Origin, Function, and Implications of Intestinal and Hepatic Macrophages in the Pathogenesis of Alcohol-Associated Liver Disease
Abstract
1. Introduction
1.1. History of Alcohol Use
1.2. Epidemiology and Drinking Patterns
1.3. Alcohol-Associated Liver Disease
2. Macrophage Heterogeneity
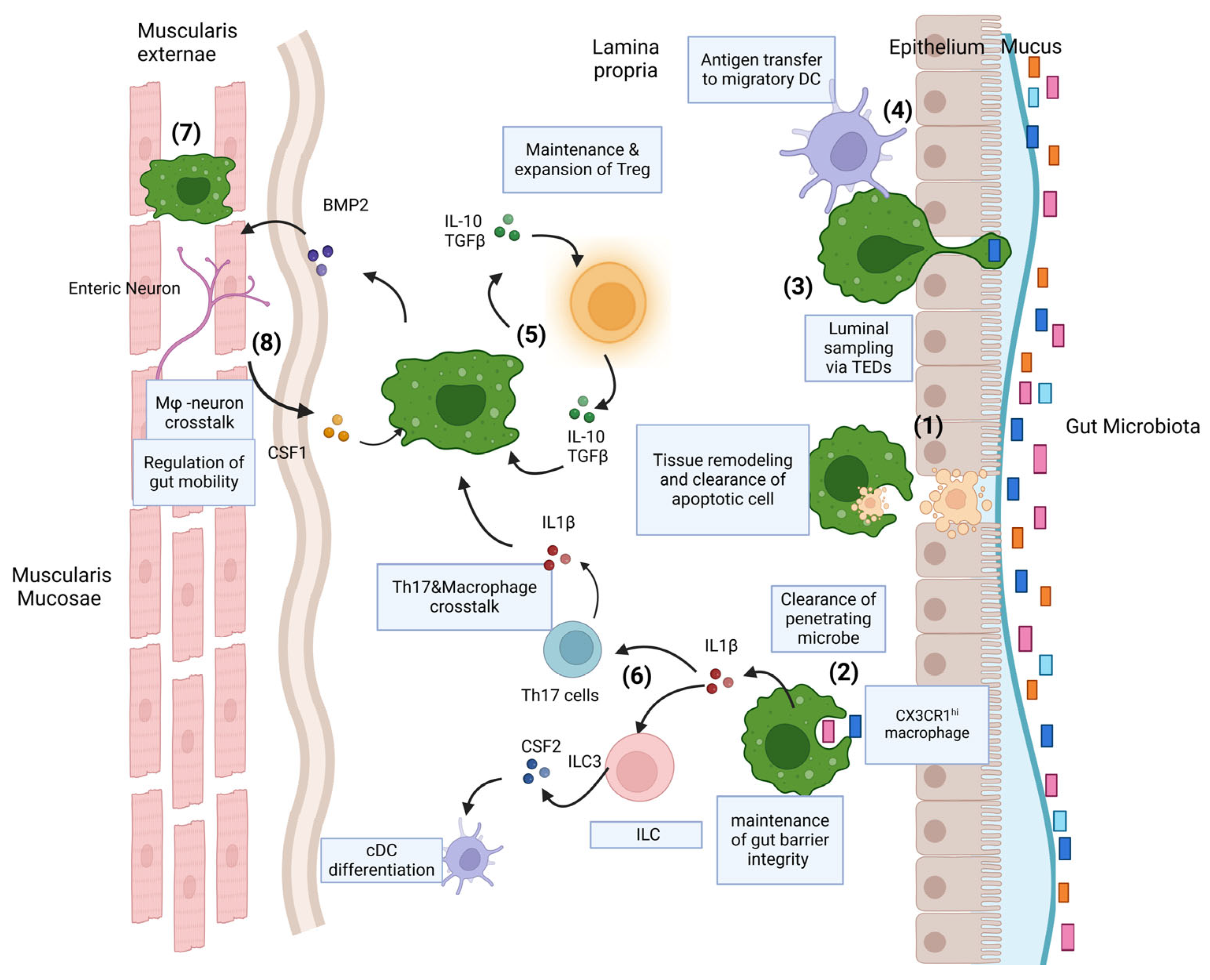
3. The Dynamics of Intestinal Macrophages
3.1. Function of Gut Macrophages
3.2. Gut Macrophages and Crosstalk with Other Immune Cells
3.3. Gut Macrophage Crosstalk with Enteric Nervous System
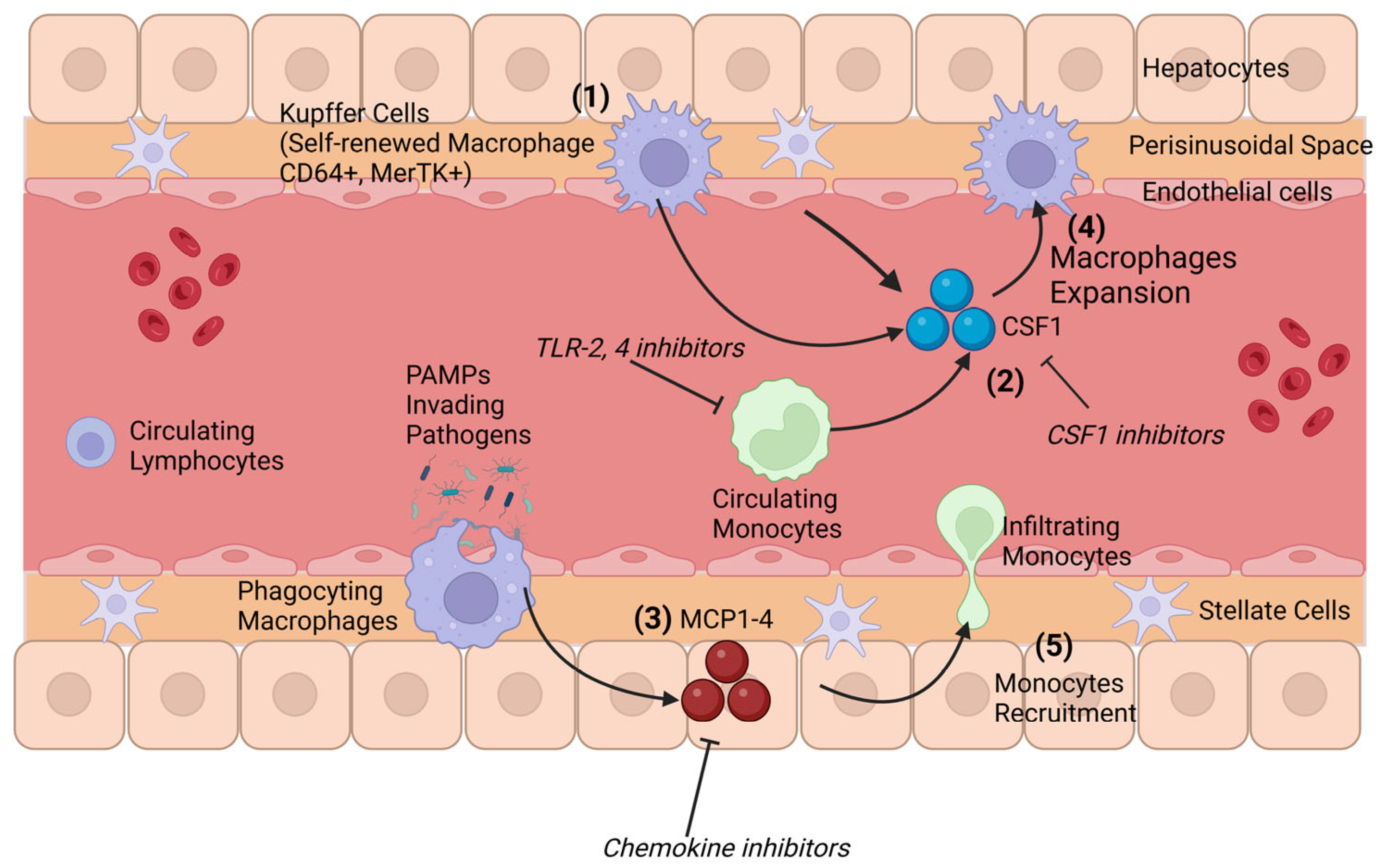
4. Origin and Composition of Hepatic Macrophages
Hepatic Macrophages and Crosstalk with the Gut Microbiota
5. Identifying Macrophages in the Liver and Intestines
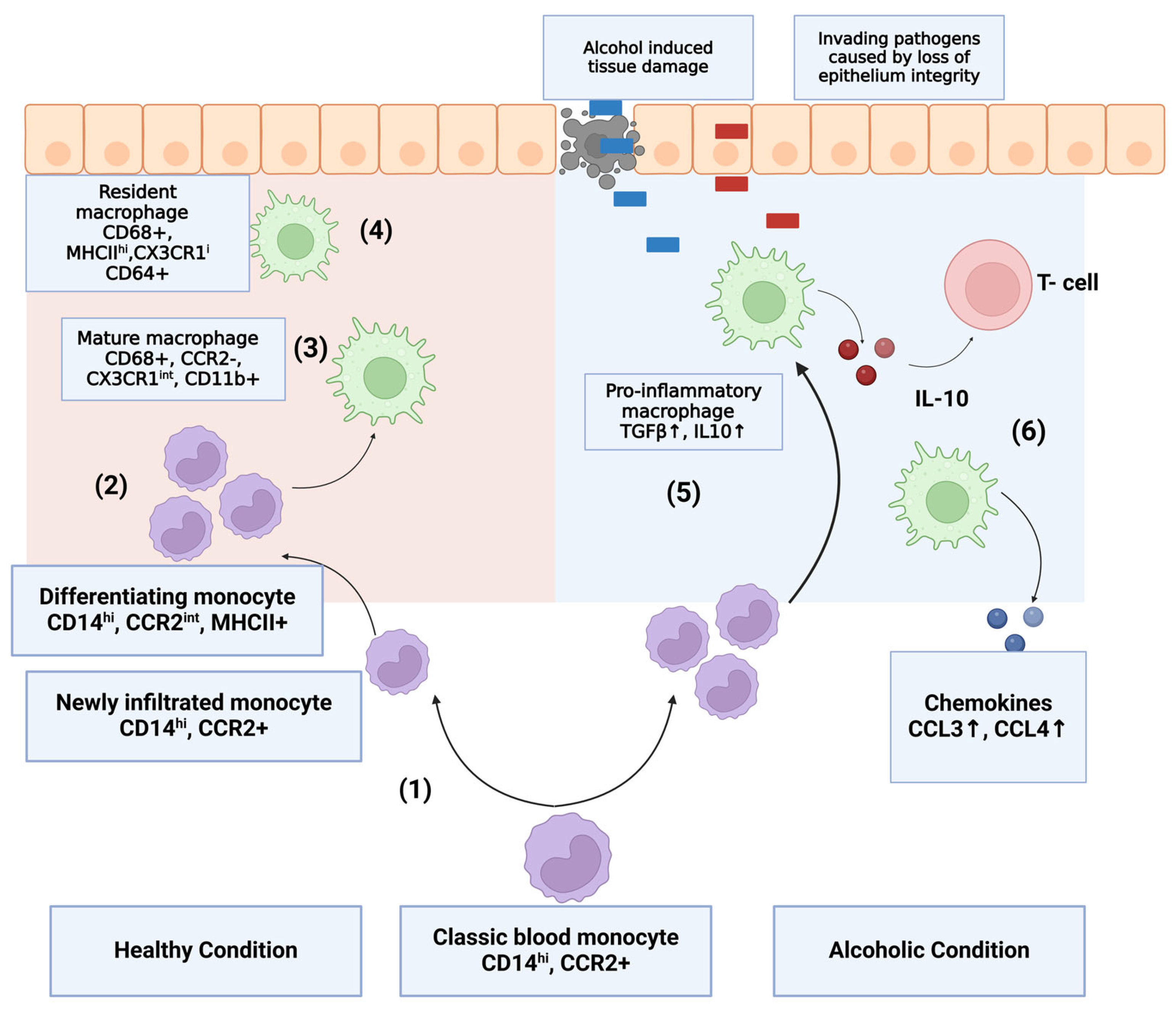
6. Monocytes and Macrophages in Alcohol Use Disorder and Alcohol-Induced Liver Disease
6.1. Implication in Intestinal and Hepatic Inflammation
6.2. Macrophage Activation in Alcohol-Induced Liver Disease
6.3. Micro RNA as a Modulator of Alcohol Response in Macrophages
7. Limitation of Animal Models in ALD Studies
8. Therapeutic Interventions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Jiang, L.; Sun, H. Early evidence for beer drinking in a 9000-year-old platform mound in southern China. PLoS ONE 2021, 16, e0255833. [Google Scholar] [CrossRef]
- Joffe, A.H. Alcohol and Social Complexity in Ancient Western Asia. Curr. Anthropol. 1998, 39, 297–322. [Google Scholar] [CrossRef]
- Khaderi, S.A. Introduction: Alcohol and Alcoholism. Clin. Liver Dis. 2019, 23, 1–10. [Google Scholar] [CrossRef]
- The Alcohol Industry in Data. Available online: https://alcohol.org/guides/the-alcohol-industry-in-data/ (accessed on 9 March 2023).
- Bosron, W.F.; Crabb, D.W.; Ting-Kai, L.i. Relationship between kinetics of liver alcohol dehydrogenase and alcohol metabolism. Pharmacol. Biochem. Behav. 1983, 18 (Suppl. 1), 223–227. [Google Scholar] [CrossRef]
- Hingson, R.W.; Heeren, T.; Winter, M.R. Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Arch. Pediatr. Adolesc. Med. 2006, 160, 739–746. [Google Scholar] [CrossRef]
- Chrostek, L.; Panasiuk, A. Liver fibrosis markers in alcoholic liver disease. World J. Gastroenterol. 2014, 20, 8018. [Google Scholar] [CrossRef]
- Agarwal, K.; Kontorinis, N.; Dieterich, D.T. Alcoholic Hepatitis. Curr. Treat. Options Gastroenterol. 2023, 7, 451–458. [Google Scholar] [CrossRef]
- Maccioni, L.; Gao, B.; Leclercq, S.; Pirlot, B.; Horsmans, Y.; De Timary, P.; Leclercq, I.; Fouts, D.; Schnabl, B.; Stärkel, P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020, 12, 1782157. [Google Scholar] [CrossRef]
- Van Furth, R.; Cohn, Z.A.; Hirsch, J.G.; Humphrey, J.H.; Spector, W.G.; Langevoort, H.L. The mononuclear phagocyte system: A new classification of macrophages, monocytes, and their precursor cells. Bull. World Health Organ. 1972, 46, 845. [Google Scholar]
- Van Furth, R.; Cohn, Z.A. The Origin and Kinetics of Mononuclear Phagocytes. J. Exp. Med. 1968, 128, 415–435. [Google Scholar] [CrossRef]
- Kuziel, W.A.; Morgan, S.J.; Dawson, T.C.; Griffin, S.; Smithies, O.; Ley, K.; Maeda, N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA 1997, 94, 12053–12058. [Google Scholar] [CrossRef]
- Takahashi, K. Development and Differentiation of Macrophages and Related Cells: Historical Review and Current Concepts. J. Clin. Exp. Hematop. 2001, 41, 1–31. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef]
- Hattori, Y. The microglia-blood vessel interactions in the developing brain. Neurosci. Res. 2023, 187, 58–66. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2014, 518, 547–551. [Google Scholar] [CrossRef]
- Kang, B.; Alvarado, L.J.; Kim, T.; Lehmann, M.; Cho, H.; He, J.; Li, P.; Larochelle, A.; Kelsall, B.L. Commensal microbiota drive the functional diversification of colon macrophages. Mucosal Immunol. 2020, 13, 216. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Zhang, J.; He, Y.; Yang, X.; Nie, Y.; Sun, L. Crosstalk between gut microbiota and gut resident macrophages in inflammatory bowel disease. J. Transl. Intern. Med. 2023, 11, 382. [Google Scholar] [CrossRef]
- Chen, Q.; Nair, S.; Ruedl, C. Microbiota regulates the turnover kinetics of gut macrophages in health and inflammation. Life Sci. Alliance 2022, 5, e202101178. [Google Scholar] [CrossRef]
- Scott, N.A.; Andrusaite, A.; Andersen, P.; Lawson, M.; Alcon-Giner, C.; Leclaire, C.; Caim, S.; Le Gall, G.; Shaw, T.; Connolly, J.P.R.; et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci. Transl. Med. 2018, 10, eaao4755. [Google Scholar] [CrossRef]
- Bander ZAl Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, X.; Li, L. Crosstalk between liver macrophages and gut microbiota: An important component of inflammation-associated liver diseases. Front. Cell Dev. Biol. 2022, 10, 1070208. [Google Scholar] [CrossRef]
- Schridde, A.; Bain, C.C.; Mayer, J.U.; Montgomery, J.; Pollet, E.; Denecke, B.; Milling, S.W.F.; Jenkins, S.J.; Dalod, M.; Henri, S.; et al. Tissue-specific differentiation of colonic macrophages requires TGFβ receptor-mediated signaling. Mucosal Immunol. 2017, 10, 1387–1399. [Google Scholar] [CrossRef]
- Kumawat, A.K.; Yu, C.; Mann, E.A.; Schridde, A.; Finnemann, S.C.; Mowat, A.M.I. Expression and characterization of αvβ5 integrin on intestinal macrophages. Eur. J. Immunol. 2018, 48, 1181–1187. [Google Scholar] [CrossRef]
- Bujko, A.; Atlasy, N.; Landsverk, O.J.B.; Richter, L.; Yaqub, S.; Horneland, R.; Øyen, O.; Aandahl, E.M.; Aabakken, L.; Stunnenberg, H.G.; et al. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J. Exp. Med. 2018, 215, 441–458. [Google Scholar] [CrossRef]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef]
- Branchett, W.J.; Saraiva, M.; O’Garra, A. Regulation of inflammation by Interleukin-10 in the intestinal and respiratory mucosa. Curr. Opin. Immunol. 2024, 91, 102495. [Google Scholar] [CrossRef]
- Niess, J.H.; Brand, S.; Gu, X.; Landsman, L.; Jung, S.; McCormick, B.A.; Vyas, J.M.; Boes, M.; Ploegh, H.L.; Fox, J.G.; et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005, 307, 254–258. [Google Scholar] [CrossRef]
- Chieppa, M.; Rescigno, M.; Huang, A.Y.C.; Germain, R.N. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 2006, 203, 2841–2852. [Google Scholar] [CrossRef]
- Kim, K.W.; Vallon-Eberhard, A.; Zigmond, E.; Farache, J.; Shezen, E.; Shakhar, G.; Ludwig, A.; Lira, S.A.; Jung, S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 2011, 118, e156–e167. [Google Scholar] [CrossRef]
- Ulevitch, R.J.; Tobias, P.S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 1995, 13, 437–457. [Google Scholar] [CrossRef]
- Mazzini, E.; Massimiliano, L.; Penna, G.; Rescigno, M. Oral Tolerance Can Be Established via Gap Junction Transfer of Fed Antigens from CX3CR1+ Macrophages to CD103+ Dendritic Cells. Immunity 2014, 40, 248–261. [Google Scholar] [CrossRef]
- He, J.; Song, Y.; Li, G.; Xiao, P.; Liu, Y.; Xue, Y.; Cao, Q.; Tu, X.; Pan, T.; Jiang, Z.; et al. Fbxw7 increases CCL2/7 in CX3CR1hi macrophages to promote intestinal inflammation. J. Clin. Investig. 2019, 129, 3877. [Google Scholar] [CrossRef]
- Asano, K.; Takahashi, N.; Ushiki, M.; Monya, M.; Aihara, F.; Kuboki, E.; Moriyama, S.; Iida, M.; Kitamura, H.; Qiu, C.; et al. Intestinal CD169+ macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat. Commun. 2015, 6, 7802. [Google Scholar] [CrossRef]
- Viola, M.F.; Boeckxstaens, G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut 2021, 70, 1383. [Google Scholar] [CrossRef]
- Lewis, S.A.; Sureshchandra, S.; Doratt, B.; Jimenez, V.A.; Stull, C.; Grant, K.A.; Messaoudi, I. Transcriptional, Epigenetic, and Functional Reprogramming of Monocytes from Non-Human Primates Following Chronic Alcohol Drinking. Front. Immunol. 2021, 12, 724015. [Google Scholar] [CrossRef]
- Maccioni, L.; Gao, B.; Leclercq, S.; Stärkel, P. Host Factors in Dysregulation of the Gut Barrier Function during Alcohol-Associated Liver Diseasent. J. Mol. Sci. 2021, 22, 12687. [Google Scholar] [CrossRef]
- Purohit, V.; Bode, J.C.; Bode, C.; Brenner, D.A.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, Intestinal Bacterial Growth, Intestinal Permeability to Endotoxin, and Medical Consequences: Summary of a Symposium. Alcohol 2008, 42, 349. [Google Scholar] [CrossRef]
- Tuomisto, S.; Pessi, T.; Collin, P.; Vuento, R.; Aittoniemi, J.; Karhunen, P.J. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Cosovanu, C.; Neumann, C. The Many Functions of Foxp3+ Regulatory T Cells in the Intestine. Front. Immunol. 2020, 11, 600973. [Google Scholar] [CrossRef]
- Worbs, T.; Bode, U.; Yan, S.; Hoffmann, M.W.; Hintzen, G.; Bernhardt, G.; Förster, R.; Pabst, O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 2006, 203, 519–527. [Google Scholar] [CrossRef]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3+ Regulatory T Cells in the Lamina Propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef]
- Kim, M.; Galan, C.; Hill, A.A.; Wu, W.J.; Fehlner-Peach, H.; Song, H.W.; Schady, D.; Bettini, M.L.; Simpson, K.W.; Longman, R.S.; et al. Critical Role for the Microbiota in CX3CR1+ Intestinal Mononuclear Phagocyte Regulation of Intestinal T Cell Responses. Immunity 2018, 49, 151–163. [Google Scholar] [CrossRef]
- Zigmond, E.; Bernshtein, B.; Friedlander, G.; Walker, C.R.; Yona, S.; Kim, K.W.; Brenner, O.; Krauthgamer, R.; Varol, C.; Müller, W.; et al. Macrophage-Restricted Interleukin-10 Receptor Deficiency, but Not IL-10 Deficiency, Causes Severe Spontaneous Colitis. Immunity 2014, 40, 720–733. [Google Scholar] [CrossRef]
- Shaw, M.H.; Kamada, N.; Kim, Y.G.; Núñez, G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 2012, 209, 251–258. [Google Scholar] [CrossRef]
- Panea, C.; Farkas, A.M.; Goto, Y.; Abdollahi-Roodsaz, S.; Lee, C.; Koscsó, B.; Gowda, K.; Hohl T., M.; Bogunovic, M.; Ivanov, I.I. Intestinal Monocyte-Derived Macrophages Control Commensal-Specific Th17 Responses. Cell Rep. 2015, 12, 1314–1324. [Google Scholar] [CrossRef]
- Schulz, O.; Jaensson, E.; Persson, E.K.; Liu, X.; Worbs, T.; Agace, W.W.; Pabst, O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 2009, 206, 3101–3114. [Google Scholar] [CrossRef]
- Gao, B.; Ahmad, M.F.; Nagy, L.E.; Tsukamoto, H. Inflammatory pathways in alcoholic steatohepatitis. J. Hepatol. 2019, 70, 249–259. [Google Scholar] [CrossRef]
- Mikkelsen, H.B.; Rumessen, J.J. Characterization of macrophage-like cells in the external layers of human small and large intestine. Cell Tissue Res. 1992, 270, 273–279. [Google Scholar] [CrossRef]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Schmidt, I.; Boeckx, B.; Dierckx de Casterlé, I.; et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 2018, 175 (Suppl. 1), 400–415.e13. [Google Scholar] [CrossRef]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef]
- Muller, P.A.; Koscsó, B.; Rajani, G.M.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Schmidt, I.; Boeckx, B.; Dierckx de Casterlé, I.; et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 2014, 158, 300–313. [Google Scholar] [CrossRef]
- Blériot, C.; Ginhoux, F. Understanding the Heterogeneity of Resident Liver Macrophages. Front. Immunol. 2019, 10, 2694. [Google Scholar] [CrossRef]
- Ishibashi, H.; Nakamura, M.; Komori, A.; Migita, K.; Shimoda, S. Liver architecture, cell function, and disease. Semin. Immunopathol. 2009, 31, 399–409. [Google Scholar] [CrossRef]
- Scott, C.L.; Zheng, F.; De Baetselier, P.; Martens, L.; Saeys, Y.; De Prijck, S.; Lippens, S.; Abels, C.; Schoonooghe, S.; Raes, G.; et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 2016, 7, 10321. [Google Scholar] [CrossRef]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef]
- Schulz, C.; Perdiguero, E.G.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.; Pollard, J.W.; et al. A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science 2012, 335, 86–90. [Google Scholar] [CrossRef]
- David, B.A.; Rezende, R.M.; Antunes, M.M.; Santos, M.M.; Freitas Lopes, M.A.; Diniz, A.B.; Sousa Pereira, R.V.; Marchesi, S.C.; Alvarenga, D.M.; Nakagaki, B.N.; et al. Combination of Mass Cytometry and Imaging Analysis Reveals Origin, Location, and Functional Repopulation of Liver Myeloid Cells in Mice. Gastroenterology 2016, 151, 1176–1191. [Google Scholar] [CrossRef]
- Sierro, F.; Evrard, M.; Rizzetto, S.; Melino, M.; Mitchell, A.J.; Florido, M.; Beattie, L.; Walters, S.B.; Tay, S.S.; Lu, B.; et al. A Liver Capsular Network of Monocyte-Derived Macrophages Restricts Hepatic Dissemination of Intraperitoneal Bacteria by Neutrophil Recruitment. Immunity 2017, 47, 374–388. [Google Scholar] [CrossRef]
- Nolan, J.P. The role of intestinal endotoxin in liver injury: A long and evolving history. Hepatology 2010, 52, 1829–1835. [Google Scholar] [CrossRef]
- Soares, J.B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659. [Google Scholar] [CrossRef]
- Kawaratani, H.; Tsujimoto, T.; Douhara, A.; Takaya, H.; Moriya, K.; Namisaki, T.; Noguchi, R.; Yoshiji, H.; Fujimoto, M.; Fukui, H. The effect of inflammatory cytokines in alcoholic liver disease. Mediat. Inflamm. 2013, 2013, 495156. [Google Scholar] [CrossRef]
- Slevin, E.; Baiocchi, L.; Wu, N.; Ekser, B.; Sato, K.; Lin, E.; Ceci, L.; Chen, L.; Lorenzo, S.R.; Xu, W.; et al. Kupffer Cells: Inflammation Pathways and Cell-Cell Interactions in Alcohol-Associated Liver Disease. Am. J. Pathol. 2020, 190, 2185–2193. [Google Scholar] [CrossRef]
- Singh, V.; Huang, E.; Pathak, V.; Willard, B.B.; Allende, D.S.; Nagy, L.E. Phosphoproteomics identifies pathways underlying the role of receptor-interaction protein kinase 3 in alcohol-associated liver disease and uncovers apoptosis signal-regulating kinase 1 as a target. Hepatol. Commun. 2022, 6, 2022–2041. [Google Scholar] [CrossRef]
- Roychowdhury, S.; McMullen, M.R.; Pisano, S.G.; Liu, X.; Nagy, L.E. Absence of receptor-interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 2013, 57, 1773. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; McMullen, M.R.; Miyata, T.; Kim, A.; Pathak, V.; Wu, J.; Day, L.Z.; Hardesty, J.E.; Welch, N.; et al. Macrophage-derived MLKL in alcohol-associated liver disease: Regulation of phagocytosis. Hepatology 2023, 77, 902. [Google Scholar] [CrossRef]
- Duan, Y.; Chu, H.; Brandl, K.; Jiang, L.; Zeng, S.; Meshgin, N.; Papachristoforou, E.; Argemi, J.; Mendes, B.G.; Wang, Y.; et al. CRIg on liver macrophages clears pathobionts and protects against alcoholic liver disease. Nat. Commun. 2021, 12, 7172. [Google Scholar] [CrossRef]
- Luo, Z.; Ji, Y.; Gao, H.; Gomes Dos Reis, F.C.; Bandyopadhyay, G.; Jin, Z.; Ly, C.; Chang, Y.J.; Zhang, D.; Kumar, D.; et al. CRIg+ Macrophages Prevent Gut Microbial DNA-Containing Extracellular Vesicle-Induced Tissue Inflammation and Insulin Resistance. Gastroenterology 2021, 160, 863–874. [Google Scholar] [CrossRef]
- Enomoto, N.; Ikejima, K.; Bradford, B.; Rivera, C.; Kono, H.; Brenner, D.A.; Thurman, R.G. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology 1998, 115, 443–451. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Toh, E.; Ross, R.A.; Heathers, L.E.; Chandler, K.; Oshodi, A.; McGee, B.; Modlik, E.; Linton, T.; Mangiacarne, D.; et al. Quantity of alcohol drinking positively correlates with serum levels of endotoxin and markers of monocyte activation. Sci. Rep. 2017, 7, 4462. [Google Scholar] [CrossRef]
- Mandrekar, P.; Bala, S.; Catalano, D.; Kodys, K.; Szabo, G. The Opposite Effects of Acute and Chronic Alcohol on Lipopolysaccharide-Induced Inflammation Are Linked to IRAK-M in Human Monocytes. J. Immunol. 2009, 183, 1320–1327. [Google Scholar] [CrossRef]
- Bala, S.; Tang, A.; Catalano, D.; Petrasek, J.; Taha, O.; Kodys, K.; Szabo, G. Induction of Bcl-3 by acute binge alcohol results in Toll-like receptor 4/LPS tolerance. J. Leukoc. Biol. 2012, 92, 611–620. [Google Scholar] [CrossRef]
- Ju, C.; Mandrekar, P. Macrophages and Alcohol-Related Liver Inflammation. Alcohol. Res. 2015, 37, 251. Available online: https://pubmed.ncbi.nlm.nih.gov/26717583/ (accessed on 3 April 2024).
- Saha, B.; Momen-Heravi, F.; Kodys, K.; Szabo, G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. J. Biol. Chem. 2016, 291, 149–159. [Google Scholar] [CrossRef]
- Szabo, G.; Petrasek, J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 387–400. [Google Scholar] [CrossRef]
- Hume, D.A.; Perry, V.H.; Gordon, S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: Macrophages associated with epithelia. Anat. Rec. 1984, 210, 503–512. [Google Scholar] [CrossRef]
- McGarry, M.P.; Stewart, C.C. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J. Leukoc. Biol. 1991, 50, 471–478. [Google Scholar] [CrossRef]
- Gottfried, E.; Kunz-Schughart, L.A.; Weber, A.; Rehli, M.; Peuker, A.; Müller, A.; Kastenberger, M.; Brockhoff, G.; Andreesen, R.; Kreutz, M. Expression of CD68 in Non-Myeloid Cell Types. Scand. J. Immunol. 2008, 67, 453–463. [Google Scholar] [CrossRef]
- Itoh, M.; Suganami, T.; Kato, H.; Kanai, S.; Shirakawa, I.; Sakai, T.; Goto, T.; Asakawa, M.; Hidaka, I.; Sakugawa, H.; et al. CD11c+ resident macrophages drive hepatocyte death-triggered liver fibrosis in a murine model of nonalcoholic steatohepatitis. JCI Insight 2017, 2, e92902. [Google Scholar] [CrossRef]
- Ju, C.; Tacke, F. Hepatic macrophages in homeostasis and liver diseases: From pathogenesis to novel therapeutic strategies. Cell. Mol. Immunol. 2016, 13, 316–327. [Google Scholar] [CrossRef]
- Pabst, O.; Bernhardt, G. The puzzle of intestinal lamina propria dendritic cells and macrophages. Eur. J. Immunol. 2010, 40, 2107–2111. [Google Scholar] [CrossRef]
- Gautiar, E.L.; Shay, T.; Miller, J.; Kanai, S.; Shirakawa, I.; Sakai, T.; Goto, T.; Asakawa, M.; Hidaka, I.; Sakugawa, H.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Tamoutounour, S.; Henri, S.; Lelouard, H.; de Bovis, B.; de Haar, C.; van der Woude, C.J.; Woltman, A.M.; Reyal, Y.; Bonnet, D.; Sichien, D.; et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012, 42, 3150–3166. [Google Scholar] [CrossRef]
- Langlet, C.; Tamoutounour, S.; Henri, S.; de Bovis, B.; de Haar, C.; van der Woude, C.J.; Woltman, A.M.; Reyal, Y.; Bonnet, D.; Sichien, D.; et al. CD64 Expression Distinguishes Monocyte-Derived and Conventional Dendritic Cells and Reveals Their Distinct Role during Intramuscular Immunization. J. Immunol. 2012, 188, 1751–1760. [Google Scholar] [CrossRef]
- González-Domínguez érika Samaniego, R.; Flores-Sevilla, J.L.; Campos-Campos, S.F.; Gómez-Campos, G.; Salas, A.; Campos-Peña, V.; Corbí, Á.L.; Sánchez-Mateos, P.; Sánchez-Torres, C. CD163L1 and CLEC5A discriminate subsets of human resident and inflammatory macrophages in vivo. J. Leukoc. Biol. 2015, 98, 453–466. [Google Scholar] [CrossRef]
- Scott, C.L.; Bain, C.C.; Wright, P.B.; Sichien, D.; Kotarsky, K.; Persson, E.K.; Luda, K.; Guilliams, M.; Lambrecht, B.N.; Agace, W.W.; et al. CCR2+CD103− intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol. 2015, 8, 327–339. [Google Scholar] [CrossRef]
- Schlitzer, A.; McGovern, N.; Teo, P.; Zelante, T.; Atarashi, K.; Low, D.; Ho, A.W.; See, P.; Shin, A.; Wasan, P.S.; et al. IRF4 Transcription Factor-Dependent CD11b+ Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity 2013, 38, 970–983. [Google Scholar] [CrossRef]
- Bohaud, C.; Johansen, M.D.; Jorgensen, C.; Kremer, L.; Ipseiz, N.; Djouad, F. The Role of Macrophages During Mammalian Tissue Remodeling and Regeneration Under Infectious and Non-Infectious Conditions. Front. Immunol. 2021, 12, 707856. [Google Scholar] [CrossRef]
- Cerovic, V.; Houston, S.A.; Scott, C.L.; Aumeunier, A.; Yrlid, U.; Mowat, A.M.; Milling, S.W. Intestinal CD103− dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 2013, 6, 104–113. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Artis, D.; Mowat, A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef]
- Varol, C.; Vallon-Eberhard, A.; Elinav, E.; Aychek, T.; Shapira, Y.; Luche, H.; Fehling, H.J.; Hardt, W.D.; Shakhar, G.; Jung, S. Intestinal Lamina Propria Dendritic Cell Subsets Have Different Origin and Functions. Immunity 2009, 31, 502–512. [Google Scholar] [CrossRef]
- Bogunovic, M.; Ginhoux, F.; Helft, J.; Shang, L.; Hashimoto, D.; Greter, M.; Liu, K.; Jakubzick, C.; Ingersoll, M.A.; Leboeuf, M.; et al. Origin of the Lamina Propria Dendritic Cell Network. Immunity 2009, 31, 513–525. [Google Scholar] [CrossRef]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K.; et al. Unique CD14+ intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Investig. 2008, 118, 2269–2280. [Google Scholar] [CrossRef]
- Zigmond, E.; Varol, C.; Farache, J.; Elmaliah, E.; Satpathy, A.T.; Friedlander, G.; Mack, M.; Shpigel, N.; Boneca, I.G.; Murphy, K.M.; et al. Ly6Chi Monocytes in the Inflamed Colon Give Rise to Proinflammatory Effector Cells and Migratory Antigen-Presenting Cells. Immunity 2012, 37, 1076–1090. [Google Scholar] [CrossRef]
- Shaw, T.N.; Houston, S.A.; Wemyss, K.; Bridgeman, H.M.; Barbera, T.A.; Zangerle-Murray, T.; Strangward, P.; Ridley, A.J.L.; Wang, P.; Tamoutounour, S.; et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med. 2018, 215, 1507–1518. [Google Scholar] [CrossRef]
- Hume, D.A.; Allan, W.; Hogan, P.G.; Doe, W.F. Immunohistochemical Characterisation of Macrophages in Human Liver and Gastrointestinal Tract: Expression of CD4, HLA-DR, OKM1, and the Mature Macrophage Marker 25F9 in Normal and Diseased Tissue. J. Leukoc. Biol. 1987, 42, 474–484. [Google Scholar] [CrossRef]
- Landsman, L.; Liat, B.O.; Zernecke, A.; Kim, K.W.; Krauthgamer, R.; Shagdarsuren, E.; Lira, S.A.; Weissman, I.L.; Weber, C.; Jung, S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 2009, 113, 963–972. [Google Scholar] [CrossRef]
- Collison, J.L.; Carlin, L.M.; Eichmann, M.; Geissmann, F.; Peakman, M. Heterogeneity in the Locomotory Behavior of Human Monocyte Subsets over Human Vascular Endothelium In Vitro. J. Immunol. 2015, 195, 1162–1170. [Google Scholar] [CrossRef]
- Enomoto, N.; Ikejima, K.; Yamashina, S.; Hirose, M.; Shimizu, H.; Kitamura, T.; Takei, Y.; Sato And, N.; Thurman, R.G. Kupffer Cell Sensitization by Alcohol Involves Increased Permeability to Gut-Derived Endotoxin. Alcohol Clin. Exp. Res. 2001, 25 (Suppl. 6), S51–S54. [Google Scholar] [CrossRef]
- Nagy, L.E. Recent Insights into the Role of the Innate Immune System in the Development of Alcoholic Liver Disease. Exp. Biol. Med. 2003, 228, 882–890. [Google Scholar] [CrossRef]
- Mandrekar, P.; Ambade, A.; Lim, A.; Szabo, G.; Catalano, D. An essential role for MCP-1 in alcoholic liver injury: Regulation of pro-inflammatory cytokines and hepatic steatosis. Hepatology 2011, 54, 2185. [Google Scholar] [CrossRef]
- Maccioni, L.; Kasavuli, J.; Leclercq, S.; Pirlot, B.; Laloux, G.; Horsmans, Y.; Leclercq, I.; Schnabl, B.; Stärkel, P. Toll-like receptor 2 activation in monocytes contributes to systemic inflammation and alcohol-associated liver disease in humans. Hepatol. Commun. 2023, 7, e0107. [Google Scholar] [CrossRef]
- Antón, M.; Rodríguez-González, A.; Ballesta, A.; González, N.; Del Pozo, A.; de Fonseca, F.R.; Gómez-Lus, M.L.; Leza, J.C.; García-Bueno, B.; Caso, J.R.; et al. Alcohol binge disrupts the rat intestinal barrier: The partial protective role of oleoylethanolamide. Br. J. Pharmacol. 2018, 175, 4464–4479. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Nie, J.; Lv, D.; Wang, T.; Xu, Y. Toll-like receptor 4 increases intestinal permeability through up-regulation of membrane PKC activity in alcoholic steatohepatitis. Alcohol 2013, 47, 459–465. [Google Scholar] [CrossRef]
- Voss, J.K.; Li, Z.; Weinman, S.A. Elevated blood monocyte counts in alcohol-associated hepatitis. J. Gastroenterol. Hepatol. 2022, 6, 148. [Google Scholar] [CrossRef]
- Wang, M.; You, Q.; Lor, K.; Chen, F.; Gao, B.; Ju, C. Chronic alcohol ingestion modulates hepatic macrophage populations and functions in mice. J. Leukoc. Biol. 2014, 96, 657. [Google Scholar] [CrossRef]
- Janicova, A.; Haag, F.; Xu, B.; Garza, A.P.; Dunay, I.R.; Neunaber, C.; Nowak, A.J.; Cavalli, P.; Marzi, I.; Sturm, R.; et al. Acute Alcohol Intoxication Modulates Monocyte Subsets and Their Functions in a Time-Dependent Manner in Healthy Volunteers. Front. Immunol. 2021, 12, 652488. [Google Scholar] [CrossRef]
- Platt, A.M.; Bain, C.C.; Bordon, Y.; Sester, D.P.; Mowat, A.M. An Independent Subset of TLR Expressing CCR2-Dependent Macrophages Promotes Colonic Inflammation. J. Immunol. 2010, 184, 6843–6854. [Google Scholar] [CrossRef]
- Muzaki, A.R.B.M.; Tetlak, P.; Sheng, J.; Loh, S.C.; Setiagani, Y.A.; Poidinger, M.; Zolezzi, F.; Karjalainen, K.; Ruedl, C. Intestinal CD103+ CD11b- dendritic cells restrain colitis via IFN-γ-induced anti-inflammatory response in epithelial cells. Mucosal Immunol. 2016, 9, 336–351. [Google Scholar] [CrossRef]
- Medina-Contreras, O.; Geem, D.; Laur, O.; Williams, I.R.; Lira, S.A.; Nusrat, A.; Parkos, C.A.; Denning, T.L. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J. Clin. Investig. 2011, 121, 4787. [Google Scholar] [CrossRef]
- Alharshawi, K.; Fey, H.; Vogle, A.; Klenk, T.; Kim, M.; Aloman, C. Alcohol Consumption Accumulation of Monocyte Derived Macrophages in Female Mice Liver Is Interferon Alpha Receptor Dependent. Front. Immunol. 2021, 12, 663548. [Google Scholar] [CrossRef]
- Yip, J.L.K.; Balasuriya, G.K.; Spencer, S.J.; Hill-Yardin, E.L. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1701. [Google Scholar] [CrossRef]
- Shroka, T.M.; Kufareva, I.; Salanga, C.L.; Handel, T.M. The dual function chemokine receptor CCR2 drives migration and chemokine scavenging through distinct pathways. Sci. Signal 2023, 16, eabo4314. [Google Scholar] [CrossRef]
- Grimm, M.; Pullman, W.; Bennett, G.; Sullivan, P.; Pavli, P.; Doe, W. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J. Gastroenterol. Hepatol. 1995, 10, 387–395. [Google Scholar] [CrossRef]
- She, S.; Ren, L.; Chen, P.; Wang, M.; Chen, D.; Wang, Y.; Chen, H. Functional Roles of Chemokine Receptor CCR2 and Its Ligands in Liver Disease. Front. Immunol. 2022, 13, 812431. [Google Scholar] [CrossRef]
- Hoshi, N.; Schenten, D.; Nish, S.A.; Walther, Z.; Gagliani, N.; Flavell, R.A.; Reizis, B.; Shen, Z.; Fox, J.G.; Iwasaki, A.; et al. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nat. Commun. 2012, 3, 1120. [Google Scholar] [CrossRef]
- Weber, C.; Weber, K.S.C.; Klier, C.; Gu, S.; Wank, R.; Horuk, R.; Nelson, P.J. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and TH1-like/CD45RO+T cells. Blood 2001, 97, 1144–1146. [Google Scholar] [CrossRef]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef]
- Johnson, L.A.; Jackson, D.G. The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J. Cell Sci. 2013, 126, 5259–5270. [Google Scholar] [CrossRef]
- Ahmed, Y.A.; Lafdil, F.; Tacke, F. Ambiguous Pathogenic Roles of Macrophages in Alcohol-Associated Liver Diseases. Hepatic. Med. Evid. Res. 2023, 15, 113–127. [Google Scholar] [CrossRef]
- Tapia-Abellán, A.; Martínez-Esparza, M.; Ruiz-Alcaraz, A.J.; Hernández-Caselles, T.; Martínez-Pascual, C.; Miras-López, M.; Such, J.; Francés, R.; García-Peñarrubia, P. The peritoneal macrophage inflammatory profile in cirrhosis depends on the alcoholic or hepatitis C viral etiology and is related to ERK phosphorylation. BMC Immunol. 2012, 13, 1–9. [Google Scholar] [CrossRef]
- Luna-Casado, L.; Diez-Ruiz, A.; Gutierrez-Gea, F.; Santos-Perez, J.L.; Rico-Irles, J.; Wachter, H.; Fuchs, D. Increased peripheral mononuclear cells expression of adhesion molecules in alcoholic cirrhosis: Its relation to immune activation. J. Hepatol. 1997, 27, 477–483. [Google Scholar] [CrossRef]
- Fisher, N.C.; Neil, D.A.H.; Williams, A.; Adams, D.H. Serum concentrations and peripheral secretion of the beta chemokines monocyte chemoattractant protein 1 and macrophage inflammatory protein 1alpha in alcoholic liver disease. Gut 1999, 45, 416–420. [Google Scholar] [CrossRef]
- McClain, C.J.; Barve, S.; Deaciuc, I.; Kugelmas, M.; Hill, D. Cytokines in alcoholic liver disease. Semin. Liver Dis. 1999, 19, 205–219. [Google Scholar] [CrossRef]
- Zhang, Z.; Bagby, G.J.; Stoltz, D.; Oliver, P.; Schwarzenberger, P.O.; Kolls, J.K. Prolonged Ethanol Treatment Enhances Lipopolysaccharide/Phorbol Myristate Acetate-Induced Tumor Necrosis Factor-α Production in Human Monocytic Cells. Alcohol Clin. Exp. Res. 2001, 25, 444–449. [Google Scholar] [CrossRef]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef]
- Koop, D.R.; Klopfenstein, B.; Iimuro, Y.; Thurman, R.G. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol. Pharmacol. 1997, 51, 944–950. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Barve, S.; Joshi-Barve, S.; Uriarte, S.; Song, Z.; McClain, C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: Relevance to alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G681–G688. [Google Scholar] [CrossRef]
- Herrnreiter, C.J.; Luck, M.E.; Cannon, A.R.; Li, X.; Choudhry, M.A. Reduced Expression of miR-146a Potentiates Intestinal Inflammation following Alcohol and Burn Injury. J. Immunol. 2024, 212, 881–893. [Google Scholar] [CrossRef]
- Torres, J.L.; Novo-Veleiro, I.; Manzanedo, L.; Alvela-Suárez, L.; Macías, R.; Laso, F.J.; Marcos, M. Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 4104. [Google Scholar] [CrossRef]
- Bala, S.; Marcos, M.; Kodys, K.; Csak, T.; Catalano, D.; Mandrekar, P.; Szabo, G. Up-regulation of MicroRNA-155 in Macrophages Contributes to Increased Tumor Necrosis Factor α (TNFα) Production via Increased mRNA Half-life in Alcoholic Liver Disease. J. Biol. Chem. 2011, 286, 1436–1444. [Google Scholar] [CrossRef]
- Yin, H.; Liang, X.; Jogasuria, A.; Davidson, N.O.; You, M. miR-217 Regulates Ethanol-Induced Hepatic Inflammation by Disrupting Sirtuin 1–Lipin-1 Signaling. Am. J. Pathol. 2015, 185, 1286. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Catalano, D.; Talis, A.; Szabo, G.; Bala, S. Protective effect of LNA-anti-miR-132 therapy on liver fibrosis in mice. Mol. Ther. Nucleic Acids. 2021, 25, 155. [Google Scholar] [CrossRef]
- Bala, S.; Csak, T.; Kodys, K.; Catalano, D.; Ambade, A.; Furi, I.; Lowe, P.; Cho, Y.; Iracheta-Vellve, A.; Szabo, G. Alcohol-induced miR-155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J. Leukoc. Biol. 2017, 102, 487. [Google Scholar] [CrossRef]
- Bala, S.; Petrasek, J.; Csak, T.; Catalano, D.; Kodys, K.; Mundkur, S.; Szabo, G. MicroRNA-155 regulates inflammation in alcoholic liver disease via targeting SOCS1 and SHIP1 (54.15). J. Immunol. 2012, 188 (Suppl. 1), 15–54. [Google Scholar] [CrossRef]
- Bala, S.; Szabo, G. MicroRNA Signature in Alcoholic Liver Disease. Int. J. Hepatol. 2012, 2012, 498232. [Google Scholar] [CrossRef]
- Saikia, P.; Roychowdhury, S.; Bellos, D.; Pollard, K.A.; McMullen, M.R.; McCullough, R.L.; McCullough, A.J.; Gholam, P.; de la Motte, C.; Nagy, L.E. Hyaluronic acid 35 normalizes TLR4 signaling in Kupffer cells from ethanol-fed rats via regulation of microRNA291b and its target Tollip. Sci. Rep. 2017, 7, 15671. [Google Scholar] [CrossRef]
- Saikia, P.; Bellos, D.; McMullen, M.R.; Pollard, K.A.; de la Motte, C.; Nagy, L.E. miR181b-3p and its target importin α5 regulate TLR4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology 2017, 66, 602. [Google Scholar] [CrossRef]
- Klieser, E.; Mayr, C.; Kiesslich, T.; Wissniowski, T.; Fazio, P.D.; Neureiter, D.; Ocker, M. The Crosstalk of miRNA and Oxidative Stress in the Liver: From Physiology to Pathology and Clinical Implications. Int. J. Mol. Sci. 2019, 20, 5266. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, L.; Forsyth, C.B.; Shaikh, M.; Song, S.; Keshavarzian, A. The Role of miR-212 And iNOS in Alcohol-Induced Intestinal Barrier Dysfunction and Steatohepatitis. Alcohol Clin. Exp. Res. 2015, 39, 1632. [Google Scholar] [CrossRef]
- Kim, A.; Saikia, P.; Nagy, L.E. MiRNAs involved in M1/M2 hyperpolarization are clustered and coordinately expressed in alcoholic hepatitis. Front. Immunol. 2019, 10, 457889. [Google Scholar] [CrossRef]
- Sureshchandra, S.; Rais, M.; Stull, C.; Grant, K.; Messaoudi, I. Transcriptome Profiling Reveals Disruption of Innate Immunity in Chronic Heavy Ethanol Consuming Female Rhesus Macaques. PLoS ONE 2016, 11, e0159295. [Google Scholar] [CrossRef]
- Demetrius, L. Of mice and men. EMBO Rep. 2005, 6 (Suppl. 1), S39. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Chen, J.Y.J.; Qiao, Y.; Komisar, J.L.; Baze, W.B.; Hsu, I.C.; Tseng, J. Increased susceptibility to staphylococcal enterotoxin B intoxication in mice primed with actinomycin D. Infect. Immun. 1994, 62, 4626. [Google Scholar] [CrossRef]
- Warren, H.S.; Fitting, C.; Hoff, E.; Adib-Conquy, M.; Beasley-Topliffe, L.; Tesini, B.; Liang, X.; Valentine, C.; Hellman, J.; Hayden, D.; et al. Resilience to bacterial infection: Difference between species could be due to proteins in serum. J. Infect. Dis. 2010, 201, 223. [Google Scholar] [CrossRef]
- Coers, J.; Starnbach, M.N.; Howard, J.C. Modeling Infectious Disease in Mice: Co-Adaptation and the Role of Host-Specific IFNγ Responses. PLoS Pathog. 2009, 5, e1000333. [Google Scholar] [CrossRef]
- Singh, S.B.; Davis, A.S.; Taylor, G.A.; Deretic, V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 2006, 313, 1438–1441. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef]
- Barish, G.D.; Downes, M.; Alaynick, W.A.; Yu, R.T.; Ocampo, C.B.; Bookout, A.L.; Mangelsdorf, D.J.; Evans, R.M. A Nuclear Receptor Atlas: Macrophage Activation. Mol. Endocrinol. 2005, 19, 2466–2477. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Schneemann, M.; Schoeden, G. Macrophage biology and immunology: Man is not a mouse. J. Leukoc. Biol. 2007, 81, 579. [Google Scholar] [CrossRef]
- Randal Bollinger, R.; Barbas, A.S.; Bush, E.L.; Lin, S.S.; Parker, W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J. Theor. Biol. 2007, 249, 826–831. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Song, M.; Chen, T.; Prough, R.A.; Cave, M.C.; Mcclain, C.J. Chronic alcohol consumption causes liver injury in high-fructose-fed male mice through enhanced hepatic inflammatory response. Alcohol Clin. Exp. Res. 2016, 40, 518. [Google Scholar] [CrossRef]
- Guo, F.; Zheng, K.; Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. The Lieber-DeCarli diet—a flagship model for experimental alcoholic liver disease. Alcohol Clin. Exp. Res. 2018, 42, 1828–1840. [Google Scholar] [CrossRef]
- Fujimoto, M.; Uemura, M.; Nakatani, Y.; Tsujita, S.; Hoppo, K.; Tamagawa, T.; Kitano, H.; Kikukawa, M.; Ann, T.; Ishii, Y.; et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: Relation to severity of liver disturbance. Alcohol Clin. Exp. Res. 2000, 24 (Suppl. S4), 48S–54S. [Google Scholar] [CrossRef]
- Marrs, T.; Walter, J. Pros and cons: Is faecal microbiota transplantation a safe and efficient treatment option for gut dysbiosis? Allergy 2021, 76, 2312–2317. [Google Scholar] [CrossRef]
- Bertolotti, M.; Ferrari, A.; Vitale, G.; Stefani, M.; Trenti, T.; Loria, P.; Carubbi, F.; Carulli, N.; Sternieri, E. Effect of liver cirrhosis on the systemic availability of naltrexone in humans. J. Hepatol. 1997, 27, 505–511. [Google Scholar] [CrossRef]
- Anton, R.F.; O’Malley, S.S.; Ciraulo, D.A.; Cisler, R.A.; Couper, D.; Donovan, D.M.; Gastfriend, D.R.; Hosking, J.D.; Johnson, B.A.; LoCastro, J.S.; et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA 2006, 295, 2003–2017. [Google Scholar] [CrossRef]
- Mason, B.J.; Heyser, C.J. Acamprosate: A prototypic neuromodulator in the treatment of alcohol dependence. CNS Neurol. Disord. Drug Targets 2010, 9, 23. [Google Scholar] [CrossRef]
- Bellar, A.; Welch, N.; Dasarathy, J.; Attaway, A.; Musich, R.; Kumar, A.; Sekar, J.; Mishra, S.; Sandlers, Y.; Streem, D.; et al. Peripheral blood mononuclear cell mitochondrial dysfunction in acute alcohol-associated hepatitis. Clin. Transl. Med. 2023, 13, e1276. [Google Scholar] [CrossRef]
- Ren, A.; He, W.; Rao, J.; Ye, D.; Cheng, P.; Jian, Q.; Fu, Z.; Zhang, X.; Deng, R.; Gao, Y.; et al. Dysregulation of innate cell types in the hepatic immune microenvironment of alcoholic liver cirrhosis. Front. Immunol. 2023, 14, 1034356. [Google Scholar] [CrossRef]
- Fan, N.; Zhang, X.; Zhao, W.; Zhao, J.; Luo, D.; Sun, Y.; Li, D.; Zhao, C.; Wang, Y.; Zhang, H.; et al. Covalent inhibition of pyruvate kinase M2 reprograms metabolic and inflammatory pathways in hepatic macrophages against non-alcoholic fatty liver disease. Int. J. Biol. Sci. 2022, 18, 5260–5275. [Google Scholar] [CrossRef]
- Lee, S.; Usman, T.O.; Yamauchi, J.; Chhetri, G.; Wang, X.; Coudriet, G.M.; Zhu, C.; Gao, J.; McConnell, R.; Krantz, K. Myeloid FoxO1 depletion attenuates hepatic inflammation and prevents nonalcoholic steatohepatitis. J. Clin. Investig. 2022, 132, e154333. [Google Scholar] [CrossRef]
- Pant, R.; Kabeer, S.W.; Sharma, S.; Kumar, V.; Patra, D.; Pal, D.; Tikoo, K. Pharmacological inhibition of DNMT1 restores macrophage autophagy and M2 polarization in Western diet–induced nonalcoholic fatty liver disease. J. Biol. Chem. 2023, 299, 104779. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Schnabl, B.; Stärkel, P. Origin, Function, and Implications of Intestinal and Hepatic Macrophages in the Pathogenesis of Alcohol-Associated Liver Disease. Cells 2025, 14, 207. https://doi.org/10.3390/cells14030207
Hu Y, Schnabl B, Stärkel P. Origin, Function, and Implications of Intestinal and Hepatic Macrophages in the Pathogenesis of Alcohol-Associated Liver Disease. Cells. 2025; 14(3):207. https://doi.org/10.3390/cells14030207
Chicago/Turabian StyleHu, Yifan, Bernd Schnabl, and Peter Stärkel. 2025. "Origin, Function, and Implications of Intestinal and Hepatic Macrophages in the Pathogenesis of Alcohol-Associated Liver Disease" Cells 14, no. 3: 207. https://doi.org/10.3390/cells14030207
APA StyleHu, Y., Schnabl, B., & Stärkel, P. (2025). Origin, Function, and Implications of Intestinal and Hepatic Macrophages in the Pathogenesis of Alcohol-Associated Liver Disease. Cells, 14(3), 207. https://doi.org/10.3390/cells14030207






