocetto, F.; Cuomo, V.; Santoro, C.; Fiorillo, L.; Barone, B.; Arcaniolo, D.; Fedele, T.; Imbimbo, C.; Esposito, R. New Possibilities in Heart Failure: The Effects of Tadalafil on Diastolic Function in Patients Undergoing Robot-Assisted Radical Prostatectomy. Appl. Sci. 2022, 12, 5629. [Google Scholar] [CrossRef]
- Foresta, C.; Caretta, N.; Zuccarello, D.; Poletti, A.; Biagioli, A.; Caretti, L.; Galan, A. Expression of the PDE5 enzyme on human retinal tissue: New aspects of PDE5 inhibitors ocular side effects. Eye 2008, 22, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Teich, A.F.; Sakurai, M.; Patel, M.; Holman, C.; Saeed, F.; Fiorito, J.; Arancio, O. PDE5 Exists in Human Neurons and is a Viable Therapeutic Target for Neurologic Disease. J. Alzheimers Dis. 2016, 52, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sokanovic, S.J.; Capo, I.; Medar, M.M.; Andric, S.A.; Kostic, T.S. Long-term inhibition of PDE5 ameliorates aging-induced changes in rat testis. Exp. Gerontol. 2018, 108, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Fujishige, K.; Kotera, J.; Michibata, H.; Yuasa, K.; Takebayashi, S.; Okumura, K.; Omori, K. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J. Biol. Chem. 1999, 274, 18438–18445. [Google Scholar] [CrossRef] [PubMed]
- Scipioni, A.; Stefanini, S.; Santone, R.; Giorgi, M. Immunohistochemical localisation of PDE5 in Leydig and myoid cells of prepuberal and adult rat testis. Histochem. Cell Biol. 2005, 124, 401–407. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Tsounapi, P.; Saito, M.; Watanabe, T.; Sylakos, A.; Tsabalas, S.; Miyagawa, I.; Sofikitis, N. Is there a role for PDE5 inhibitors in the management of male infertility due to defects in testicular or epididymal function? Curr. Pharm. Des. 2009, 15, 3506–3520. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.; Filippi, S.; Marini, M.; Morelli, A.; Vignozzi, L.; Salonia, A.; Montorsi, F.; Mondaini, N.; Vannelli, G.B.; Donati, S.; et al. Expression and functional activity of phosphodiesterase type 5 in human and rabbit vas deferens. Mol. Hum. Reprod. 2005, 11, 107–115. [Google Scholar] [CrossRef][Green Version]
- ElHady, A.K.; El-Gamil, D.S.; Abdel-Halim, M.; Abadi, A.H. Advancements in Phosphodiesterase 5 Inhibitors: Unveiling Present and Future Perspectives. Pharmaceuticals 2023, 16, 1266. [Google Scholar] [CrossRef] [PubMed]
- Benau, D.; Szabo, E.I.; Terner, C. Endogenous inhibitors of cyclic adenosine 3′,5′-monophosphate-phosphodiesterase in rat epididymis. Biol. Reprod. 1986, 35, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Djoseland, O.; Gordeladze, J.O.; Hoglo, S.; Halse, J.I.; Haugen, H.N. Evidence for androgen-dependent phosphodiesterase activity in rat seminal vesicle and epididymis. Int. J. Androl. 1980, 3, 363–366. [Google Scholar] [CrossRef]
- Razzaboni, B.; Terner, C. Cyclic adenosine 3′,5′-monophosphate-phosphodiesterases in epididymis and prostate of castrate and of aged rats. Mech. Ageing Dev. 1988, 43, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sofikitis, N.; Kaltsas, A.; Dimitriadis, F.; Rassweiler, J.; Grivas, N.; Zachariou, A.; Kaponis, A.; Tsounapi, P.; Paterakis, N.; Karagiannis, A.; et al. The Effect of PDE5 Inhibitors on the Male Reproductive Tract. Curr. Pharm. Des. 2021, 27, 2697–2713. [Google Scholar] [CrossRef]
- Davidoff, M.S.; Breucker, H.; Holstein, A.F.; Seidl, K. Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res. 1990, 262, 253–261. [Google Scholar] [CrossRef]
- Zhang, C.; Yeh, S.; Chen, Y.T.; Wu, C.C.; Chuang, K.H.; Lin, H.Y.; Wang, R.S.; Chang, Y.J.; Mendis-Handagama, C.; Hu, L.; et al. Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc. Natl. Acad. Sci. USA 2006, 103, 17718–17723. [Google Scholar] [CrossRef]
- Lefievre, L.; de Lamirande, E.; Gagnon, C. Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol. Reprod. 2002, 67, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Mewe, M.; Bauer, C.K.; Muller, D.; Middendorff, R. Regulation of spontaneous contractile activity in the bovine epididymal duct by cyclic guanosine 5′-monophosphate-dependent pathways. Endocrinology 2006, 147, 2051–2062. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Begum, N.; Shen, W.; Manganiello, V. Role of PDE3A in regulation of cell cycle progression in mouse vascular smooth muscle cells and oocytes: Implications in cardiovascular diseases and infertility. Curr. Opin. Pharmacol. 2011, 11, 725–729. [Google Scholar] [CrossRef]
- Wayman, C.; Phillips, S.; Lunny, C.; Webb, T.; Fawcett, L.; Baxendale, R.; Burgess, G. Phosphodiesterase 11 (PDE11) regulation of spermatozoa physiology. Int. J. Impot. Res. 2005, 17, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Faja, F.; Finocchi, F.; Carlini, T.; Rizzo, F.; Pallotti, F.; Spaziani, M.; Balercia, G.; Lenzi, A.; Paoli, D.; Lombardo, F. PDE11A gene polymorphism in testicular cancer: Sperm parameters and hormonal profile. J. Endocrinol. Investig. 2021, 44, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, J.V.; Joseph, D.R.; Conti, M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: Evidence for a family of genes. Proc. Natl. Acad. Sci. USA 1989, 86, 5325–5329. [Google Scholar] [CrossRef] [PubMed]
- Geremia, R.; Rossi, P.; Pezzotti, R.; Conti, M. Cyclic nucleotide phosphodiesterase in developing rat testis. Identification of somatic and germ-cell forms. Mol. Cell. Endocrinol. 1982, 28, 37–53. [Google Scholar] [CrossRef]
- Morena, A.R.; Boitani, C.; de Grossi, S.; Stefanini, M.; Conti, M. Stage and cell-specific expression of the adenosine 3′,5′ monophosphate-phosphodiesterase genes in the rat seminiferous epithelium. Endocrinology 1995, 136, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Coskran, T.M.; Morton, D.; Menniti, F.S.; Adamowicz, W.O.; Kleiman, R.J.; Ryan, A.M.; Strick, C.A.; Schmidt, C.J.; Stephenson, D.T. Immunohistochemical localization of phosphodiesterase 10A in multiple mammalian species. J. Histochem. Cytochem. 2006, 54, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; McCarthy, S.A.; Chapin, D.S.; Fujiwara, R.A.; James, L.C.; Williams, R.D.; Stock, J.L.; McNeish, J.D.; Strick, C.A.; Menniti, F.S.; et al. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: Evidence for altered striatal function. Neuropharmacology 2006, 51, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H. Phosphodiesterase 11 (PDE11): Is it a player in human testicular function? Int. J. Impot. Res. 2005, 17, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Middendorff, R.; Muller, D.; Mewe, M.; Mukhopadhyay, A.K.; Holstein, A.F.; Davidoff, M.S. The tunica albuginea of the human testis is characterized by complex contraction and relaxation activities regulated by cyclic GMP. J. Clin. Endocrinol. Metab. 2002, 87, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Mukhopadhyay, A.K.; Speth, R.C.; Guidone, G.; Potthast, R.; Potter, L.R.; Middendorff, R. Spatiotemporal regulation of the two atrial natriuretic peptide receptors in testis. Endocrinology 2004, 145, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, K.; Omori, K.; Yanaka, N. Binding and phosphorylation of a novel male germ cell-specific cGMP-dependent protein kinase-anchoring protein by cGMP-dependent protein kinase Ialpha. J. Biol. Chem. 2000, 275, 4897–4905. [Google Scholar] [CrossRef] [PubMed]
- Silberbach, M.; Roberts, C.T., Jr. Natriuretic peptide signalling: Molecular and cellular pathways to growth regulation. Cell Signal 2001, 13, 221–231. [Google Scholar] [CrossRef]
- Cameron, V.A.; Ellmers, L.J. Minireview: Natriuretic peptides during development of the fetal heart and circulation. Endocrinology 2003, 144, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Middendorff, R.; Muller, D.; Paust, H.J.; Davidoff, M.S.; Mukhopadhyay, A.K. Natriuretic peptides in the human testis: Evidence for a potential role of C-type natriuretic peptide in Leydig cells. J. Clin. Endocrinol. Metab. 1996, 81, 4324–4328. [Google Scholar] [CrossRef]
- Middendorff, R.; Kumm, M.; Davidoff, M.S.; Holstein, A.F.; Muller, D. Generation of cyclic guanosine monophosphate by heme oxygenases in the human testis—A regulatory role for carbon monoxide in Sertoli cells? Biol. Reprod. 2000, 63, 651–657. [Google Scholar] [CrossRef]
- Middendorff, R.; Muller, D.; Wichers, S.; Holstein, A.F.; Davidoff, M.S. Evidence for production and functional activity of nitric oxide in seminiferous tubules and blood vessels of the human testis. J. Clin. Endocrinol. Metab. 1997, 82, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Ojatula, A.O.; Nwanja, N. Effects of a Conventional Aphrodisiac: A Twenty Eight Day Repeated Administration of Sildenafil Citrate Graded Doses on Experimental Rats. Dutse J. Pure Appl. Sci. 2022, 8, 115–123. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Tsiriopoulos, I.; Baltogiannis, D.; Tsounapi, P.; Gratsias, S.; Vlachopoulou, E.; Tsambalas, S.; Saito, M.; Watanabe, T.; Giannakis, D.; et al. Effects of phosphodiesterase-5 inhibitors on Leydig cell secretory function. 24th annual EUA Congress. March 17th–21st 2009, Stockholm. Eur. Urol. Suppl. 2009, 8, 215. [Google Scholar] [CrossRef]
- Saez, J.M. Leydig cells: Endocrine, paracrine, and autocrine regulation. Endocr. Rev. 1994, 15, 574–626. [Google Scholar] [CrossRef]
- Stocco, D.M.; Wang, X.; Jo, Y.; Manna, P.R. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol. Endocrinol. 2005, 19, 2647–2659. [Google Scholar] [CrossRef] [PubMed]
- Catt, K.J.; Dufau, M.L. Spare gonadotrophin receptors in rat testis. Nat. New Biol. 1973, 244, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, C.; Dufau, M.; Catt, K. Gonadotropin binding and stimulation of cyclic adenosine 3′:5′-monophosphate and testosterone production in isolated Leydig cells. J. Biol. Chem. 1975, 250, 8818–8823. [Google Scholar] [CrossRef] [PubMed]
- Vasta, V.; Shimizu-Albergine, M.; Beavo, J.A. Modulation of Leydig cell function by cyclic nucleotide phosphodiesterase 8A. Proc. Natl. Acad. Sci. USA 2006, 103, 19925–19930. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Smith, J.F.; Pillar, J.S.; St Denis, S.H.; Cheng, J.B. Isolation and characterization of PDE8A, a novel human cAMP-specific phosphodiesterase. Biochem. Biophys. Res. Commun. 1998, 246, 570–577. [Google Scholar] [CrossRef]
- Soderling, S.H.; Bayuga, S.J.; Beavo, J.A. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc. Natl. Acad. Sci. USA 1998, 95, 8991–8996. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, R.W.; Fraser, L.R. Mammalian sperm phosphodiesterases and their involvement in receptor-mediated cell signaling important for capacitation. Mol. Reprod. Dev. 2005, 71, 495–508. [Google Scholar] [CrossRef]
- Glavas, N.A.; Ostenson, C.; Schaefer, J.B.; Vasta, V.; Beavo, J.A. T cell activation up-regulates cyclic nucleotide phosphodiesterases 8A1 and 7A3. Proc. Natl. Acad. Sci. USA 2001, 98, 6319–6324. [Google Scholar] [CrossRef] [PubMed]
- Janjic, M.M.; Stojkov, N.J.; Bjelic, M.M.; Mihajlovic, A.I.; Andric, S.A.; Kostic, T.S. Transient rise of serum testosterone level after single sildenafil treatment of adult male rats. J. Sex. Med. 2012, 9, 2534–2543. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Baltogiannis, D.; Koukos, S.; Giannakis, D.; Tsounapi, P.; Seminis, G.; Saito, M.; Takenaka, A.; Sofikitis, N. The Role of PDE5 Inhibitors in the Treatment of Testicular Dysfunction. Male Infertil. 2012, 1, 77–106. [Google Scholar]
- Vlachopoulou, E.; Tsiriopoulos, I.; Dimitriadis, F.; Baltogiannis, D.; Miyagawa, I.; Sofikitis, N. Effects of vardenafil on Sertoli cell secretory function. 24th annual EUA Congress. March 17th–21st 2009, Stockholm. Eur. Urol. Suppl. 2009, 8, 148. [Google Scholar] [CrossRef]
- Zhao, A.Z.; Yan, C.; Sonnenburg, W.K.; Beavo, J.A. Recent advances in the study of Ca2+/CaM-activated phosphodiesterases: Expression and physiological functions. Adv. Second. Messenger Phosphoprot. Res. 1997, 31, 237–251. [Google Scholar]
- Zhang, X.; Yan, G.; Ji, J.; Wu, J.; Sun, X.; Shen, J.; Jiang, H.; Wang, H. PDE5 inhibitor promotes melanin synthesis through the PKG pathway in B16 melanoma cells. J. Cell Biochem. 2012, 113, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, R.; Burslem, F.; Phillips, S. Phosphodiesterase type 11 (PDE11) cellular localisation: Progress towards defining a physiological role in testis and/or reproduction. J. Urol. 2001, 165 (Suppl. S5), 340. [Google Scholar]
- Yan, C.; Zhao, A.Z.; Sonnenburg, W.K.; Beavo, J.A. Stage and cell-specific expression of calmodulin-dependent phosphodiesterases in mouse testis. Biol. Reprod. 2001, 64, 1746–1754. [Google Scholar] [CrossRef][Green Version]
- Salanova, M.; Chun, S.Y.; Iona, S.; Puri, C.; Stefanini, M.; Conti, M. Type 4 cyclic adenosine monophosphate-specific phosphodiesterases are expressed in discrete subcellular compartments during rat spermiogenesis. Endocrinology 1999, 140, 2297–2306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farooqui, S.M.; Al-Bagdadi, F.; Houslay, M.D.; Bolger, G.B.; Stout, R.; Specian, R.D.; Cherry, J.A.; Conti, M.; O’Donnell, J.M. Surgically induced cryptorchidism-related degenerative changes in spermatogonia are associated with loss of cyclic adenosine monophosphate-dependent phosphodiesterases type 4 in abdominal testes of rats. Biol. Reprod. 2001, 64, 1583–1589. [Google Scholar] [CrossRef]
- Reinhardt, R.R.; Chin, E.; Zhou, J.; Taira, M.; Murata, T.; Manganiello, V.C.; Bondy, C.A. Distinctive anatomical patterns of gene expression for cGMP-inhibited cyclic nucleotide phosphodiesterases. J. Clin. Investig. 1995, 95, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Kayik, G.; Tuzun, N.S.; Durdagi, S. Investigation of PDE5/PDE6 and PDE5/PDE11 selective potent tadalafil-like PDE5 inhibitors using combination of molecular modeling approaches, molecular fingerprint-based virtual screening protocols and structure-based pharmacophore development. J. Enzyme Inhib. Med. Chem. 2017, 32, 311–330. [Google Scholar] [CrossRef]
- Ricker, D.D. The autonomic innervation of the epididymis: Its effects on epididymal function and fertility. J. Androl. 1998, 19, 1–4. [Google Scholar] [CrossRef]
- Mewe, M.; Bauer, C.K.; Schwarz, J.R.; Middendorff, R. Mechanisms regulating spontaneous contractions in the bovine epididymal duct. Biol. Reprod. 2006, 75, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Mulryan, K.; Gitterman, D.P.; Lewis, C.J.; Vial, C.; Leckie, B.J.; Cobb, A.L.; Brown, J.E.; Conley, E.C.; Buell, G.; Pritchard, C.A.; et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 2000, 403, 86–89. [Google Scholar] [CrossRef]
- Elbashir, S.; Magdi, Y.; Rashed, A.; Henkel, R.; Agarwal, A. Epididymal contribution to male infertility: An overlooked problem. Andrologia 2021, 53, e13721. [Google Scholar] [CrossRef]
- Elfgen, V.; Mietens, A.; Mewe, M.; Hau, T.; Middendorff, R. Contractility of the epididymal duct: Function, regulation and potential drug effects. Reproduction 2018, 156, R125–R141. [Google Scholar] [CrossRef] [PubMed]
- Mietens, A.; Eichner, G.; Tasch, S.; Feuerstacke, C.; Schneider-Hüther, I.; Müller, D.; Middendorff, R. Video microscopy as a tool to visualize cGMP effects on contractility and sperm transport in seminiferous tubules and the epididymal duct. BMC Pharmacol. Toxicol. 2013, 14, P44. [Google Scholar] [CrossRef]
- Lucas, K.A.; Pitari, G.M.; Kazerounian, S.; Ruiz-Stewart, I.; Park, J.; Schulz, S.; Chepenik, K.P.; Waldman, S.A. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 2000, 52, 375–414. [Google Scholar] [CrossRef]
- Muller, D.; Mukhopadhyay, A.K.; Davidoff, M.S.; Middendorff, R. Cyclic GMP signaling in rat urinary bladder, prostate, and epididymis: Tissue-specific changes with aging and in response to Leydig cell depletion. Reproduction 2011, 142, 333–343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mietens, A.; Tasch, S.; Feuerstacke, C.; Eichner, G.; Volkmann, J.; Schermuly, R.T.; Grimminger, F.; Muller, D.; Middendorff, R. Phosphodiesterase 5 (PDE5) inhibition, ANP and NO rapidly reduce epididymal duct contractions, but long-term PDE5 inhibition in vivo does not. Mol. Cell. Endocrinol. 2012, 349, 145–153. [Google Scholar] [CrossRef]
- Alp, H.; Cirit, U.; Tas, M.; Rifaioglu, M.M.; Hatipoglu, N.K.; Aytekin, I.; Yucel, M.; Firat, U.; Ozmen, M.F.; Seker, U.; et al. Effects of Sildenafil Citrate, Isoniazid, and Streptomycin on Testicular Tissue and Epididymal Semen Quality in Rats. Urology 2012, 80, 953.E9–953.E14. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, I.A. Phosphodiesterase 5 inhibitors in rapid ejaculation: Potential use and possible mechanisms of action. Drugs 2004, 64, 13–26. [Google Scholar] [CrossRef]
- Puscasu, C.; Zanfirescu, A.; Negres, S.; Seremet, O.C. Exploring the Multifaceted Potential of Sildenafil in Medicine. Medicina 2023, 59, 2190. [Google Scholar] [CrossRef]
- Chen, J.; Mabjeesh, N.J.; Matzkin, H.; Greenstein, A. Efficacy of sildenafil as adjuvant therapy to selective serotonin reuptake inhibitor in alleviating premature ejaculation. Urology 2003, 61, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Sundkvist, E.; Jaeger, R.; Sager, G. Pharmacological characterization of the ATP-dependent low K(m) guanosine 3′,5′-cyclic monophosphate (cGMP) transporter in human erythrocytes. Biochem. Pharmacol. 2002, 63, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Sager, G.; Orbo, A.; Pettersen, R.H.; Kjorstad, K.E. Export of guanosine 3′,5′-cyclic monophosphate (cGMP) from human erythrocytes characterized by inside-out membrane vesicles. Scand. J. Clin. Lab. Investig. 1996, 56, 289–293. [Google Scholar] [CrossRef]
- Bilge, S.S.; Kesim, Y.; Kurt, M.; Aksoz, E.; Celik, S. Possible role of sildenafil in inhibiting rat vas deferens contractions by influencing the purinergic system. Int. J. Urol. 2005, 12, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Medina, P.; Segarra, G.; Torondel, B.; Chuan, P.; Domenech, C.; Vila, J.M.; Lluch, S. Inhibition of neuroeffector transmission in human vas deferens by sildenafil. Br. J. Pharmacol. 2000, 131, 871–874. [Google Scholar] [CrossRef]
- Rosevear, H.M.; Krishnamachari, Y.; Ariza, C.A.; Mallapragada, S.K.; Salem, A.K.; Griffith, T.S.; De Young, B.R.; Wald, M. Effect of combined locally delivered growth factors and systemic sildenafil citrate on microrecanalization in biodegradable conduit for vas deferens reconstruction. Urology 2012, 79, 967.e1–967.e4. [Google Scholar] [CrossRef]
- Holoch, P.A.; Mallapragada, S.K.; Ariza, C.A.; Griffith, T.S.; Deyoung, B.R.; Wald, M. Micro-recanalization in a biodegradable graft for reconstruction of the vas deferens is enhanced by sildenafil citrate. Asian J. Androl. 2010, 12, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Gur, S.; Sikka, S.C.; Knight, G.E.; Burnstock, G.; Hellstrom, W.J. Purinergic contraction of the rat vas deferens in L-NAME-induced hypertension: Effect of sildenafil. Asian J. Androl. 2010, 12, 415–421. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.; Lotti, F.; Favilla, V.; Morgia, G.; Maggi, M.; Calogero, A.E. Seminal vesicles and diabetic neuropathy: Ultrasound evaluation after prolonged treatment with a selective phosphodiesterase-5 inhibitor. Andrology 2013, 1, 245–250. [Google Scholar] [CrossRef]
- La Vignera, S. Seminal vesicles of infertile patients with male accessory gland infection: Ultrasound evaluation after prolonged treatment with tadalafil, a selective phosphodiesterase-5 inhibitor. Andrologia 2013, 45, 386–391. [Google Scholar] [CrossRef]
- Birowo, P.; Uckert, S.; Kedia, G.T.; Sonnenberg, J.E.; Sandner, P.; Thon, W.F.; Scheller, F.; Rahardjo, D.; Kuczyk, M.A. Exposure of human seminal vesicle tissue to phosphodiesterase (PDE) inhibitors antagonizes the contraction induced by norepinephrine and increases production of cyclic nucleotides. Urology 2010, 76, 1518.e1–1518.e6. [Google Scholar] [CrossRef] [PubMed]
- Meares, E.M., Jr. Prostatitis. Med. Clin. N. Am. 1991, 75, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tang, R.; Shapiro, E.; Burnett, A.L.; Lepor, H. Effects of nitric oxide on human and canine prostates. Urology 1995, 45, 440–446. [Google Scholar] [CrossRef]
- Uckert, S.; Kuthe, A.; Jonas, U.; Stief, C.G. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J. Urol. 2001, 166, 2484–2490. [Google Scholar] [CrossRef]
- Grimsley, S.J.; Khan, M.H.; Jones, G.E. Mechanism of Phosphodiesterase 5 inhibitor relief of prostatitis symptoms. Med. Hypotheses 2007, 69, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, R.; Kawai, Y.; Oka, M.; Fuchikami, C.; Oyama, T. Effect of a single treatment with tadalafil on blood flow in lower urinary tract tissues in rat models of bladder overdistension/emptying and abdominal aorta clamping/release. Eur. J. Pharmacol. 2015, 754, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Mitchell, C.; Mayton, E.; Hoke, N.N.; Salloum, F.N.; Park, M.A.; Qureshi, I.; Lee, R.; Dent, P.; et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 18202–18207. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.O.; Carvalho Mda, C.; Saraiva, K.L.; Ribeiro, E.L.; AK, E.S.; Donato, M.A.; Rocha, S.W.; Santos e Silva, B.; Peixoto, C.A. Effect of chronic Sildenafil treatment on the prostate of C57Bl/6 mice. Tissue Cell 2014, 46, 439–449. [Google Scholar] [CrossRef]
- Morelli, G.; Pagni, R.; Mariani, C.; Minervini, R.; Morelli, A.; Gori, F.; Ferdeghini, E.M.; Paterni, M.; Mauro, E.; Guidi, E.; et al. Results of vardenafil mediated power Doppler ultrasound, contrast enhanced ultrasound and systematic random biopsies to detect prostate cancer. J. Urol. 2011, 185, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Kim, S.H.; Lee, S.W.; Jeon, J.H.; Kang, K.K.; Choi, S.B.; Park, J.K. Activity of phosphodiesterase type 5 inhibitors in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. BJU Int. 2011, 107, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Zenzmaier, C.; Sampson, N.; Pernkopf, D.; Plas, E.; Untergasser, G.; Berger, P. Attenuated proliferation and trans-differentiation of prostatic stromal cells indicate suitability of phosphodiesterase type 5 inhibitors for prevention and treatment of benign prostatic hyperplasia. Endocrinology 2010, 151, 3975–3984. [Google Scholar] [CrossRef][Green Version]
- Lee, J.G.; Moon, D.G.; Kang, S.H.; Cho, D.Y.; Park, H.S.; Bae, J.H. Relaxation effect of phosphodiesterase-5 inhibitor on the animal bladder and prostatic urethra: In vitro and in vivo study. Urol. Int. 2010, 84, 231–235. [Google Scholar] [CrossRef]
- Fibbi, B.; Morelli, A.; Vignozzi, L.; Filippi, S.; Chavalmane, A.; De Vita, G.; Marini, M.; Gacci, M.; Vannelli, G.B.; Sandner, P.; et al. Characterization of phosphodiesterase type 5 expression and functional activity in the human male lower urinary tract. J. Sex. Med. 2010, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Oger, S.; Behr-Roussel, D.; Gorny, D.; Lebret, T.; Denoux, Y.; Alexandre, L.; Giuliano, F. Combination of alfuzosin and tadalafil exerts an additive relaxant effect on human detrusor and prostatic tissues in vitro. Eur. Urol. 2010, 57, 699–707. [Google Scholar] [CrossRef]
- Tsounapi, P.; Honda, M.; Dimitriadis, F.; Koukos, S.; Hikita, K.; Zachariou, A.; Sofikitis, N.; Takenaka, A. Effects of a micronutrient supplementation combined with a phosphodiesterase type 5 inhibitor on sperm quantitative and qualitative parameters, percentage of mature spermatozoa and sperm capacity to undergo hyperactivation: A randomised controlled trial. Andrologia 2018, 50, e13071. [Google Scholar] [CrossRef]
- Yang, Y.T.; Yan, B.; Li, Y.H.; Guo, L.N.; Wang, W.W.; Liu, L.J.; Yu, H.G.; Diao, H. Phosphodiesterase 10A inhibitor PF-2545920 as a prospective agent for the clinical promotion of sperm motility. Asian J. Androl. 2023, 25, 608–615. [Google Scholar] [CrossRef]
- Mostafa, T. Oral phosphodiesterase-5 inhibitors and sperm functions. Int. J. Impot. Res. 2008, 20, 530–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nasimi Doost Azgomi, R.; Nazemiyeh, H.; Sadeghi Bazargani, H.; Fazljou, S.M.B.; Nejatbakhsh, F.; Moini Jazani, A.; Ahmadi AsrBadr, Y.; Zomorrodi, A. Comparative evaluation of the effects of Withania somnifera with pentoxifylline on the sperm parameters in idiopathic male infertility: A triple-blind randomised clinical trial. Andrologia 2018, 50, e13041. [Google Scholar] [CrossRef]
- Mahaldashtian, M.; Khalili, M.A.; Nottola, S.A.; Woodward, B.; Macchiarelli, G.; Miglietta, S. Does in vitro application of pentoxifylline have beneficial effects in assisted male reproduction? Andrologia 2021, 53, e13722. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.C.; Diniz, S.A.; Viegas, R.N.; Cortes, S.F.; Costa, E.D.; Freitas, M.M.; Martins-Filho, O.A.; Araujo, M.S.S.; Lana, A.M.Q.; Wenceslau, R.R.; et al. Addition of caffeine to equine thawed sperm increases motility and decreases nitrite concentration. Andrologia 2021, 53, e13918. [Google Scholar] [CrossRef]
- Gala, B.; Badge, A.; Bawaskar, P.; Gajbe, U.; Singh, B.R.; Kohale, M. The Potential of Theophylline and Pentoxifylline in Sperm Optimization and Its Intracytoplasmic Sperm Injection Outcomes. Cureus 2023, 15, e48192. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, I.; Zareba, K.; Wolczynski, S.; Czerniecki, J. Endocrine-disrupting chemicals-Mechanisms of action on male reproductive system. Toxicol. Ind. Health 2017, 33, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Schill, W.B. Caffeine- and kallikrein-induced stimulation of human sperm motility: A comparative study. Andrologia 1975, 7, 229–236. [Google Scholar] [CrossRef] [PubMed]
- De Turner, E.; Aparicio, N.J.; Turner, D.; Schwarzstein, L. Effect of two phosphodiesterase inhibitors, cyclic adenosine 3′:5′-monophosphate, and a beta-blocking agent on human sperm motility. Fertil. Steril. 1978, 29, 328–331. [Google Scholar] [CrossRef]
- Haesungcharern, A.; Chulavatnatol, M. Stimulation of human spermatozoal motility by caffeine. Fertil. Steril. 1973, 24, 662–665. [Google Scholar] [CrossRef]
- Purvis, K.; Muirhead, G.J.; Harness, J.A. The effects of sildenafil on human sperm function in healthy volunteers. Br. J. Clin. Pharmacol. 2002, 53 (Suppl. S1), 53S–60S. [Google Scholar] [CrossRef]
- Aversa, A.; Mazzilli, F.; Rossi, T.; Delfino, M.; Isidori, A.M.; Fabbri, A. Effects of sildenafil (Viagra) administration on seminal parameters and post-ejaculatory refractory time in normal males. Hum. Reprod. 2000, 15, 131–134. [Google Scholar] [CrossRef]
- du Plessis, S.S.; de Jongh, P.S.; Franken, D.R. Effect of acute in vivo sildenafil citrate and in vitro 8-bromo-cGMP treatments on semen parameters and sperm function. Fertil. Steril. 2004, 81, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Lombardo, F.; Salacone, P.; Gandini, L.; Lenzi, A. Treatment of sexual dysfunctions secondary to male infertility with sildenafil citrate. Fertil. Steril. 2004, 81, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Lombardo, F.; Salacone, P.; Gandini, L.; Jannini, E.A. Stress, sexual dysfunctions, and male infertility. J. Endocrinol. Investig. 2003, 26, 72–76. [Google Scholar]
- Pomara, G.; Morelli, G.; Canale, D.; Turchi, P.; Caglieresi, C.; Moschini, C.; Liguori, G.; Selli, C.; Macchia, E.; Martino, E.; et al. Alterations in sperm motility after acute oral administration of sildenafil or tadalafil in young, infertile men. Fertil. Steril. 2007, 88, 860–865. [Google Scholar] [CrossRef]
- Hellstrom, W.J.; Overstreet, J.W.; Yu, A.; Saikali, K.; Shen, W.; Beasley, C.M., Jr.; Watkins, V.S. Tadalafil has no detrimental effect on human spermatogenesis or reproductive hormones. J. Urol. 2003, 170, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, K.; Dula, E.; Drehobl, M.; Pryor, J.; Shapiro, J.; Seger, M. Daily vardenafil for 6 months has no detrimental effects on semen characteristics or reproductive hormones in men with normal baseline levels. J. Urol. 2008, 179, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.T.; Rakkah, N.I. Neurophysiological role of sildenafil citrate (Viagra) on seminal parameters in diabetic males with and without neuropathy. Pak. J. Pharm. Sci. 2007, 20, 36–42. [Google Scholar]
- Dimitriadis, F.; Tsambalas, S.; Tsounapi, P.; Kawamura, H.; Vlachopoulou, E.; Haliasos, N.; Gratsias, S.; Watanabe, T.; Saito, M.; Miyagawa, I.; et al. Effects of phosphodiesterase-5 inhibitors on Leydig cell secretory function in oligoasthenospermic infertile men: A randomized trial. BJU Int. 2010, 106, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Rago, R.; Salacone, P.; Caponecchia, L.; Marcucci, I.; Fiori, C.; Sebastianelli, A. Effect of vardenafil on semen parameters in infertile men: A pilot study evaluating short-term treatment. J. Endocrinol. Investig. 2012, 35, 897–900. [Google Scholar] [CrossRef]
- Woolf, B.; Rajasundaram, S.; Cronje, H.T.; Yarmolinsky, J.; Burgess, S.; Gill, D. A drug target for erectile dysfunction to help improve fertility, sexual activity, and wellbeing: Mendelian randomisation study. BMJ 2023, 383, e076197. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Liu, L.; Wei, S.; Tang, Z.; Yang, L.; Wei, Q. The Effect of Oral Phosphodiesterase-5 Inhibitors on Sperm Parameters: A Meta-analysis and Systematic Review. Urology 2017, 105, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, X.; Yan, X.; Shen, Y.; Li, Y.; Yu, X. Effect of Phosphodiesterase-5 Inhibitors on the Treatment of Male Infertility: A Systematic Review and Meta-Analysis. World J. Mens. Health 2021, 39, 776–796. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, D.S.; Shim, S.H.; Lim, J.J.; Yang, S.C. Usage and perceptions of phosphodiesterase type 5 inhibitors among the male partners of infertile couples. Clin. Exp. Reprod. Med. 2016, 43, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Gokce, A.; Halis, F.; Demirtas, A.; Ekmekcioglu, O. The effects of three phosphodiesterase type 5 inhibitors on ejaculation latency time in lifelong premature ejaculators: A double-blind laboratory setting study. BJU Int. 2011, 107, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, W.J.; Gittelman, M.; Jarow, J.; Steidle, C.; McMurray, J.; Talley, D.; Watts, S.; Mitchell, C.L.; McGill, J.M. An evaluation of semen characteristics in men 45 years of age or older after daily dosing with tadalafil 20mg: Results of a multicenter, randomized, double-blind, placebo-controlled, 9-month study. Eur. Urol. 2008, 53, 1058–1065. [Google Scholar] [CrossRef]
- Khalaf, M.A.; Abbas, M.F.; El-Fakahany, H.M. Effects of chronic tadalafil use on the testes and sperm parameters of old albino rats. Andrologia 2012, 44 (Suppl. S1), 370–375. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Badr El Dine, F.M.; Nabil, I.M. Histopathologic and Ultrastructural Changes in Seminiferous Tubules of Adult Male Albino Rats Following Daily Administration of Different Doses of Tadalafil. Urology 2016, 90, 89–96. [Google Scholar] [CrossRef]
- Wang, L.; Chopp, M.; Szalad, A.; Liu, Z.; Bolz, M.; Alvarez, F.M.; Lu, M.; Zhang, L.; Cui, Y.; Zhang, R.L.; et al. Phosphodiesterase-5 is a therapeutic target for peripheral neuropathy in diabetic mice. Neuroscience 2011, 193, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, Y.; Hu, J.; Li, X.; Zhang, H.; You, L.; Chen, Z.J. Combined use of phosphodiesterase-5 inhibitors and selective serotonin reuptake inhibitors for temporary ejaculation failure in couple undergoing assisted reproductive technologies. Fertil. Steril. 2009, 91, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.R.; Traboulsi, A.; Hussain, A.; Dubin, N.H. In vitro effects of sildenafil and phentolamine, drugs used for erectile dysfunction, on human sperm motility. Am. J. Obstet. Gynecol. 2000, 182, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Vacquier, V.D. Cyclic GMP-specific phosphodiesterase-5 regulates motility of sea urchin spermatozoa. Mol. Biol. Cell 2006, 17, 114–121. [Google Scholar] [CrossRef][Green Version]
- Burger, M.; Sikka, S.C.; Bivalacqua, T.J.; Lamb, D.J.; Hellstrom, W.J. The effect of sildenafil on human sperm motion and function from normal and infertile men. Int. J. Impot. Res. 2000, 12, 229–234. [Google Scholar] [CrossRef]
- Lefievre, L.; De Lamirande, E.; Gagnon, C. The cyclic GMP-specific phosphodiesterase inhibitor, sildenafil, stimulates human sperm motility and capacitation but not acrosome reaction. J. Androl. 2000, 21, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Cuadra, D.L.; Chan, P.J.; Patton, W.C.; Stewart, S.C.; King, A. Type 5 phosphodiesterase regulation of human sperm motility. Am. J. Obstet. Gynecol. 2000, 182, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Glenn, D.R.; McVicar, C.M.; McClure, N.; Lewis, S.E. Sildenafil citrate improves sperm motility but causes a premature acrosome reaction in vitro. Fertil. Steril. 2007, 87, 1064–1070. [Google Scholar] [CrossRef]
- Mostafa, T. Tadalafil as an in vitro sperm motility stimulant. Andrologia 2007, 39, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, Y.; Yang, H.; Jin, Y.; Hu, K.; Wang, H.X.; Wang, Y.X.; Huang, Y.R.; Chen, B. Effect of acute tadalafil on sperm motility and acrosome reaction: In vitro and in vivo studies. Andrologia 2014, 46, 417–422. [Google Scholar] [CrossRef]
- Biel, M.; Sautter, A.; Ludwig, A.; Hofmann, F.; Zong, X. Cyclic nucleotide-gated channels—Mediators of NO:cGMP-regulated processes. Naunyn Schmiedebergs Arch. Pharmacol. 1998, 358, 140–144. [Google Scholar] [CrossRef]
- Tur-Kaspa, I.; Segal, S.; Moffa, F.; Massobrio, M.; Meltzer, S. Viagra for temporary erectile dysfunction during treatments with assisted reproductive technologies. Hum. Reprod. 1999, 14, 1783–1784. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zachariou, A.; Dimitriadis, F.; Chrisofos, M.; Sofikitis, N. Empirical Treatments for Male Infertility: A Focus on Lifestyle Modifications and Medicines. Diseases 2024, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- DeVilbiss, E.A.; Sjaarda, L.A.; Peterson, C.M.; Hotaling, J.M.; Mills, J.L.; Mendola, P.; Carrell, D.T.; Johnstone, E.; Chen, Z.; Perkins, N.J.; et al. Longitudinal semen parameter assessments and live birth: Variability and implications for treatment strategies. Fertil. Steril. 2022, 118, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Lambert, S.M.; Brown, M.; Shabsigh, R. PDE-5 inhibitors: Current status and future trends. Urol. Clin. N. Am. 2005, 32, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.O.; Turk, A.; Kunej, T. Towards a Multi-Omics of Male Infertility. World J. Mens. Health 2023, 41, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Zikopoulos, A.; Markou, E.; Zachariou, A.; Stavropoulos, M.; Kratiras, Z.; Symeonidis, E.N.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M. Proteomics and Metabolomics in Varicocele-Associated Male Infertility: Advancing Precision Diagnostics and Therapy. J. Clin. Med. 2024, 13, 7390. [Google Scholar] [CrossRef]
- Selvaraj, C.; Chandra, I.; Singh, S.K. Artificial intelligence and machine learning approaches for drug design: Challenges and opportunities for the pharmaceutical industries. Mol. Divers. 2022, 26, 1893–1913. [Google Scholar] [CrossRef]

| Study Info | Outcome | Infertile Men | Normozoospermic Men |
|---|---|---|---|
| 2017 Tan et al. N = 1317 [143] | Semen Volume | No significant effect (MD = 0.08; 95% CI: –0.25 to 0.40; p = 0.65) | No significant effect (MD = 0.08; 95% CI: –0.47 to 0.64; p = 0.76) |
| Sperm Concentration | No significant effect (MD = 2.02; 95% CI: –4.43 to 8.47; p = 0.54) | No significant effect (MD = 1.36; 95% CI: –5.16 to 7.88; p = 0.68) | |
| % Motile Spermatozoa | Significant increase (MD = 6.89; 95% CI: 3.55–10.23; p < 0.001) | No significant effect (MD = 0.67; 95% CI: –2.83 to 4.17; p = 0.71) | |
| % Total Progressive Motility | Significant increase (MD = 6.64; 95% CI: 2.55–10.74; p = 0.001) | No significant effect (MD = 2.11; p > 0.05) (95% CI not explicitly reported) | |
| % Rapid Progressive Motility | Significant increase (MD = 3.89; 95% CI: 0.17–7.61; p = 0.04) | No significant effect (MD = 0.92; 95% CI: –2.45 to 4.28; p = 0.59) | |
| % Morphologically Normal Spermatozoa | Significant increase (MD = 12.15; 95% CI: 5.16–19.15; p = 0.0007) | No significant effect | |
| Reproductive Hormones | No significant effect on total T, free T, LH, or FSH levels | No significant effect on total T, free T, LH, or FSH levels | |
| 2021 Dong et al. N = 1121 [144] | Semen Volume | Contradictory findings | Not analyzed separately |
| Sperm Number (WHO 1999/2010) | Contradictory findings | Not analyzed separately | |
| Sperm Concentration (WHO 1999) | Significant improvement (MD = 1.96; 95% CI: 1.70–2.21; p < 0.001) | Not analyzed separately | |
| Sperm Concentration (WHO 2010) | Significant improvement (MD = 3.22; 95% CI: 1.96–4.48; p < 0.001) | Not analyzed separately | |
| % Straight Progressive Motility (Grade A, WHO 1999) | Significant improvement (MD = 3.71; 95% CI: 2.21–5.20; p < 0.001) | Not analyzed separately | |
| Total Sperm Motility (WHO 1999) | Significant improvement (MD = 8.09; 95% CI: 7.83–8.36; p < 0.001) | Not analyzed separately | |
| Progressive Motile Sperm (WHO 2010) | Significant improvement (MD = 5.34; 95% CI: 3.87–6.81; p < 0.001) | Not analyzed separately | |
| % Morphologically Normal Spermatozoa (WHO 1999) | Significant improvement (MD = 0.67; 95% CI: 0.20–1.15; p = 0.005) | Not analyzed separately | |
| % Morphologically Normal Spermatozoa (WHO 2010) | Significant improvement (MD = 1.27; 95% CI: 0.02–2.52; p = 0.05) | Not analyzed separately | |
| Sperm Abnormalities (%) (WHO 1999) | Significant improvement (MD = –0.64; 95% CI: –0.81 to –0.47; p < 0.001) | Not analyzed separately |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Dimitriadis, F.; Zachariou, A.; Sofikitis, N.; Chrisofos, M. Phosphodiesterase Type 5 Inhibitors in Male Reproduction: Molecular Mechanisms and Clinical Implications for Fertility Management. Cells 2025, 14, 120. https://doi.org/10.3390/cells14020120
Kaltsas A, Dimitriadis F, Zachariou A, Sofikitis N, Chrisofos M. Phosphodiesterase Type 5 Inhibitors in Male Reproduction: Molecular Mechanisms and Clinical Implications for Fertility Management. Cells. 2025; 14(2):120. https://doi.org/10.3390/cells14020120
Chicago/Turabian StyleKaltsas, Aris, Fotios Dimitriadis, Athanasios Zachariou, Nikolaos Sofikitis, and Michael Chrisofos. 2025. "Phosphodiesterase Type 5 Inhibitors in Male Reproduction: Molecular Mechanisms and Clinical Implications for Fertility Management" Cells 14, no. 2: 120. https://doi.org/10.3390/cells14020120
APA StyleKaltsas, A., Dimitriadis, F., Zachariou, A., Sofikitis, N., & Chrisofos, M. (2025). Phosphodiesterase Type 5 Inhibitors in Male Reproduction: Molecular Mechanisms and Clinical Implications for Fertility Management. Cells, 14(2), 120. https://doi.org/10.3390/cells14020120









