RyR1 Is Involved in the Control of Myogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Cell Culture
2.3. Cell Treatment
2.4. Quantitative Real-Time PCR
2.5. Western Blot Analysis and Quantification
2.6. Calcium Imaging
2.7. Immunofluorescence Staining
2.8. Statistics
3. Results
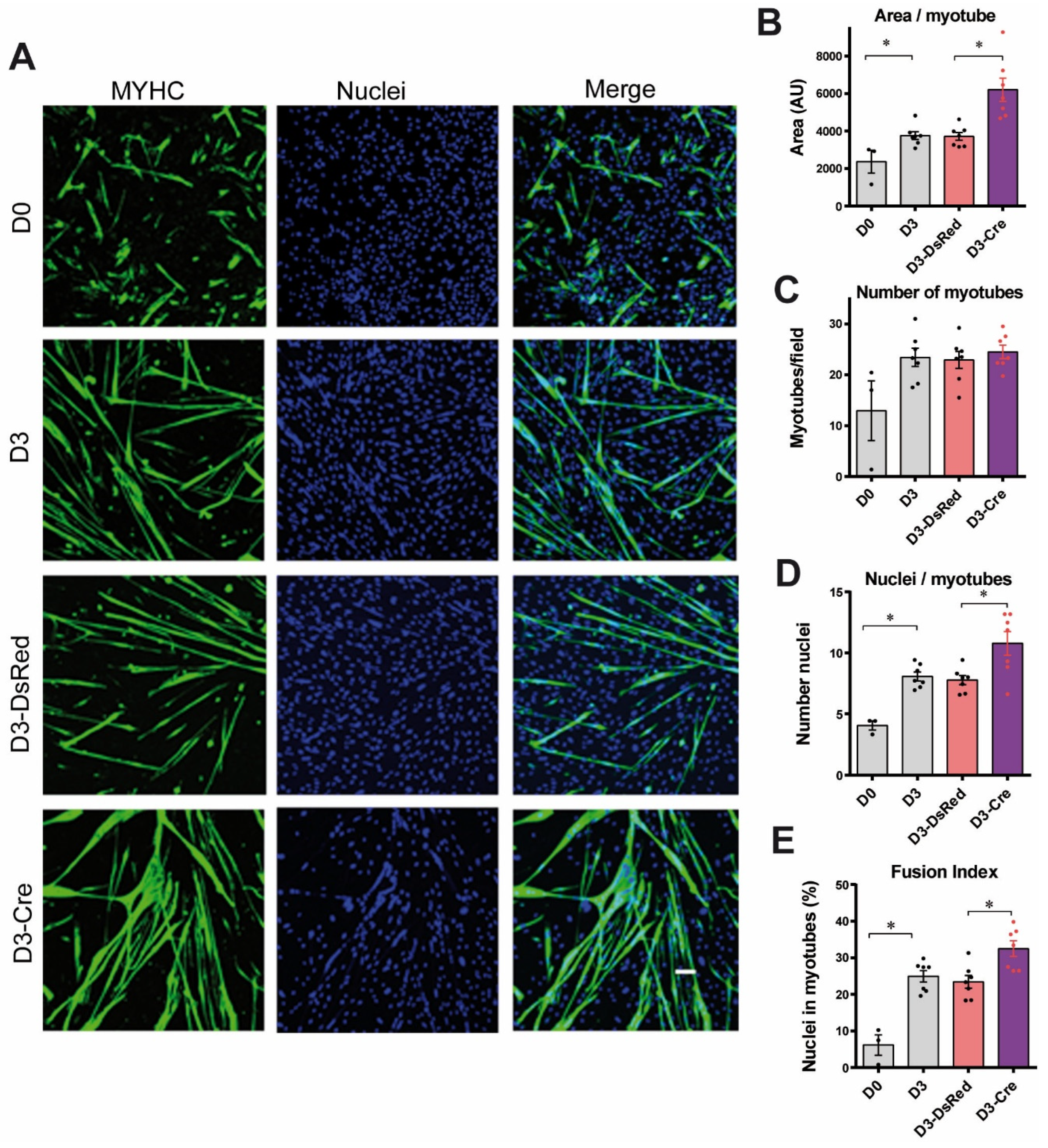
3.1. Reduction in RyR1 Amount and Calcium Channel Activity in RyR1-Rec Primary Myotubes
3.2. Reduction in RyR1 Amount in Primary Myotubes Is Associated with an Increase in Myotubes Differentiation Without Affecting Myogenic Factors
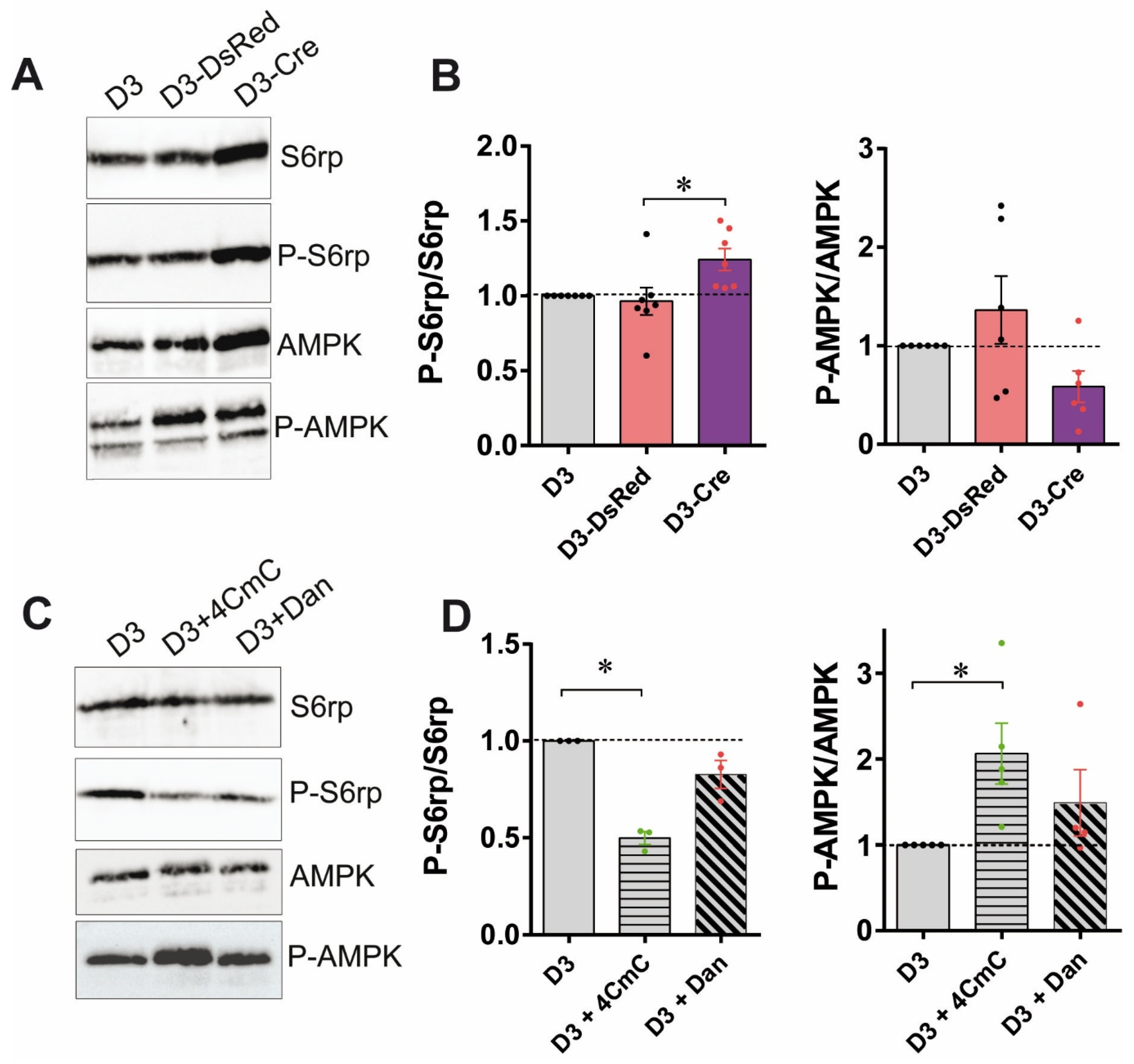
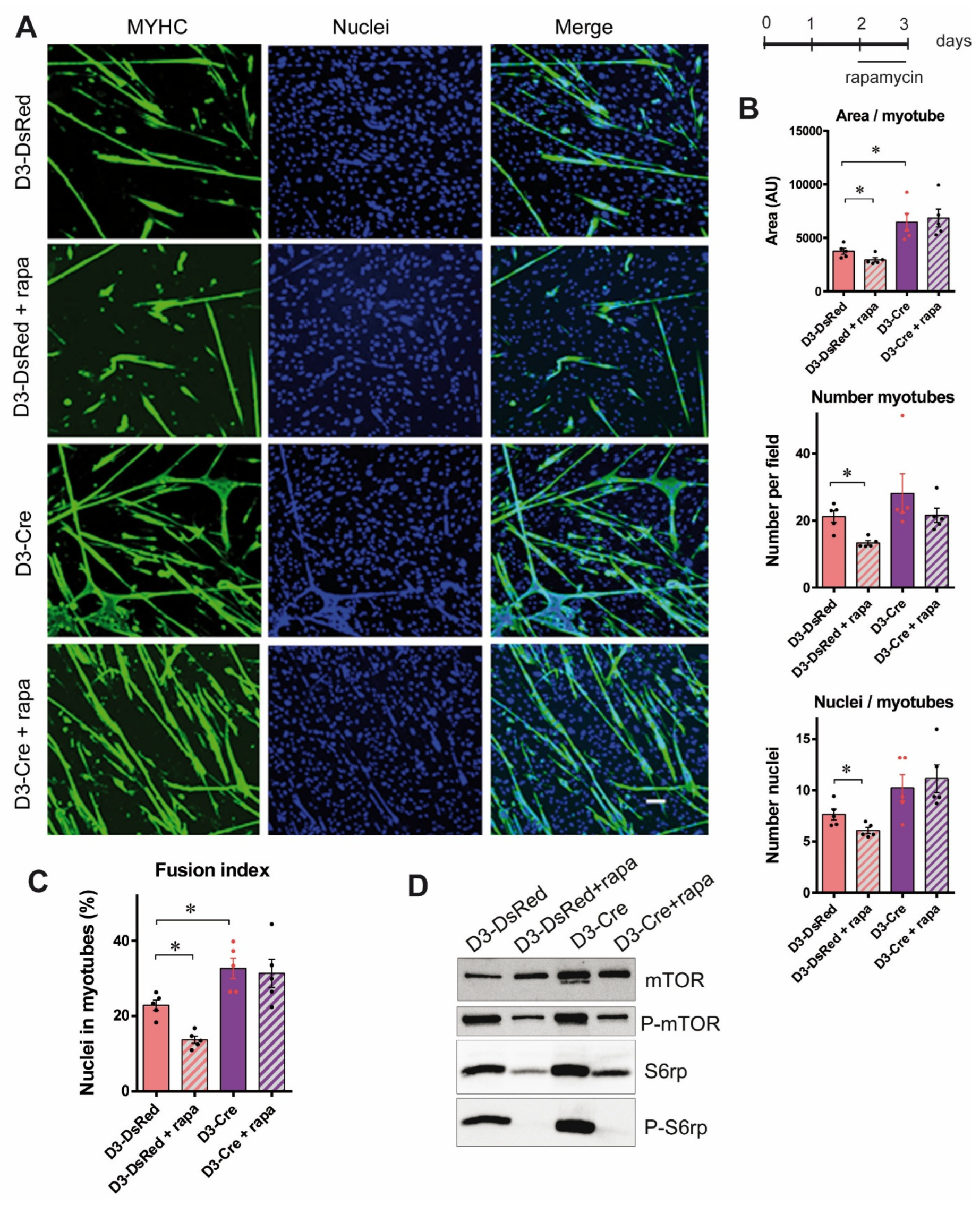
3.3. RyR1 Quantity and Calcium Channel Activity Modulate the mTOR Pathway
3.4. The Increased Myotube Differentiation in RyR1-Rec Myotubes Is Independent of the mTOR Pathway
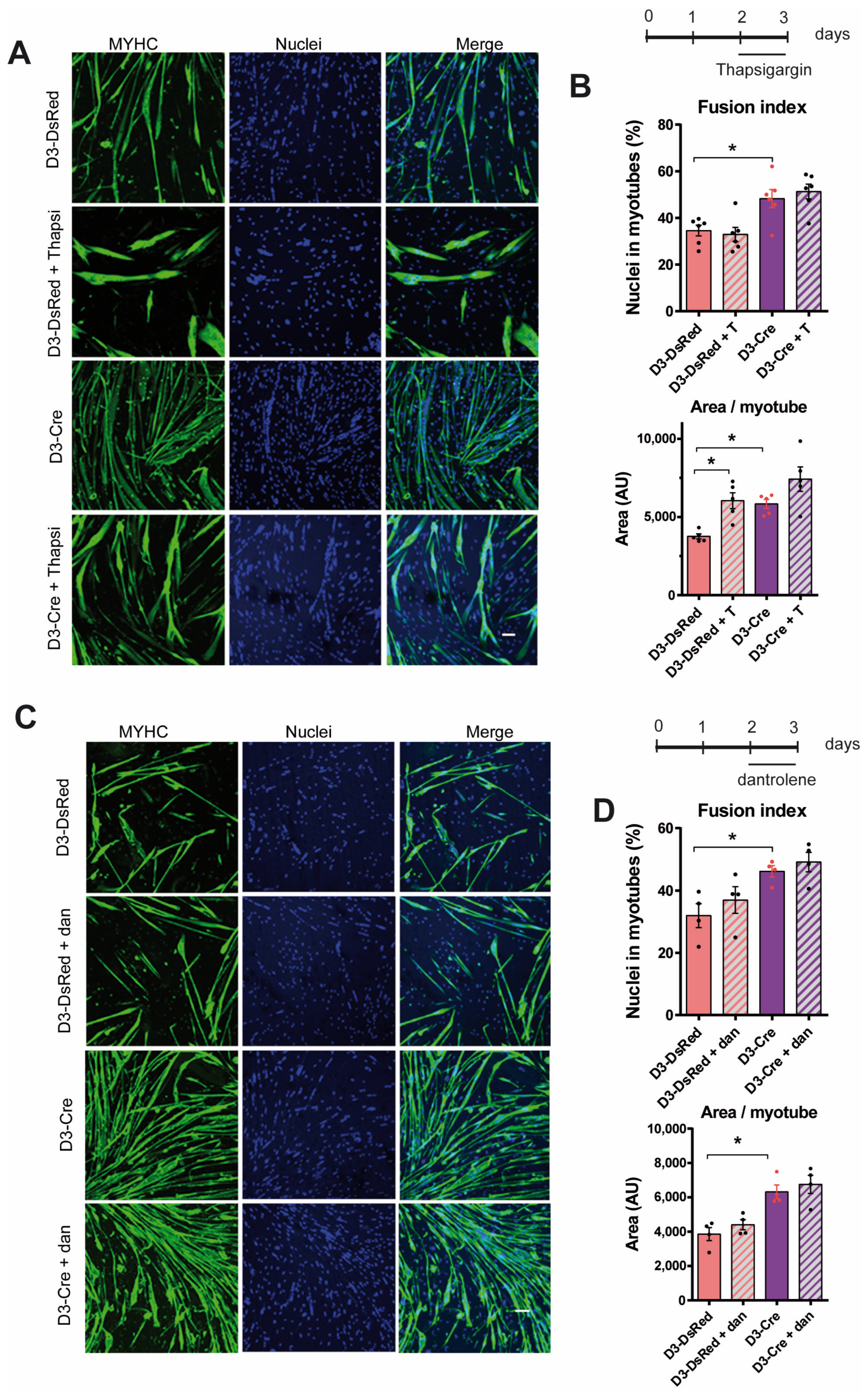
3.5. RyR1 Function on Myotube Differentiation Is Independent of RyR1-Mediated Calcium Release
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearce, L.; Meizoso-Huesca, A.; Seng, C.; Lamboley, C.R.; Singh, D.P.; Launikonis, B.S. Ryanodine receptor activity and store-operated Ca2+ entry: Critical regulators of Ca2+ content and function in skeletal muscle. J. Physiol. 2023, 601, 4183–4202. [Google Scholar] [CrossRef] [PubMed]
- Marty, I.; Fauré, J. Excitation-Contraction Coupling Alterations in Myopathies. J. Neuromuscul. Dis. 2016, 3, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Lawal, T.A.; Todd, J.J.; Witherspoon, J.W.; Bönnemann, C.G.; Dowling, J.J.; Hamilton, S.L.; Meilleur, K.G.; Dirksen, R.T. Ryanodine receptor 1-related disorders: An historical perspective and proposal for a unified nomenclature. Skelet. Muscle 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M. A Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef] [PubMed]
- Abmayr, S.M.; Pavlath, G.K. Myoblast fusion: Lessons from flies and mice. Development 2012, 139, 641–656. [Google Scholar] [CrossRef]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, J. Mammalian target of rapamycin (mTOR) signaling network in skeletal myogenesis. J. Biol. Chem. 2012, 287, 43928–43935. [Google Scholar]
- Rion, N.; Castets, P.; Lin, S.; Enderle, L.; Reinhard, J.R.; Eickhorst, C.; Rüegg, M.A. mTOR controls embryonic and adult myogenesis via mTORC1. Development 2019, 146, dev.172460. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Goh, K.Y.; Lee, W.X.; Choy, S.M.; Tang, H.-W. The Importance of mTORC1-Autophagy Axis for Skeletal Muscle Diseases. Int. J. Mol. Sci. 2022, 24, 297. [Google Scholar] [CrossRef]
- Leikina, E.; Gamage, D.G.; Prasad, V.; Goykhberg, J.; Crowe, M.; Diao, J.; Kozlov, M.M.; Chernomordik, L.V.; Millay, D.P. Myomaker and Myomerger Work Independently to Control Distinct Steps of Membrane Remodeling during Myoblast Fusion. Dev. Cell 2018, 46, 767–780.e7. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Elbaz-Alon, Y.; Avinoam, O. Ca2+ as a coordinator of skeletal muscle differentiation, fusion and contraction. FEBS J. 2022, 289, 6531–6542. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Notarnicola, C.; Meli, A.C.; Matecki, S.; Hugon, G.; Salvador, J.; Khalil, M.; Féasson, L.; Cances, C.; Cottalorda, J.; et al. Skeletal Ryanodine Receptors Are Involved in Impaired Myogenic Differentiation in Duchenne Muscular Dystrophy Patients. Int. J. Mol. Sci. 2021, 22, 12985. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Wang, Y.; Xu, D.; He, L.; Zhang, X.; Yan, E.; Wang, L.; Yin, J. Ryanodine receptor RyR1-mediated elevation of Ca(2+) concentration is required for the late stage of myogenic differentiation and fusion. J. Anim. Sci. Biotechnol. 2022, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Fernandez, E.A.; Wohlwend, M.; Laurila, P.; Lopez-Mejia, A.; Ochala, J.; Lobrinus, A.J.; Kayser, B.; Lopez-Mejia, I.C.; Place, N.; et al. Ryanodine receptor type 1 content decrease-induced endoplasmic reticulum stress is a hallmark of myopathies. J. Cachexia Sarcopenia Muscle 2023, 14, 2882–2897. [Google Scholar] [CrossRef]
- Eigler, T.; Zarfati, G.; Amzallag, E.; Sinha, S.; Segev, N.; Zabary, Y.; Zaritsky, A.; Shakked, A.; Umansky, K.B.; Schejter, E.D.; et al. ERK1/2 inhibition promotes robust myotube growth via CaMKII activation resulting in myoblast-to-myotube fusion. Dev. Cell 2021, 56, 3349–3363.e6. [Google Scholar] [CrossRef]
- Marty, I.; Robert, M.; Villaz, M.; De Jongh, K.; Lai, Y.; Catterall, W.A.; Ronjat, M. Biochemical evidence for a complex involving dihydropyridine receptor and ryanodine receptor in triad junctions of skeletal muscle. Proc. Natl. Acad. Sci. USA 1994, 91, 2270–2274. [Google Scholar] [CrossRef]
- Pelletier, L.; Petiot, A.; Brocard, J.; Giannesini, B.; Giovannini, D.; Sanchez, C.; Travard, L.; Chivet, M.; Beaufils, M.; Kutchukian, C.; et al. In vivo RyR1 reduction in muscle triggers a core-like myopathy. Acta Neuropathol. Commun. 2020, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Falcone, S.; Roman, W.; Hnia, K.; Gache, V.; Didier, N.; Lainé, J.; Auradé, F.; Marty, I.; Nishino, I.; Charlet-Berguerand, N.; et al. N-WASP is required for Amphiphysin-2/BIN1-dependent nuclear positioning and triad organization in skeletal muscle and is involved in the pathophysiology of centronuclear myopathy. EMBO Mol. Med. 2014, 6, 1455–1475. [Google Scholar] [CrossRef] [PubMed]
- Oddoux, S.; Brocard, J.; Schweitzer, A.; Szentesi, P.; Giannesini, B.; Brocard, J.; Fauré, J.; Pernet-Gallay, K.; Bendahan, D.; Lunardi, J.; et al. Triadin deletion induces impaired skeletal muscle function. J. Biol. Chem. 2009, 284, 34918–34929. [Google Scholar] [CrossRef] [PubMed]
- Garza-Lombó, C.; Schroder, A.; Reyes-Reyes, E.M.; Franco, R. mTOR/AMPK signaling in the brain: Cell metabolism, proteostasis and survival. Curr. Opin. Toxicol. 2018, 8, 102–110. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, D.; Li, Y.; Dai, T.; Liu, F.; Yan, C. TGM2 positively regulates myoblast differentiation via enhancing the mTOR signaling. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1869, 119173. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Li, Y.; Greensmith, D.J.; Eisner, D.A.; Venetucci, L. Biphasic decay of the Ca transient results from increased sarcoplasmic reticulum Ca leak. J. Physiol. 2016, 594, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Bertocchini, F.; Ovitt, C.E.; Conti, A.; Barone, V.; Schöler, H.R.; Bottinelli, R.; Reggiani, C.; Sorrentino, V. Requirement for the ryanodine receptor type 3 for efficient contraction in neonatal skeletal muscles. EMBO J. 1997, 16, 6956–6963. [Google Scholar] [CrossRef]
- Filipova, D.; Walter, A.M.; Gaspar, J.A.; Brunn, A.; Linde, N.F.; Ardestani, M.A.; Deckert, M.; Hescheler, J.; Pfitzer, G.; Sachinidis, A.; et al. Gene profiling of embryonic skeletal muscle lacking type I ryanodine receptor Ca(2+) release channel. Sci. Rep. 2016, 6, 20050. [Google Scholar]
- Marks, A.R. Intracellular calcium-release channels: Regulators of cell life and death. Am. J. Physiol. Circ. Physiol. 1997, 272, H597–H605. [Google Scholar] [CrossRef] [PubMed]
- Rosemblit, N.; Moschella, M.C.; Gutstein, D.E.; Marks, A.R. Intracellular calcium release channel expression during embryogenesis. Dev. Biol. 1999, 206, 163–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Constantin, B.; Cognard, C.; Raymond, G. Myoblast fusion requires cytosolic calcium elevation but not activation of voltage-dependent calcium channels. Cell Calcium 1996, 19, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Matsunobe, M.; Motohashi, N.; Aoki, E.; Tominari, T.; Inada, M.; Aoki, Y. Caveolin-3 regulates the activity of Ca2+/calmodulin-dependent protein kinase II in C2C12 cells. Am. J. Physiol. Cell Physiol. 2022, 323, C1137–C1148. [Google Scholar] [CrossRef]
- Qiu, K.; Xu, D.; Wang, L.; Zhang, X.; Jiao, N.; Gong, L.; Yin, J. Association Analysis of Single-Cell RNA Sequencing and Proteomics Reveals a Vital Role of Ca2+ Signaling in the Determination of Skeletal Muscle Development Potential. Cells 2020, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Turishcheva, E.; Vildanova, M.; Onishchenko, G.; Smirnova, E. The Role of Endoplasmic Reticulum Stress in Differentiation of Cells of Mesenchymal Origin. Biochemistry 2022, 87, 916–931. [Google Scholar] [CrossRef]
- Afroze, D.; Kumar, A. ER stress in skeletal muscle remodeling and myopathies. FEBS J. 2019, 286, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kakiguchi, K.; Yonemura, S.; Nakano, A.; Morishima, N. Transient Ca2+ depletion from the endoplasmic reticulum is critical for skeletal myoblast differentiation. FASEB J. 2015, 29, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, K.R.; McMillan, J.D.; Kumar, A. Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J. Cell. Physiol. 2018, 233, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Hindi, S.M.; Mann, A.K.; Gallot, Y.S.; Bohnert, K.R.; Cavener, D.R.; Whittemore, S.R.; Kumar, A.; States, U. The PERK arm of the unfolded protein response regulates satellite cell-mediated skeletal muscle regeneration. eLife 2017, 6, e22871. [Google Scholar] [CrossRef] [PubMed]
- Tokutake, Y.; Yamada, K.; Hayashi, S.; Arai, W.; Watanabe, T.; Yonekura, S. IRE1-XBP1 Pathway of the Unfolded Protein Response Is Required during Early Differentiation of C2C12 Myoblasts. Int. J. Mol. Sci. 2019, 21, 182. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Zhang, X.; Laubry, L.; Brunetti, J.; Koenig, S.; Wang, X.; Castelbou, C.; Hetz, C.; Liu, Y.; Frieden, M.; et al. The ER stress sensor IRE1 interacts with STIM1 to promote store-operated calcium entry, T cell activation, and muscular differentiation. Cell Rep. 2023, 42, 113540. [Google Scholar] [CrossRef]
- Joshi, A.S.; da Silva, M.T.; Roy, A.; Koike, T.E.; Wu, M.; Castillo, M.B.; Gunaratne, P.H.; Liu, Y.; Iwawaki, T.; Kumar, A. The IRE1alpha/XBP1 signaling axis drives myoblast fusion in adult skeletal muscle. EMBO Rep. 2024, 25, 3627–3650. [Google Scholar] [CrossRef]
- Lee, C.S.; Hanna, A.D.; Wang, H.; Dagnino-Acosta, A.; Joshi, A.D.; Knoblauch, M.; Xia, Y.; Georgiou, D.K.; Xu, J.; Long, C.; et al. A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat. Commun. 2017, 8, 14659. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Chen, J. Mammalian target of rapamycin (mTOR) signaling is required for a late-stage fusion process during skeletal myotube maturation. J. Biol. Chem. 2005, 9, 32009–32017. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.-C.; Hardie, D.G. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef]
- He, L.; Zhou, X.; Huang, N.; Li, H.; Tian, J.; Li, T.; Yao, K.; Nyachoti, C.M.; Kim, S.W.; Yin, Y. AMPK Regulation of Glucose, Lipid and Protein Metabolism: Mechanisms and Nutritional Significance. Curr. Protein Pept. Sci. 2017, 18, 562–570. [Google Scholar] [CrossRef]
- Williamson, D.L.; Butler, D.C.; Alway, S.E. AMPK inhibits myoblast differentiation through a PGC-1alpha-dependent mechanism. Am. J. Physiol. Metab. 2009, 297, E304–E314. [Google Scholar] [CrossRef]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Takahashi, H.; Kitagawa, E.; Watanabe, H.; Sakurada, T.; Aso, H.; Yamaguchi, T. AMPK activation by AICAR inhibits myogenic differentiation and myostatin expression in cattle. Cell Tissue Res. 2012, 349, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhang, D.; Zhao, L.; Li, Y.; Yao, X.; Wang, H.; Zhang, S.; Liu, W.; Cao, H.; Yu, S.; et al. CaMKK2 Suppresses Muscle Regeneration through the Inhibition of Myoblast Proliferation and Differentiation. Int. J. Mol. Sci. 2016, 17, 1695. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [PubMed]
- Kneppers, A.; Ben Larbi, S.; Theret, M.; Saugues, A.; Dabadie, C.; Gsaier, L.; Ferry, A.; Rhein, P.; Gondin, J.; Sakamoto, K.; et al. AMPKα2 is a skeletal muscle stem cell intrinsic regulator of myonuclear accretion. iScience 2023, 26, 108343. [Google Scholar] [CrossRef]


| Primers | Sequence 5′-3′ |
|---|---|
| RYR1_Fw | TGTTTGACCATCTCCCCTTCTG |
| RYR1_Rv | AAGGAAGTAGCCTTGGTGTGG |
| PAX7_Fw | AGGATGATGAGACCCGGCCC |
| PAX7_Rv | GGTCGACCGTTGATGAAGACCC |
| MYOD_Fw | ACTCTCACGGCTTGGGTTGAGG |
| MYOD_Rv | TCGGGGCCTGTCAAGTCTATGTC |
| MYOG_Fw | TTGCTCAGCTCCCTCAACCAGG |
| MYOG_Rv | GAGGCGCTGTGGGAGTTGCA |
| MHC1_Fw | CTGCACCAGCTGAGGTGTAA |
| MHC1_Rv | TCTAGGAGCCCCAGAAGACC |
| Desmin_Fw | AGGCTCAAGGCCAAACTACAGGAG |
| Desmin_Rv | ATCCACATCCGCTCGGAAGG |
| MYMK_Fw | CGTGACATTCTGGAGTACTTCAGCATC |
| MYMK_Rv | GAAGGTCGATCTCTGGGGTTCATC |
| MYMX_Fw | GTTAGAACTGGTGAGCAGGAG |
| MYMX_Rv | CCATCGGGAGCAATGGAA |
| ACTB_Fw | CTAAGGCCAACCGTGAAAAG |
| ACTB_Rv | ACCAGAGGCATACAGGGACA |
| GAPDH_Fw | CGTGCCGCCTGGAGAAAC |
| GAPDH_Rv | TGGGAGTTGCTGTTGAAGTCG |
| HPRT_Fw | CCTAATCATTATGCCGAGGATTTGG |
| HPRT_Rv | CCCATCTCCTTCATGACATCTCGAG |
| RYR3_Fw | CCACTGAGCTGGTCCACTTT |
| RYR3_Rv | GGGTTCGTCCTTGCCGATAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tourel, A.; Reynaud-Dulaurier, R.; Brocard, J.; Fauré, J.; Marty, I.; Petiot, A. RyR1 Is Involved in the Control of Myogenesis. Cells 2025, 14, 158. https://doi.org/10.3390/cells14030158
Tourel A, Reynaud-Dulaurier R, Brocard J, Fauré J, Marty I, Petiot A. RyR1 Is Involved in the Control of Myogenesis. Cells. 2025; 14(3):158. https://doi.org/10.3390/cells14030158
Chicago/Turabian StyleTourel, Amandine, Robin Reynaud-Dulaurier, Julie Brocard, Julien Fauré, Isabelle Marty, and Anne Petiot. 2025. "RyR1 Is Involved in the Control of Myogenesis" Cells 14, no. 3: 158. https://doi.org/10.3390/cells14030158
APA StyleTourel, A., Reynaud-Dulaurier, R., Brocard, J., Fauré, J., Marty, I., & Petiot, A. (2025). RyR1 Is Involved in the Control of Myogenesis. Cells, 14(3), 158. https://doi.org/10.3390/cells14030158







