Effectiveness of Graphene Oxide (GO) in Activating the Mitochondrial Pathway of Oxidative Stress-Induced Apoptosis in Breast Cancer Cells
Highlights
- Graphene oxide induces oxidative stress and apoptosis in MDA-MB-231 but not in ZR-75-1 cell lines;
- Apoptosis is induced by a caspase-9-dependent mitochondrial pathway.
- Insights into the mechanism of graphene oxide action on breast cancer MDA-MB-231 cells are provided;
- Graphene oxide opens up new possibilities in the fight against breast cancer.
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures and GO—Treatment
2.3. MTT Assay
2.4. Detection of Apoptosis and Necrosis
2.5. Fluorescent Microscopy
2.6. JC-I Assay
2.7. Measurement of Caspase 3/7 and 9 Activity
2.8. Western Blot Analysis and Chemiluminescence Detection
2.9. Total Protein Content in Cells
2.10. Cell Cycle Analysis
2.11. Transmission Electron Microscopy
2.12. Total Intracellular ROS Synthesis
2.13. Statistical Analysis
3. Results
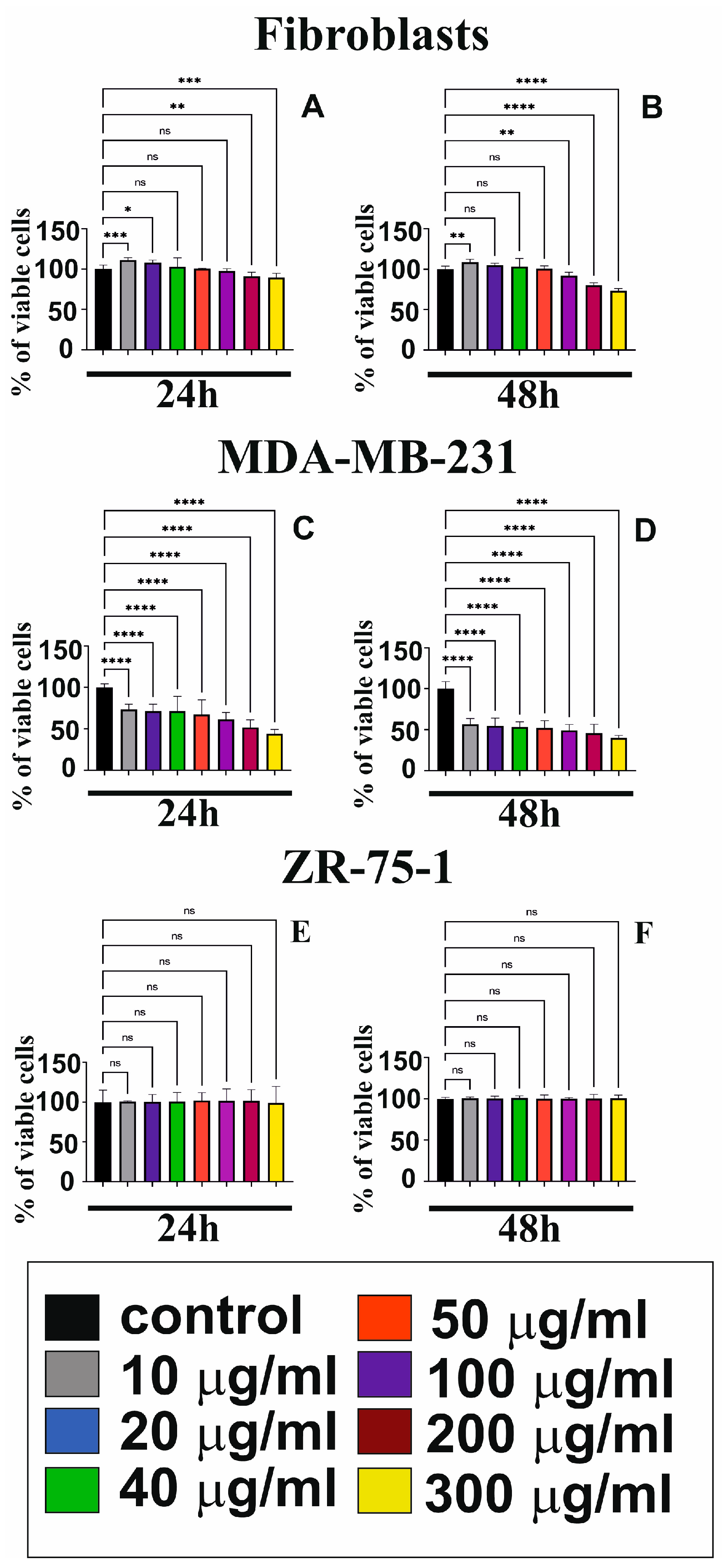
3.1. GO Cytotoxicity in Fibroblasts and Breast Cancer Cells
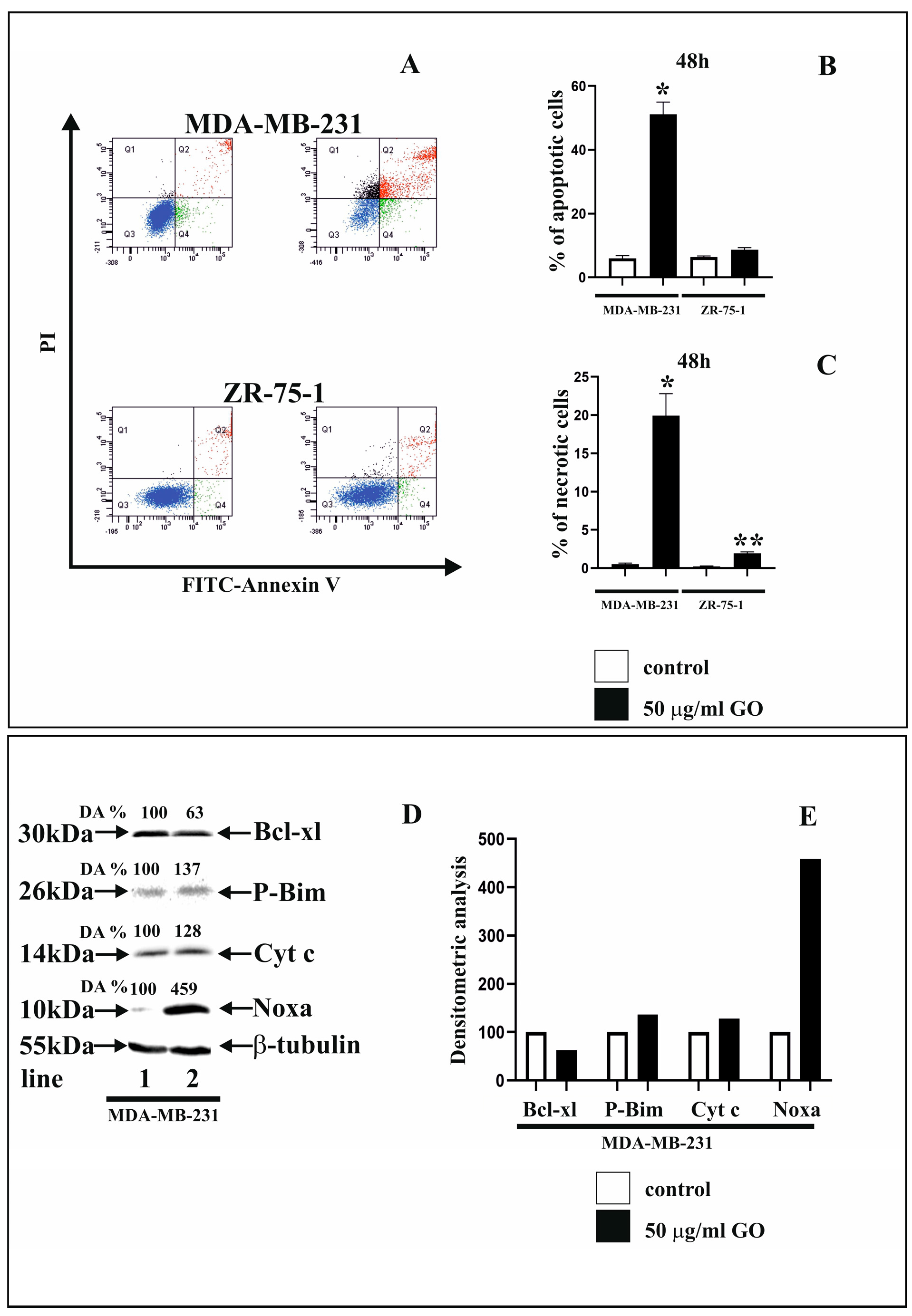
3.2. GO Influence on Apoptosis and Necrosis and Apoptosis Markers of Breast Cancer Cells
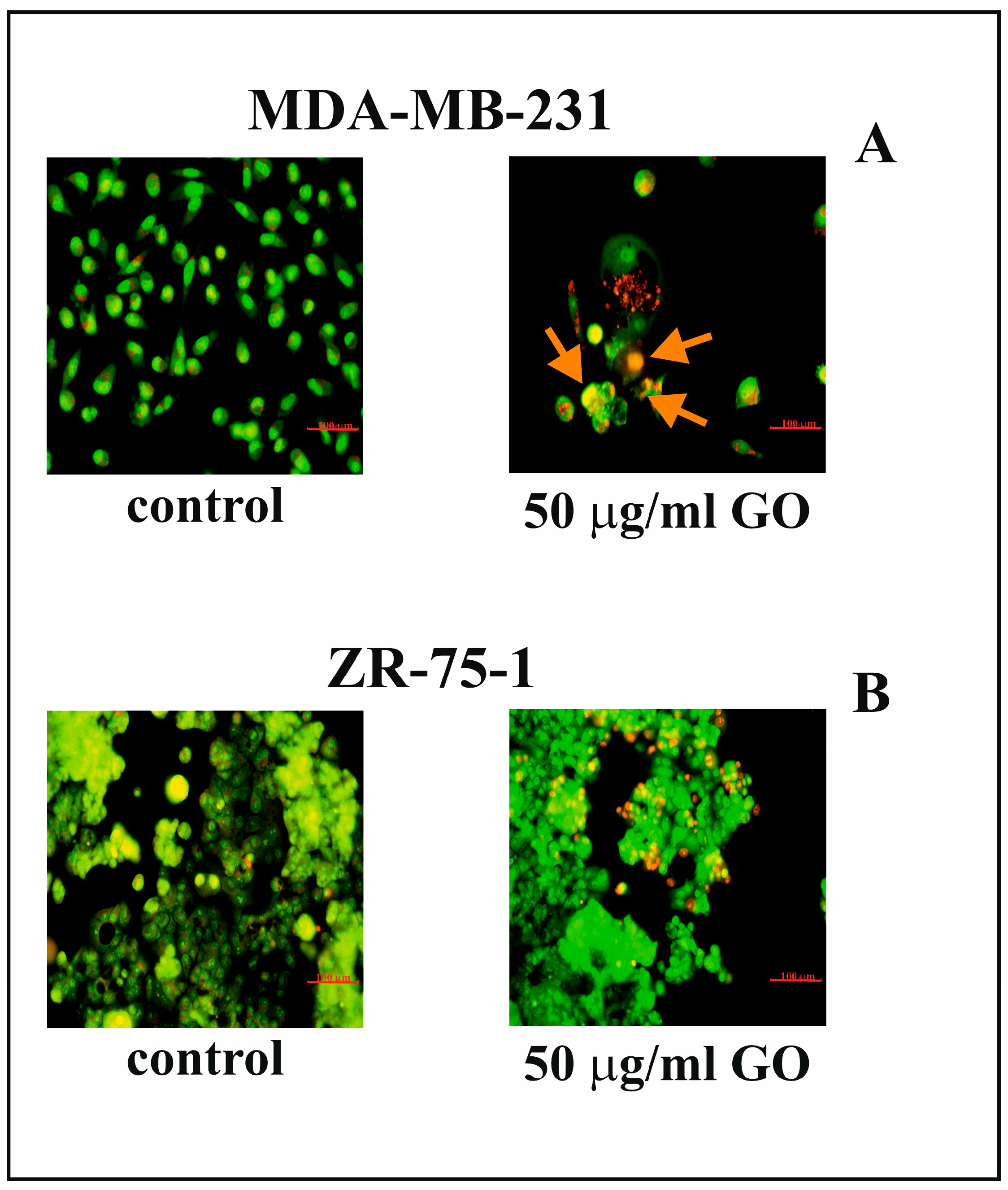
3.3. The Effect of GO on Nuclear Morphology of Breast Cancer Cells
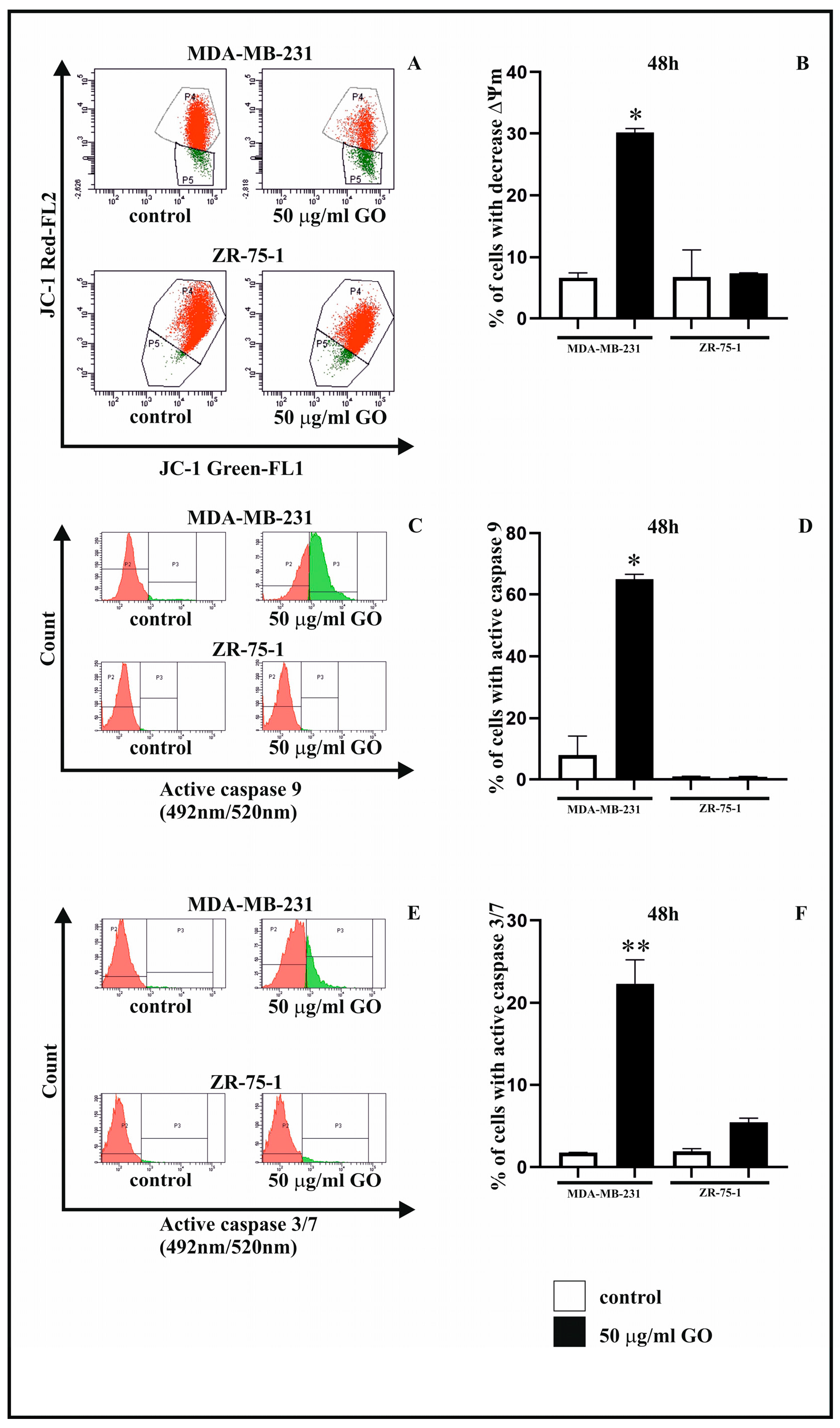
3.4. The Effect of GO on Mitochondrial Membrane Potential (∆Ψm)
3.5. The GO Induces G2/M Phase Arrest in MDA-MB-231 Cell Lines
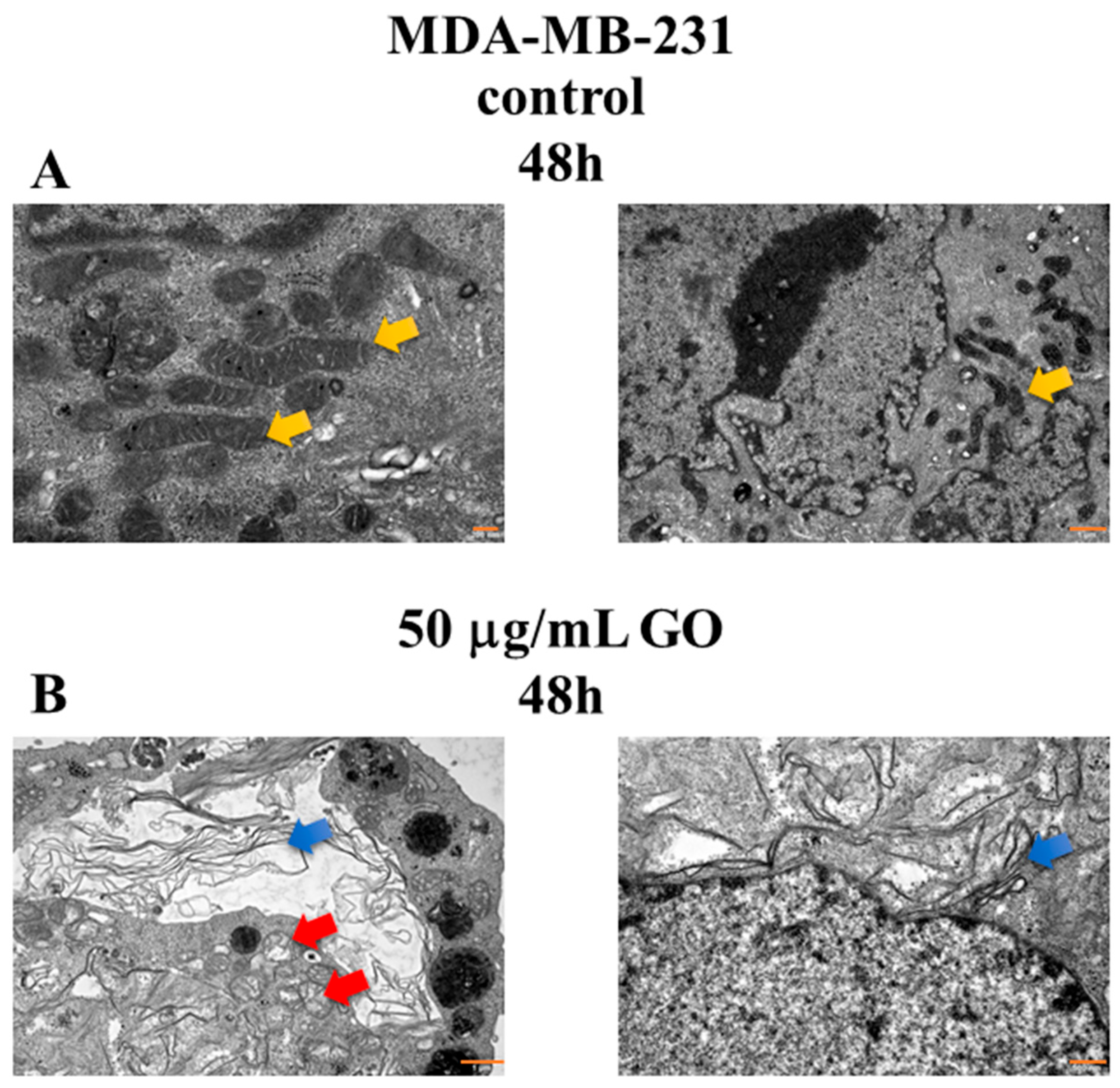
3.6. The Morphological Changes in MDA-MB-231 Cells Exposed to GO
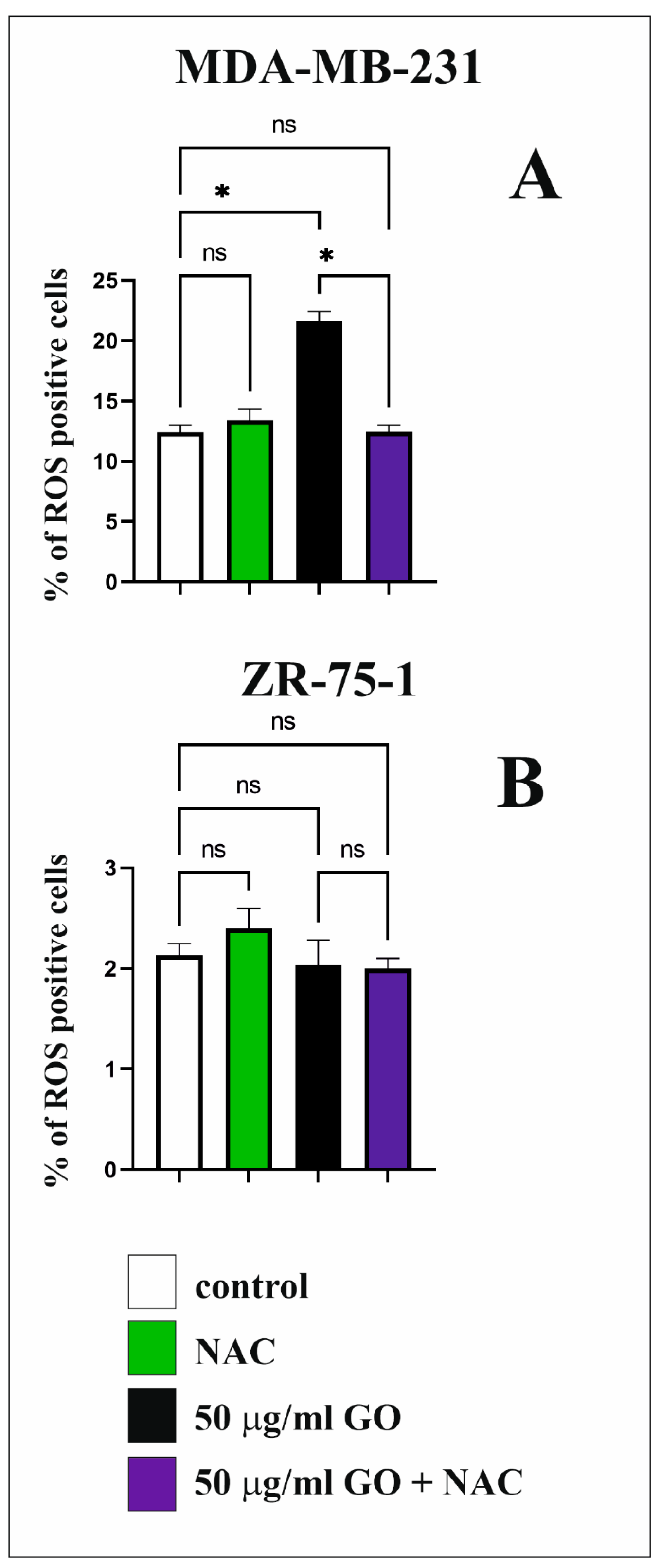
3.7. The Effect of GO on ROS Synthesis in Breast Cancer Cell Lines
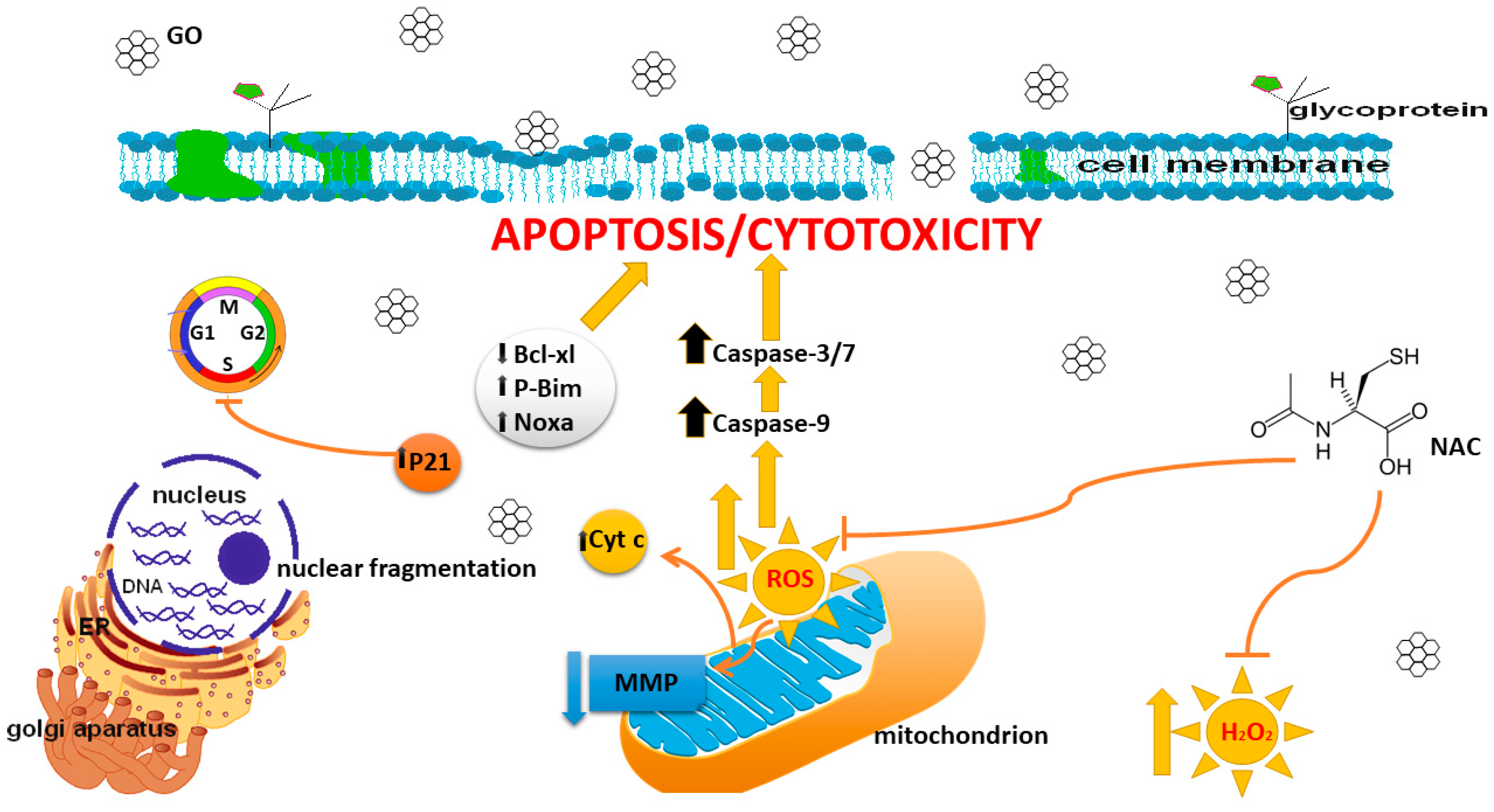
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AO | Acridine orange |
| EB | Ethidium bromide |
| GBMs | Graphene-based nanomaterials |
| GO | Graphene oxide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NAC | N-acetylcysteine |
| PCD | Programmed cell death |
| rGO | Reduced graphene oxide |
| ROS | Reactive oxygen species |
References
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Mo, Y.; Liu, W.; Liu, P.; Liu, Q.; Yuan, Z.; Wang, Q.; Yuan, D.; Chen, X.J.; Chen, T. Multifunctional Graphene Oxide Nanodelivery Platform for Breast Cancer Treatment. Int. J. Nanomed. 2022, 17, 6413–6425. [Google Scholar] [CrossRef] [PubMed]
- Al-Bader, M.; Ford, C.; Al-Ayadhy, B.; Francis, I. Analysis of estrogen receptor isoforms and variants in breast cancer cell lines. Exp. Ther. Med. 2011, 2, 537–544. [Google Scholar] [CrossRef]
- Ou, L.; Lin, S.; Song, B.; Liu, J.; Lai, R.; Shao, L. The mechanisms of graphene-based materials-induced programmed cell death: A review of apoptosis, autophagy, and programmed necrosis. Int. J. Nanomed. 2017, 12, 6633–6646. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, L.; Guo, W.; Zhang, Y.; Lai, X.; Shao, L.; Li, Y. Graphene oxide induces p62/SQSTM-dependent apoptosis through the impairment of autophagic flux and lysosomal dysfunction in PC12 cells. Acta Biomater. 2018, 81, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.H. An in vitro evaluation of graphene oxide reduced by Ganoderma spp. in human breast cancer cells (MDA-MB-231). Int. J. Nanomed. 2014, 9, 1783–1797. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kim, J.H. Graphene Oxide Enhances Biogenesis and Release of Exosomes in Human Ovarian Cancer Cells. Int. J. Nanomed. 2022, 17, 5697–5731. [Google Scholar] [CrossRef]
- Shen, J.; Dong, J.; Shao, F.; Zhao, J.; Gong, L.; Wang, H.; Chen, W.; Zhang, Y.; Cai, Y. Graphene oxide induces autophagy and apoptosis via the ROS-dependent AMPK/mTOR/ULK-1 pathway in colorectal cancer cells. Nanomedicine 2022, 17, 591–605. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; He, Y.; Liu, X.; Wang, S.; Ma, C.; Tian, X.; Wang, J.; Wu, X. Graphene oxide inhibits cell migration and invasion by destroying actin cytoskeleton in cervical cancer cells. Aging 2020, 12, 17625–17633. [Google Scholar] [CrossRef]
- Szczepaniak, J.; Strojny, B.; Chwalibog, E.S.; Jaworski, S.; Jagiello, J.; Winkowska, M.; Szmidt, M.; Wierzbicki, M.; Sosnowska, M.; Balaban, J.; et al. Effects of Reduced Graphene Oxides on Apoptosis and Cell Cycle of Glioblastoma Multiforme. Int. J. Mol. Sci. 2018, 19, 3939. [Google Scholar] [CrossRef]
- Krętowski, R.; Jabłońska-Trypuć, A.; Cechowska-Pasko, M. The Preliminary Study on the Proapoptotic Effect of Reduced Graphene Oxide in Breast Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 12593. [Google Scholar] [CrossRef]
- Krętowski, R.; Cechowska-Pasko, M. The Reduced Graphene Oxide (rGO) Induces Apoptosis, Autophagy and Cell Cycle Arrest in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 9285. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Mustafa, N.N.; El-Desouky, M.A.; Shawush, N.A.; Hanna, D.H. Apoptosis induction in ascorbic acid treated human colorectal cancer cell lines (Caco-2). J. Biol. Act. Prod. Nat. 2025, 15, 56–71. [Google Scholar] [CrossRef]
- Sayed Nady, D.; Abdel-Halim Sally, A.; Hegazy Mohamed-Elamir, F.; Aljohani, S.; AlRashidi Aljazi, A.; El-Desouky Mohamed, A.; Ahmed Hoda, A.; Jaremko, M.; Emwas, A.H.; Demiana, H. In Vitro antiproliferative potential of essential oil extract from Carica papaya L. seeds against cervical cancer. Front. Pharmacol. 2025, 16, 1676006. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.K.; Abd El-Rahman, H.A.; Mohamed, R.R.; Hanna, D.H. Multifunctional Plasticized Hyaluronic-Acid-Based Nanogel Dressing for Accelerating Diabetic and Nondiabetic Wounds. Biomacromolecules 2025, 26, 3495–3513. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.K.; Abd El-Rahman, H.A.; Mohamed, R.R.; Hanna, D.H. Hyaluronic Acid-Based pH-Sensitive Nanogel: Synthesis, Characterization, and Assessment for Acyclovir Delivery In Vitro and In Vivo. Biomacromolecules 2024, 26, 341–362. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Ahmed, S.M.; Elkhenany, H.; El-Desouky, M.A.; Magdeldin, S.; Osama, A.; Anwar, A.M.; Mohamed, I.K.; Abdelgawad, M.E.; Hanna, D.H.; et al. The cross talk between type II diabetic microenvironment and the regenerative capacities of human adipose tissue-derived pericytes: A promising cell therapy. Stem Cell Res. Ther. 2024, 15, 36. [Google Scholar] [CrossRef]
- Kozak, Y.; Finiuk, N.; Czarnomysy, R.; Gornowicz, A.; Pinyazhko, R.; Lozynskyi, A.; Holota, S.; Klyuchivska, O.; Karkhut, A.; Polovkovych, S.; et al. Juglone-Bearing Thiopyrano [2,3-d]thiazoles Induce Apoptosis in Colorectal Adenocarcinoma Cells. Cells 2025, 14, 465. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrouugh, N.J.; Far, A.L.; Randal, R.J. Protein mesaurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Khaled, M.; Ismail, S.S.; Hanna, D.H. Palladium-Imidazole Nanoparticles’ Cytotoxic Effects on Colon Cancer Cells: Induction of Cell Cycle Arrest and Apoptosis Mediated via Mitochondria. Appl. Organomet. Chem. 2024, 39, e7908. [Google Scholar] [CrossRef]
- Krętowski, R.; Szynaka, B.; Jabłońska-Trypuć, A.; Kiełtyka-Dadasiewicz, A.; Cechowska-Pasko, M. The Synergistic Effect of Reduced Graphene Oxide and Proteasome Inhibitor in the Induction of Apoptosis Through Oxidative Stress in Breast Cancer Cell Lines. Int. J. Mol. Sci. 2024, 25, 5436. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, L.; Yang, Z.; Liu, Z.; Gu, J.; Bai, B.; Liu, J.; Xu, J.; Yang, H. Mechanisms of oxidative stress, apoptosis, and autophagy involved in graphene oxide nanomaterial anti-osteosarcoma effect. Int. J. Nanomed. 2018, 13, 2907–2919. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.H. Green synthesis of graphene and its cytotoxic effects in human breast cancer cells. Int. J. Nanomed. 2013, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Z.; Qu, L. Graphene-Based Fibers: Recent Advances in Preparation and Application. Adv. Mater. 2020, 32, 1901979. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, S.; Ali, A.; Pal, M.; Chakrabarti, P. Inhibition of microtubule assembly and cytotoxic effect of graphene oxide on human colorectal carcinoma cell HCT116. Arch. Biochem. Biophys. 2021, 708, 108940. [Google Scholar] [CrossRef]
- Jiang, J.H.; Pi, J.; Jin, H.; Cai, J.Y. Functional graphene oxide as cancer-targeted drug delivery system to selectively induce oesophageal cancer cell apoptosis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 297–307. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, J.; Wu, J.; Yin, Q.; Liang, H.; Chen, A.; Shao, L. Graphene oxide and reduced graphene oxide induced neural pheochromocytoma-derived PC12 cell lines apoptosis and cell cycle alterations via the ERK signaling pathways. Int. J. Nanomed. 2017, 12, 5501–5510. [Google Scholar] [CrossRef]
- Banerjee, P.P.; Bandyopadhyay, A.; Mondal, P. Cytotoxic effect of graphene oxide-functionalized gold nanoparticles in human breast cancer cell lines. Nucleus 2019, 62, 243–250. [Google Scholar] [CrossRef]
- Ying, P.; Xiuhua, Y.; Ling, W. Protein corona reduced graphene oxide cytotoxicity by inhibiting endocytosis. Colloid Interface Sci. Commun. 2021, 45, 100514. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Deliv. Rev. 2016, 105, 145–162. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Zhang, J.; Zhang, L.; Chen, L. Inhibiting Cytoprotective Autophagy in Cancer Therapy: An Update on Pharmacological Small-Molecule Compounds. Front. Pharmacol. 2022, 13, 966012. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Elshazly, A.M.; Gewirtz, D.A. The Cytoprotective, Cytotoxic and Nonprotective Functional Forms of Autophagy Induced by Microtubule Poisons in Tumor Cells-Implications for Autophagy Modulation as a Therapeutic Strategy. Biomedicines 2022, 10, 1632. [Google Scholar] [CrossRef]
- Wu, Q.; Sharma, D. Autophagy and Breast Cancer: Connected in Growth, Progression, and Therapy. Cells 2023, 12, 1156. [Google Scholar] [CrossRef]
- Szczepaniak, J.; Jagiello, J.; Wierzbicki, M.; Nowak, D.; Sobczyk-Guzenda, A.; Sosnowska, M.; Jaworski, S.; Daniluk, K.; Szmidt, M.; Witkowska-Pilaszewicz, O.; et al. Reduced Graphene Oxides Modulate the Expression of Cell Receptors and Voltage-Dependent Ion Channel Genes of Glioblastoma Multiforme. Int. J. Mol. Sci. 2021, 22, 515. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krętowski, R.; Szynaka, B.; Borzym-Kluczyk, M.; Tyszka, N.; Jabłońska-Trypuć, A.; Gil, M.; Cechowska-Pasko, M. Effectiveness of Graphene Oxide (GO) in Activating the Mitochondrial Pathway of Oxidative Stress-Induced Apoptosis in Breast Cancer Cells. Cells 2025, 14, 1717. https://doi.org/10.3390/cells14211717
Krętowski R, Szynaka B, Borzym-Kluczyk M, Tyszka N, Jabłońska-Trypuć A, Gil M, Cechowska-Pasko M. Effectiveness of Graphene Oxide (GO) in Activating the Mitochondrial Pathway of Oxidative Stress-Induced Apoptosis in Breast Cancer Cells. Cells. 2025; 14(21):1717. https://doi.org/10.3390/cells14211717
Chicago/Turabian StyleKrętowski, Rafał, Beata Szynaka, Małgorzata Borzym-Kluczyk, Natalia Tyszka, Agata Jabłońska-Trypuć, Maciej Gil, and Marzanna Cechowska-Pasko. 2025. "Effectiveness of Graphene Oxide (GO) in Activating the Mitochondrial Pathway of Oxidative Stress-Induced Apoptosis in Breast Cancer Cells" Cells 14, no. 21: 1717. https://doi.org/10.3390/cells14211717
APA StyleKrętowski, R., Szynaka, B., Borzym-Kluczyk, M., Tyszka, N., Jabłońska-Trypuć, A., Gil, M., & Cechowska-Pasko, M. (2025). Effectiveness of Graphene Oxide (GO) in Activating the Mitochondrial Pathway of Oxidative Stress-Induced Apoptosis in Breast Cancer Cells. Cells, 14(21), 1717. https://doi.org/10.3390/cells14211717







