Cholecystokinin (CCK) Is a Mediator Between Nutritional Intake and Gonadal Development in Teleosts
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Drugs
2.2. Cell Transfection with Dual Luciferase Assay
2.3. Cell Culture
2.4. Immunofluorescence
2.5. Single-Cell RNA-Seq Library Construction and Sequencing
2.6. Real-Time PCR
2.7. Measurement of FSH Secretion and Glucose
2.8. Intraperitoneal Injection of CCK in Grass Carp
2.9. Data Transformation and Statistical Analysis
3. Results
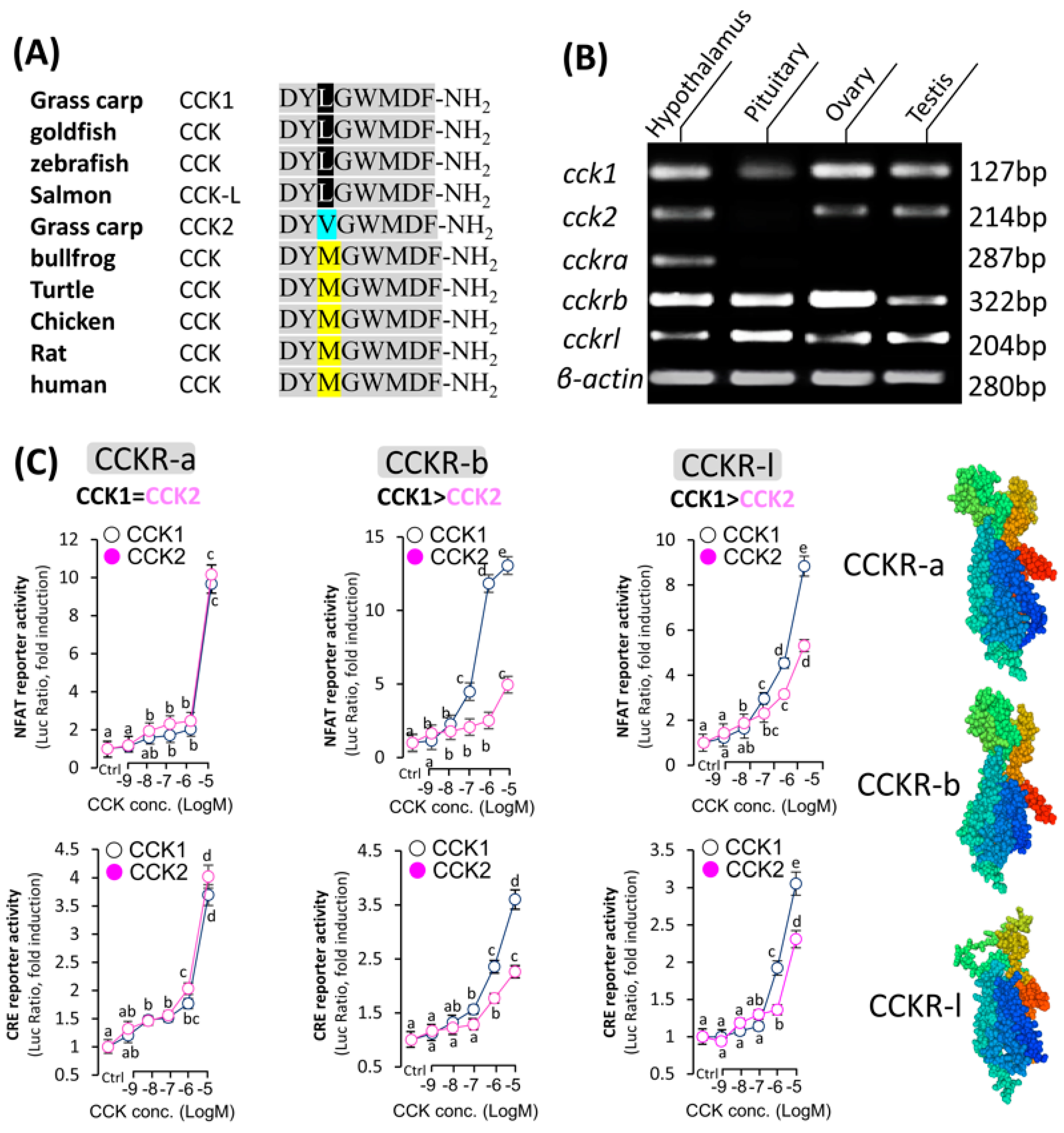
3.1. Analysis of CCK and Its Activation of the Receptor
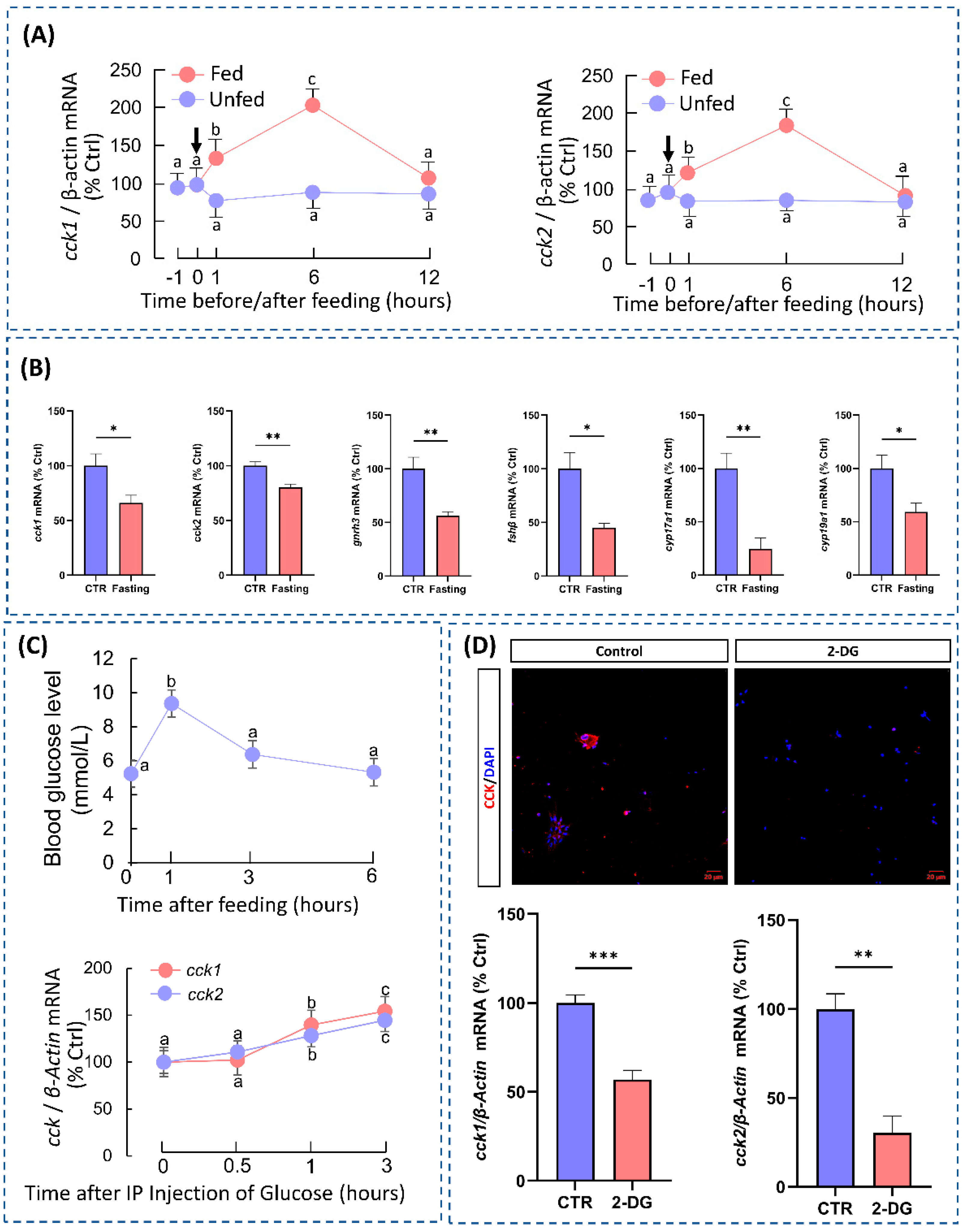
3.2. Food Intake Induces CCK Expression in the Hypothalamus
3.3. CCK Stimulates the Expression of GnRH3 in the Hypothalamus
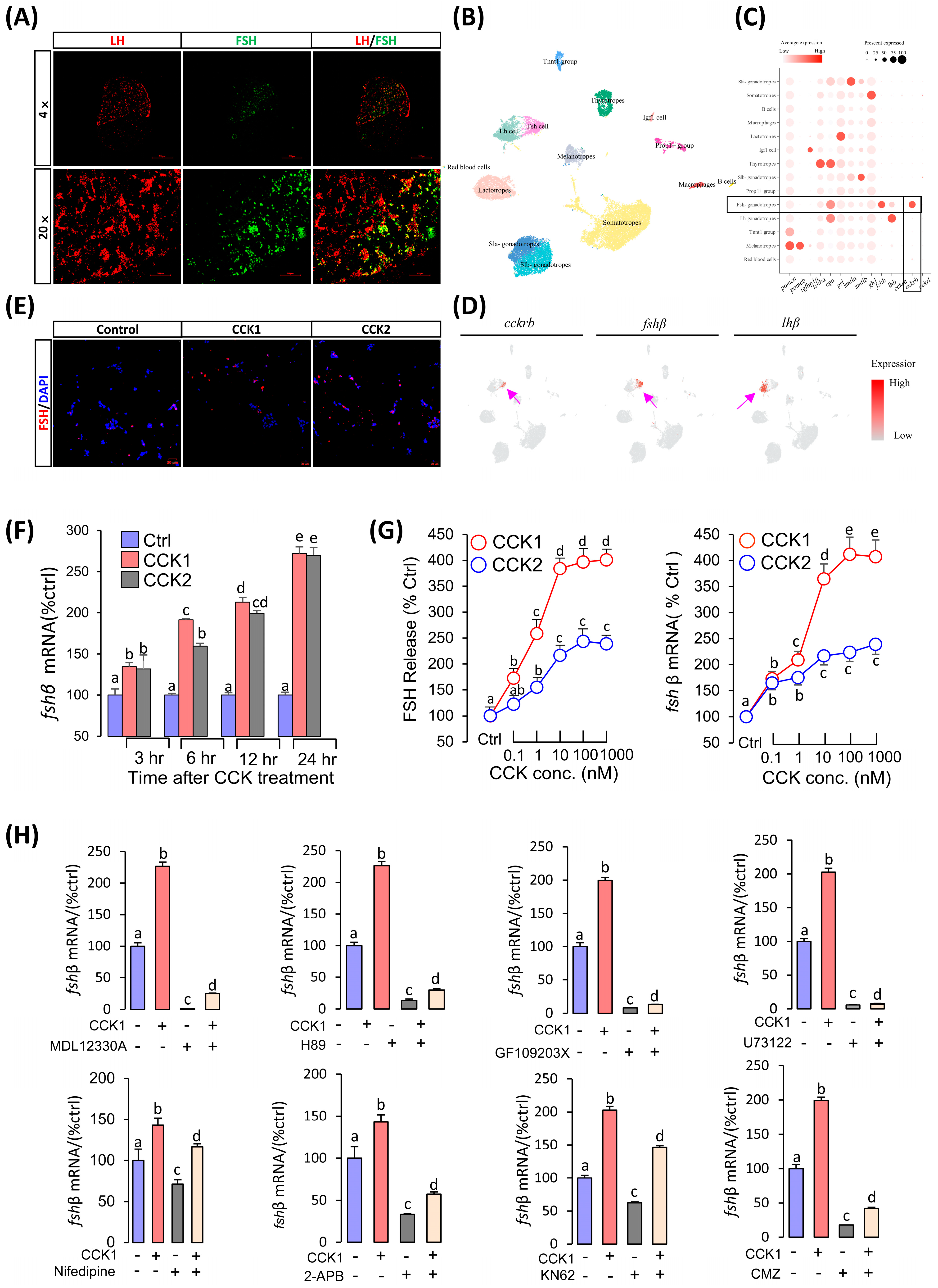
3.4. CCK Could Induce FSH Synthesis and Secretion in the Pituitary
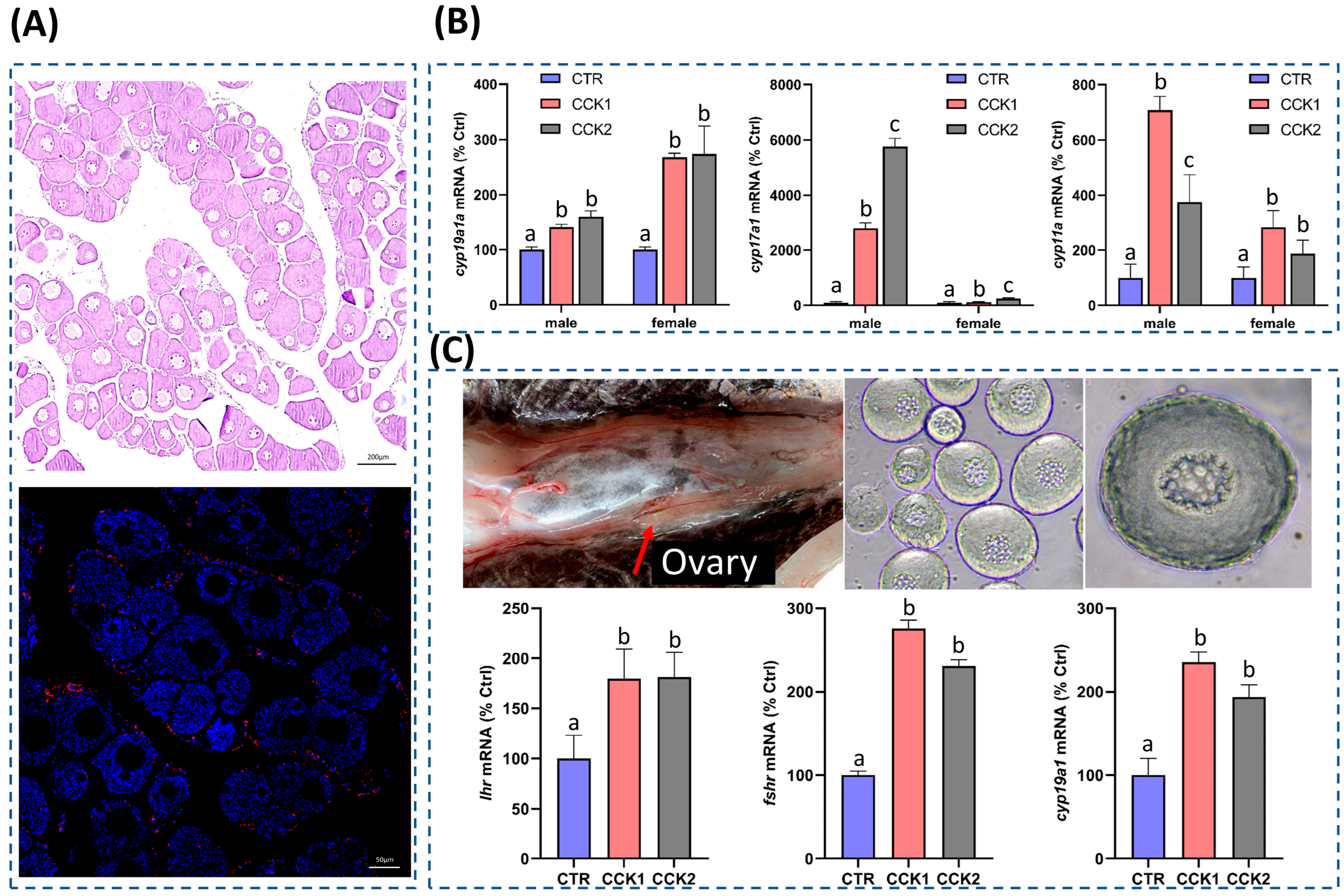
3.5. CCK Could Induce the Sexual Steroid Hormone Synthesis in the Gonad
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takumi, K.; Shimada, K.; Iijima, N.; Ozawa, H. Maternal high-fat diet during lactation increases Kiss1 mRNA expression in the arcuate nucleus at weaning and advances puberty onset in female rats. Neurosci. Res. 2015, 100, 21–28. [Google Scholar] [CrossRef]
- Fan, X.T.; Hou, T.T.; Sun, T.Z.; Zhu, L.; Zhang, S.; Tang, K.; Wang, Z.Z. Starvation stress affects the maternal development and larval fitness in zebrafish (Danio rerio). Sci. Total Environ. 2019, 695, 133897. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Spencer, S.J. Preweaning over-and underfeeding alters onset of puberty in the rat without affecting kisspeptin. Biol. Reprod. 2012, 86, 145. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, E.; Kurian, J.R.; Keen, K.L.; Shiel, N.A.; Colman, R.J.; Capuano, S.V. Body weight impact on puberty: Effects of high-calorie diet on puberty onset in female rhesus monkeys. Endocrinology 2012, 153, 1696–1705. [Google Scholar] [CrossRef]
- Villamor, E.; Jansen, E.C. Nutritional Determinants of the Timing of Puberty. Annu. Rev. Public Health 2016, 37, 33–46. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.; Dufour, S.; Karlsen, Ø.; Norberg, B.; et al. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Liddle, R.A. Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 63–67. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Cholecystokinin—From local gut hormone to ubiquitous messenger. Front. Endocrinol. 2017, 8, 47. [Google Scholar] [CrossRef]
- Coll, A.P.; Farooqi, I.S.; O’Rahilly, S. The hormonal control of food intake. Cell 2007, 129, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Finlayson, G.; Caudwell, P.; Webb, D.; Hellström, P.M.; Näslund, E.; Blundell, J.E. Postprandial profiles of CCK after high fat and high carbohydrate meals and the relationship to satiety in humans. Peptides 2016, 77, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Zhang, G.R.; Wei, K.J.; Xiong, B.X.; Liang, T.; Ping, H.C. Molecular characterization of cholecystokinin in grass carp (Ctenopharyngodon idellus): Cloning, localization, developmental profile, and effect of fasting and refeeding on expression in the brain and intestine. Fish Physiol. Biochem. 2012, 38, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Pitts, P.M.; Volkoff, H. Characterization of appetite-regulating factors in platyfish, Xiphophorus maculatus (Cyprinodontiformes Poeciliidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 208, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Garfield, A.S.; Shah, B.P.; Madara, J.C.; Burke, L.K.; Patterson, C.M.; Flak, J.; Neve, R.L.; Evans, M.L.; Lowell, B.B.; Myers, M.G.; et al. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab. 2014, 20, 1030–1037. [Google Scholar] [CrossRef]
- Costa, A.; Ai, M.; Nunn, N.; Culotta, I.; Hunter, J.; Boudjadja, M.B.; Valencia-Torres, L.; Aviello, G.; Hodson, D.J.; Snider, B.M.; et al. Anorectic and aversive effects of GLP-1 receptor agonism are mediated by brainstem cholecystokinin neurons, and modulated by GIP receptor activation. Mol. Metab. 2022, 55, 101407. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, P.; Kopin, A.S.; Beart, P.M.; Mercer, L.D.; Fasolo, A.; Wray, S. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J. Neurosci. 2004, 24, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, P.; Wray, S. Cholecystokinin directly inhibits neuronal activity of primary gonadotropin-releasing hormone cells through cholecystokinin-1 receptor. Endocrinology 2007, 148, 63–71. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, L.B.; Liu, S.Q.; Tang, J.F.; Li, F.Y.; Li, R.Y.; Song, H.D.; Chen, M.D. Expression of feeding-related peptide receptors mRNA in GT1-7 cell line and roles of leptin and orexins in control of GnRH secretion 1. Acta Pharmacol. Sin. 2005, 26, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Hollander-Cohen, L.; Golan, M.; Levavi-Sivan, B. Differential regulation of gonadotropins as revealed by transcriptomes of distinct LH and FSH cells of fish pituitary. Int. J. Mol. Sci. 2021, 22, 6478. [Google Scholar] [CrossRef]
- Himick, B.A.; Golosinski, A.A.; Jonsson, A.C.; Peter, R.E. CCK/gastrin-like immunoreactivity in the goldfish pituitary: Regulation of pituitary hormone secretion by CCK-like peptides in vitro. Gen. Comp. Endocr. 1993, 92, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Eykelbosh, A.J.; Peter, R.E. Role of leptin in the control of feeding of goldfish Carassius auratus: Interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res. 2003, 972, 90–109. [Google Scholar] [CrossRef]
- Ye, C.; Xu, S.H.; Hu, Q.Y.; Zhou, L.L.; Qin, X.F.; Jia, J.Y.; Hu, G.F. Global view of neuropeptides and their receptors in the brain and pituitary of grass carp (Ctenopharyngodon idellus). Aquaculture 2019, 512, 734360. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Qi, J.W.; Wang, S.Y.; Hao, J.; Wu, Y.B.; Chen, H.; Tian, Z.Z.; Wang, B.; Chen, D.F.; et al. CCK reduces the food intake mainly through CCK1R in Siberian sturgeon (Acipenser baerii Brandt). Sci. Rep. 2017, 7, 12413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Huang, R.; Chen, L.; Xiong, L.; He, B.; Li, Y.; Liao, L.; Zhu, Z.; Wang, Y. Computational identification of Y-linked markers and genes in the grass carp genome by using a pool-and-sequence method. Sci. Rep. 2017, 7, 8213. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.F.; He, M.; Ko, W.K.W.; Ye, C.; Hu, Q.Y. Wong AOL IGFs potentiate TAC3-induced SLα expression via upregulation of TACR3 expression in grass carp pituitary cells. Cells 2019, 8, 887. [Google Scholar] [CrossRef]
- Hu, G.; He, M.; Ko, W.K.W.; Wong, A.O.L. TAC1 Gene Products Regulate Pituitary Hormone Secretion and Gene Expression in Prepubertal Grass Carp Pituitary Cells. Endocrinology 2017, 158, 1776–1797. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.Y.; Hu, Q.Y.; Wang, L.L.; Yin, Z.; Hu, G.F. IGFBP1a is a nutrient deficient response factor that can inhibit fish reproduction through the hypothalamus-pituitary-ovary axis. Biol. Reprod. 2024, 110, 761–771. [Google Scholar] [CrossRef]
- Ballaz, S. The unappreciated roles of the cholecystokinin receptor CCK(1) in brain functioning. Rev. Neurosci. 2017, 28, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.H.; Cohen, O.; Shulman, M.; Aiznkot, T.; Fontanaud, P.; Revah, O.; Mollard, P.; Golan, M.; Sivan, B.L. The satiety hormone cholecystokinin gates reproduction in fish by controlling gonadotropin secretion. eLife 2024, 13, RP96344. [Google Scholar] [CrossRef]
- Uehara, S.K.; Nishiike, Y.; Maeda, K.; Karigo, T.; Kuraku, S.; Okubo, K.; Kanda, S. Identification of the FSH-RH as the other gonadotropin-releasing hormone. Nat. Commun. 2024, 15, 5342. [Google Scholar] [CrossRef]
- Xu, S.H. The Mechanisms of Cholecystokinin-Regulated Feeding and Reproduction in Grass Carp. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Feng, K.; Cui, X.F.; Song, Y.L.; Tao, B.B.; Chen, J.; Wang, J.; Liu, S.J.; Sun, Y.A.; Zhu, Z.Y.; Trudeau, V.L.; et al. Gnrh3 Regulates PGC Proliferation and Sex Differentiation in Developing Zebrafish. Endocrinology 2019, 161, bqz024. [Google Scholar] [CrossRef]
- Bennis, M.; Repérant, J.; Tramu, G. Evidence for co-existence of CCK-8 and GnRH in neurons of the mesencephalic tegmentum in the chameleon. Neurosci. Lett. 1998, 240, 155–158. [Google Scholar] [CrossRef]
- Pierce, J.G.; Parsons, T.F. Glycoprotein hormones: Structure and function. Annu. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef]
- Li, J.; Cheng, C.H. Evolution of gonadotropin signaling on gonad development: Insights from gene knockout studies in zebrafish. Biol. Reprod. 2018, 99, 686–694. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhu, B.; Ge, W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol. Endocrinol. 2015, 29, 76–98. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Lau, S.W.; Zhang, L.L.; Ge, W. Disruption of zebrafish follicle-stimulating hormone receptor (fshr) but not luteinizing hormone receptor (lhcgr) gene by TALEN leads to failed follicle activation in females followed by sexual reversal to males. Endocrinology 2015, 156, 3747–3762. [Google Scholar] [CrossRef]
- Wan, Y.P.; Deng, Q.Y.; Zhou, Z.C.; Deng, Y.; Zhang, J.N.; Li, J.; Wang, Y.J. Cholecystokinin (CCK) and its receptors (CCK1R and CCK2R) in chickens: Functional analysis and tissue expression. Poult. Sci. 2023, 102, 102273. [Google Scholar] [CrossRef]
- Fallah, H.P.; Rodrigues, M.S.; Corchuelo, S.; Nóbrega, R.H.; Habibi, H.R. Role of GnRH isoforms in paracrine/autocrine control of zebrafish (Danio rerio) spermatogenesis. Endocrinology 2020, 161, bqaa004. [Google Scholar] [CrossRef] [PubMed]
- Fallah, H.P.; Habibi, H.R. Role of GnRH and GnIH in paracrine/autocrine control of final oocyte maturation. Gen. Comp. Endocrinol. 2020, 299, 113619. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Salem, M.; Zhou, W.Y.; Sato-Shimizu, M.; Ye, G.; Smitz, J.; Peng, C. Neurokinin B exerts direct effects on the ovary to stimulate estradiol production. Endocrinology 2016, 157, 3355–3365. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liang, H.; Gao, X.; Zeng, X.; Zheng, S.; Wang, L.; Yuan, F.; Xu, S.; Yin, Z.; Hu, G. Cholecystokinin (CCK) Is a Mediator Between Nutritional Intake and Gonadal Development in Teleosts. Cells 2025, 14, 78. https://doi.org/10.3390/cells14020078
Li H, Liang H, Gao X, Zeng X, Zheng S, Wang L, Yuan F, Xu S, Yin Z, Hu G. Cholecystokinin (CCK) Is a Mediator Between Nutritional Intake and Gonadal Development in Teleosts. Cells. 2025; 14(2):78. https://doi.org/10.3390/cells14020078
Chicago/Turabian StyleLi, Hangyu, Hongwei Liang, Xiaowen Gao, Xiangtong Zeng, Shuo Zheng, Linlin Wang, Faming Yuan, Shaohua Xu, Zhan Yin, and Guangfu Hu. 2025. "Cholecystokinin (CCK) Is a Mediator Between Nutritional Intake and Gonadal Development in Teleosts" Cells 14, no. 2: 78. https://doi.org/10.3390/cells14020078
APA StyleLi, H., Liang, H., Gao, X., Zeng, X., Zheng, S., Wang, L., Yuan, F., Xu, S., Yin, Z., & Hu, G. (2025). Cholecystokinin (CCK) Is a Mediator Between Nutritional Intake and Gonadal Development in Teleosts. Cells, 14(2), 78. https://doi.org/10.3390/cells14020078






