Targeted Cellular Treatment of Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Stem Cell Transplantation
3. Mesenchymal Stem Cell Transplantation
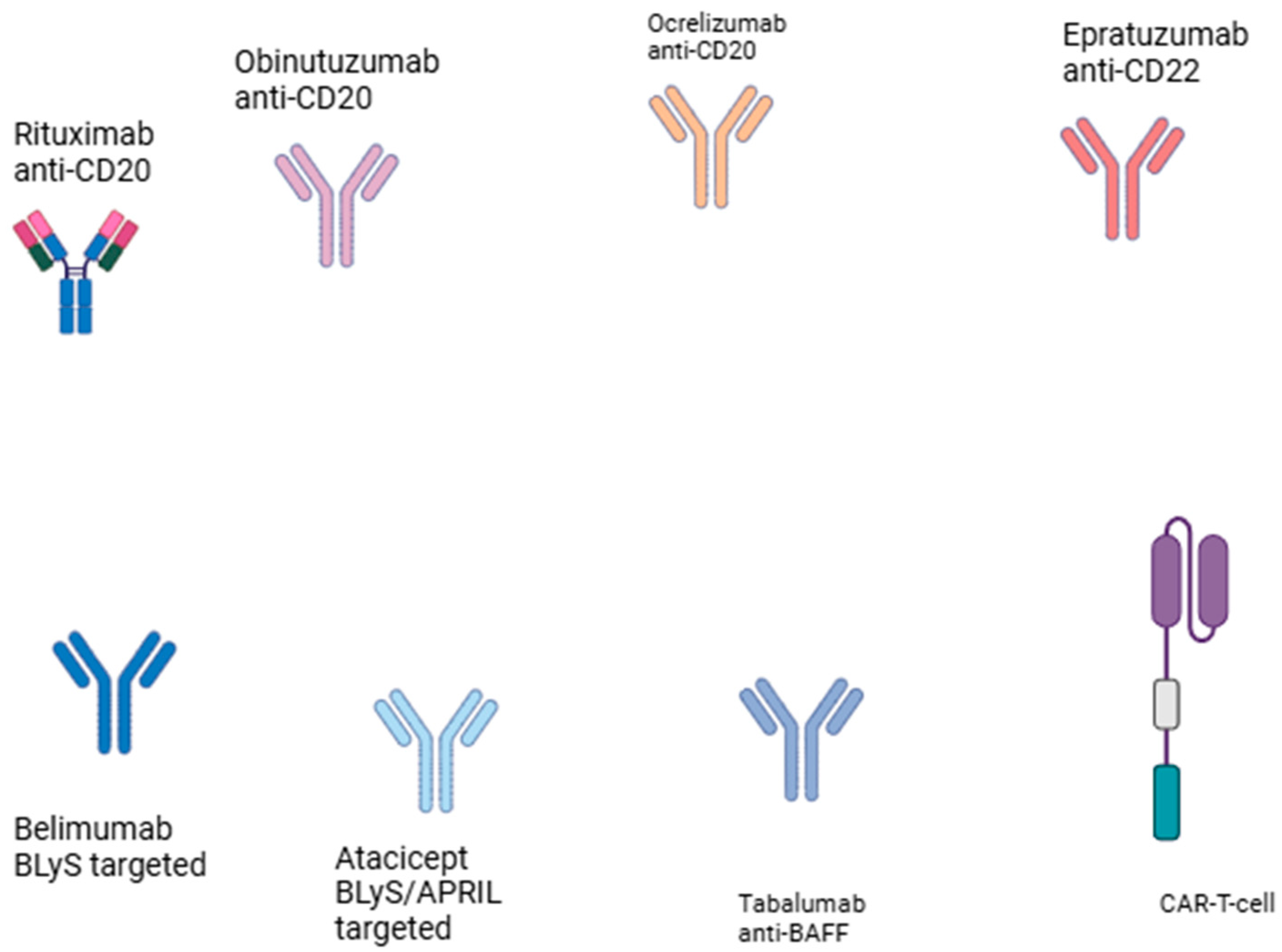
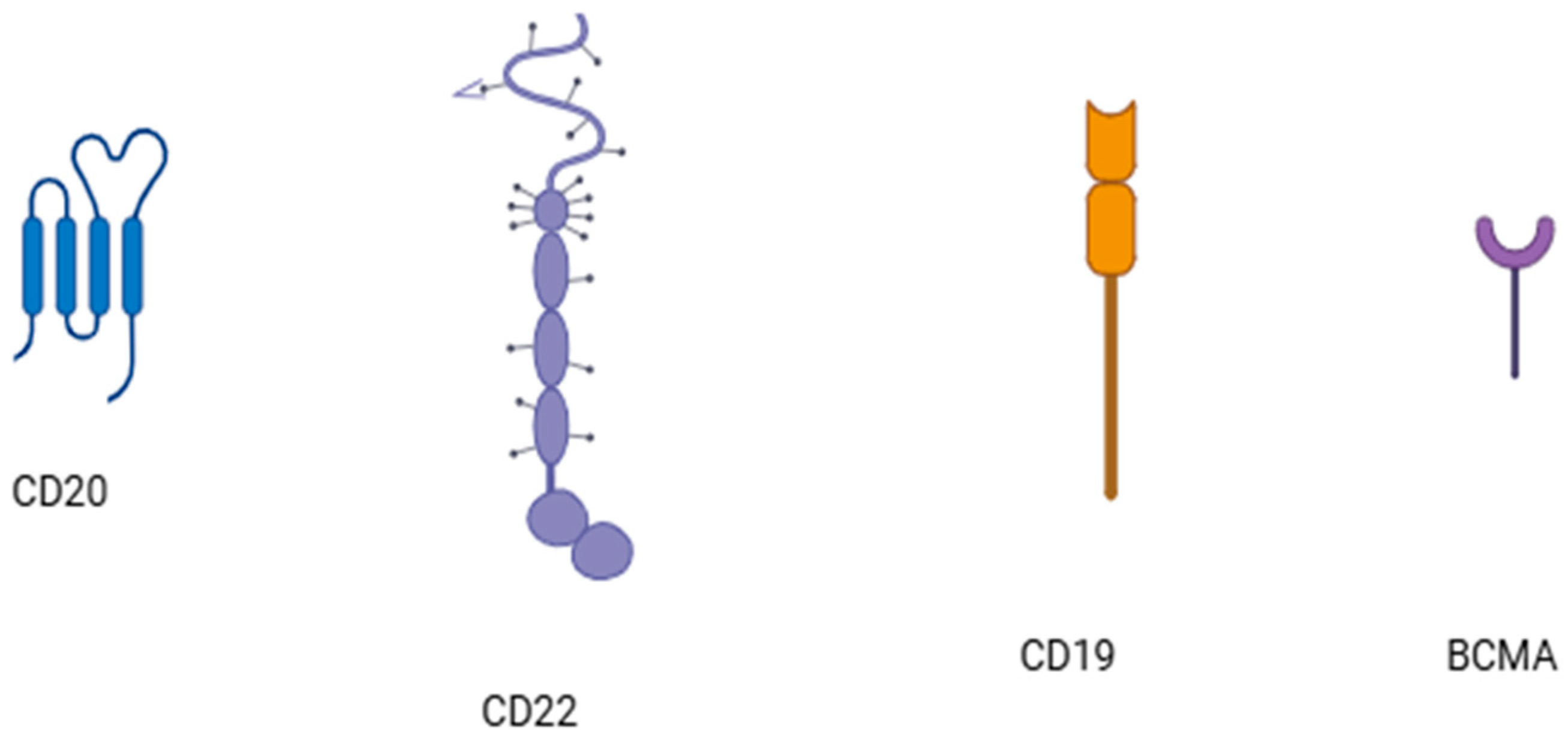
4. CAR-T Cell Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grigoriou, M.; Banos, A.; Filia, A.; Pavlidis, P.; Giannouli, S.; Karali, V.; Nikolopoulos, D.; Pieta, A.; Bertsias, G.; Verginis, P.; et al. Transcriptome reprogramming and myeloid skewing in haematopoietic stem and progenitor cells in systemic lupus erythematosus. Ann. Rheum. Dis. 2020, 79, 242–253. [Google Scholar] [CrossRef]
- Boddu, P.C.; Zeidan, A.M. Myeloid disorders after autoimmune disease. Best. Pract. Res. Clin. Haematol. 2019, 32, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; Saccardi, R.; Allez, M.; Ardizzone, S.; Arnold, R.; Cervera, R.; Denton, C.; Hawkey, C.; Labopin, M.; Mancardi, G.; et al. Haematopoietic SCT in severe autoimmune diseases: Updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012, 47, 770–790. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Kallenberg, C.; Shoenfeld, Y. Refractory disease in autoimmune diseases. Autoimmun. Rev. 2011, 10, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Delgado Alves, J.; Fortuna, J.; Faria, R.; Almeida, I.; Alves, G.; Araújo Correia, J.; Campar, A.; Brandão, M.; Crespo, J.; et al. Biological therapy in systemic lupus erythematosus, antiphospholipid syndrome, and Sjögren’s syndrome: Evidence- and practice-based guidance. Front. Immunol. 2023, 14, 1117699. [Google Scholar] [CrossRef]
- Aranow, C.; Allaart, C.F.; Amoura, Z.; Bruce, I.N.; Cagnoli, P.C.; Chatham, W.W.; Clark, K.L.; Furie, R.; Groark, J.; Urowitz, M.B.; et al. Efficacy and safety of sequential therapy with subcutaneous belimumab and one cycle of rituximab in patients with systemic lupus erythematosus: The phase 3, randomised, placebo-controlled BLISS-BELIEVE study. Ann. Rheum. Dis. 2024, 83, 1502–1512. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Lourenço, M.H.; Depascale, R.; Triantafyllias, K.; Parodis, I. Evolving Concepts in Treat-to-Target Strategies for Systemic Lupus Erythematosus. Mediterr. J. Rheumatol. 2024, 35 (Suppl. 2), 328–341. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.; Greco, R.; Snowden, J.A. Hematopoietic Stem Cell Transplantation for Autoimmune Disease. Annu. Rev. Med. 2021, 72, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.A.; Martin, R.; Mancardi, G.L.; Nicholas, R.; Sormani, M.P.; Saccardi, R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 391–405. [Google Scholar] [CrossRef]
- Balassa, K.; Danby, R.; Rocha, V. Haematopoietic stem cell transplants: Principles and indications. Br. J. Hosp. Med. 2019, 80, 33–39. [Google Scholar] [CrossRef]
- Tyndall, A. Hematopoietic stem cell transplantation for autoimmune diseases: More than just prolonged immunosuppression. Curr. Opin. Hematol. 2018, 25, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Malmegrim, K.C.R.; Lima-Júnior, J.R.; Arruda, L.C.M.; de Azevedo, J.T.C.; de Oliveira, G.L.V.; Oliveira, M.C. Autologous Hematopoietic Stem Cell Transplantation for Autoimmune Diseases: From Mechanistic Insights to Biomarkers. Front. Immunol. 2018, 9, 2602. [Google Scholar] [CrossRef]
- Atkins, H.L.; Muraro, P.A.; van Laar, J.M.; Pavletic, S.Z. Autologous hematopoietic stem cell transplantation for autoimmune disease--is it now ready for prime time? Biol. Blood Marrow Transplant. 2012, 18, S177–S183. [Google Scholar] [CrossRef] [PubMed]
- Boffa, G.; Signori, A.; Massacesi, L.; Mariottini, A.; Sbragia, E.; Cottone, S.; Amato, M.P.; Gasperini, C.; Moiola, L.; Meletti, S.; et al. Hematopoietic Stem Cell Transplantation in People with Active Secondary Progressive Multiple Sclerosis. Neurology 2023, 100, e1109–e1122. [Google Scholar] [CrossRef]
- Boffa, G.; Inglese, M.; Mancardi, G.L. Hematopoietic stem cell transplantation for multiple sclerosis. Handb. Clin. Neurol. 2024, 202, 153–167. [Google Scholar] [PubMed]
- Genc, B.; Bozan, H.R.; Genc, S.; Genc, K. Stem Cell Therapy for Multiple Sclerosis. Adv. Exp. Med. Biol. 2019, 1084, 145–174. [Google Scholar] [PubMed]
- Ross, L.A.; Stropp, L.M.; Cohen, J.A. Autologous Hematopoietic Stem Cell Transplantation to Treat Multiple Sclerosis. Neurol. Clin. 2024, 42, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, P.; Ruscitti, P.; Cipriani, P.; Giacomelli, R. Haematopoietic stem cell transplantation in systemic sclerosis: Challenges and perspectives. Autoimmun. Rev. 2020, 19, 102662. [Google Scholar] [CrossRef]
- Van Laar, J.M.; Farge, D.; Sont, J.K.; Naraghi, K.; Marjanovic, Z.; Larghero, J.; Schuerwegh, A.J.; Marijt, E.W.; Vonk, M.C.; Schattenberg, A.V.; et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: A randomized clinical trial. JAMA 2014, 311, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Reider, S.; Binder, L.; Fürst, S.; Hatzl, S.; Blesl, A. Hematopoietic Stem Cell Transplantation in Refractory Crohn’s Disease: Should It Be Considered? Cells 2022, 11, 3463. [Google Scholar] [CrossRef]
- Wang, R.; Yao, Q.; Chen, W.; Gao, F.; Li, P.; Wu, J.; Yu, J.; Cao, H. Stem cell therapy for Crohn’s disease: Systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res. Ther. 2021, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, C.J. Hematopoietic Stem Cell Transplantation in Crohn’s Disease: State-of-the-Art Treatment. Dig. Dis. 2017, 35, 107–114. [Google Scholar] [CrossRef]
- Ruiz, M.A.; Kaiser Junior, R.L.; Piron-Ruiz, L.; Peña-Arciniegas, T.; Saran, P.S.; De Quadros, L.G. Hematopoietic stem cell transplantation for Crohn’s disease: Gaps, doubts and perspectives. World J. Stem Cells. 2018, 10, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Radin, M.; Almarzooqi, A.M.; Al-Saleh, J.; Roccatello, D.; Sciascia, S.; Khamashta, M. Autologous hematopoietic stem cell transplantation in Systemic Lupus Erythematosus and antiphospholipid syndrome: A systematic review. Autoimmun. Rev. 2017, 16, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Sui, W.; Hou, X.; Che, W.; Chen, J.; Ou, M.; Xue, W.; Dai, Y. Hematopoietic and mesenchymal stem cell transplantation for severe and refractory systemic lupus erythematosus. Clin. Immunol. 2013, 148, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Marmont du Haut Champ, A.M. Hematopoietic stem cell transplantation for systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 2012, 380391. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.; Arnold, R.; Hiepe, F. Autologous hematopoietic stem cell transplantation in systemic lupus erythematosus. Z. Rheumatol. 2016, 75, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Askanase, A.; Khalili, L.; Tang, W.; Mertz, P.; Scherlinger, M.; Sebbag, E.; Chasset, F.; Felten, R.; Arnaud, L. New and future therapies: Changes in the therapeutic armamentarium for SLE. Best. Pract. Res. Clin. Rheumatol. 2023, 37, 101865. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.C.; Clave, E.; Moins-Teisserenc, H.; Douay, C.; Farge, D.; Toubert, A. Resetting the immune response after autologous hematopoietic stem cell transplantation for autoimmune diseases. Curr. Res. Transl. Med. 2016, 64, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Swart, J.F.; Lindemans, C.A.; van Royen, A.; Boelens, J.J.; Prakken, B.J.; Wulffraat, N. Changing winds in refractory autoimmune disease in children: Clearing the road for tolerance with cellular therapies. Curr. Opin. Rheumatol. 2012, 24, 267–273. [Google Scholar] [CrossRef]
- Snowden, J.A.; Badoglio, M.; Labopin, M.; Giebel, S.; McGrath, E.; Marjanovic, Z.; Burman, J.; Moore, J.; Rovira, M.; Wulffraat, N.M.; et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017, 1, 2742–2755. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.; Badoglio, M.; Henes, J.; Heesen, C.; Arnold, R.; Radbruch, A.; Snowden, J.A.; Hiepe, F. Autologous hematopoietic stem cell transplantation for autoimmune diseases: Current indications and mode of action, a review on behalf of the EBMT Autoimmune Diseases Working Party (ADWP). Z. Rheumatol. 2020, 79, 419–428. [Google Scholar] [CrossRef]
- Kelsey, P.J.; Oliveira, M.C.; Badoglio, M.; Sharrack, B.; Farge, D.; Snowden, J.A. Haematopoietic stem cell transplantation in autoimmune diseases: From basic science to clinical practice. Curr. Res. Transl. Med. 2016, 64, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Doglio, M.; Alexander, T.; Del Papa, N.; Snowden, J.A.; Greco, R. New insights in systemic lupus erythematosus: From regulatory T cells to CAR-T-cell strategies. J. Allergy Clin. Immunol. 2022, 150, 1289–1301. [Google Scholar] [CrossRef]
- Snowden, J.A.; Sánchez-Ortega, I.; Corbacioglu, S.; Basak, G.W.; Chabannon, C.; de la Camara, R.; Dolstra, H.; Duarte, R.F.; Glass, B.; Greco, R.; et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2022. Bone Marrow Transplant. 2022, 57, 1217–1239. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Donnelly, M.; Merscher-Gomez, S.; Chang, Y.H.; Franz, S.; Delfgaauw, J.; Chang, J.-M.; Choi, H.Y.; Campbell, K.N.; Kim, K.; et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 2008, 14, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Cui, M.; Kong, N.; Jing, J.; Xu, Y.; Liu, X.; Yang, F.; Xu, Z.; Yan, Y.; Zhao, D.; et al. Cytotoxic CD161−CD8+ TEMRA cells contribute to the pathogenesis of systemic lupus erythematosus. eBioMedicine 2023, 90, 104507. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Pereira, M.I.; Dreger, P. The role of hematopoietic stem cell transplantation in the treatment of relapsed/refractory Hodgkin’s lymphoma. Curr. Opin. Oncol. 2012, 24, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Capria, S.; Vignetti, M.; Mandelli, F.; Modena, V. Blast crisis of chronic myelogenous leukemia in long-lasting systemic lupus erythematosus: Regression of both diseases after autologous bone marrow transplantation. Blood 1997, 89, 4659. [Google Scholar] [CrossRef]
- Lowenthal, R.M.; Cohen, M.L.; Atkinson, K.; Biggs, J.C. Apparent cure of rheumatoid arthritis by bone marrow transplantation. J. Rheumatol. 1993, 20, 137–140. [Google Scholar]
- Yin, J.A.; Jowitt, S.N. Resolution of immune-mediated diseases following allogeneic bone marrow transplantation for leukaemia. Bone Marrow Transplant. 1992, 9, 31–33. [Google Scholar]
- Milanetti, F.; Bucha, J.; Testori, A.; Burt, R.K. Autologous hematopoietic stem cell transplantation for systemic sclerosis. Curr. Stem Cell Res. Ther. 2011, 6, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Burt, R.K.; Han, X.; Quigley, K.; Arnautovic, I.; Shah, S.J.; Lee, D.C.; Freed, B.H.; Jovanovic, B.; Helenowski, I.B. Cardiac safe hematopoietic stem cell transplantation for systemic sclerosis with poor cardiac function: A pilot safety study that decreases neutropenic interval to 5 days. Bone Marrow Transplant. 2021, 56, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Brierley, C.K.; Castilla-Llorente, C.; Labopin, M.; Badoglio, M.; Rovira, M.; Ricart, E.; Dierickx, D.; Vermeire, S.; Hasselblatt, P.; Finke, J.; et al. Autologous Haematopoietic Stem Cell Transplantation for Crohn’s Disease: A Retrospective Survey of Long-term Outcomes From the European Society for Blood and Marrow Transplantation. J. Crohns Colitis. 2018, 12, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Bregante, S.; Gualandi, F.; van Lint, M.T.; Schenone, A.; Bacigalupo, A.; Marmont, A.M. Sjögren’s syndrome associated chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) treated with autologous and subsequently allogeneic haematopoietic SCT (HSCT). Bone Marrow Transplant. 2013, 48, 1139–1140. [Google Scholar] [CrossRef]
- Loh, Y.; Oyama, Y.; Statkute, L.; Quigley, K.; Yaung, K.; Gonda, E.; Barr, W.; Jovanovic, B.; Craig, R.; Stefoski, D.; et al. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: Role of conditioning regimen used. Blood 2007, 109, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Bohgaki, T.; Atsumi, T.; Koike, T. Multiple autoimmune diseases after autologous stem-cell transplantation. N. Engl. J. Med. 2007, 357, 2734–2736. [Google Scholar] [CrossRef] [PubMed]
- Daikeler, T.; Labopin, M.; Di Gioia, M.; Abinun, M.; Alexander, T.; Miniati, I.; Gualandi, F.; Fassas, A.; Martin, T.; Schwarze, C.P.; et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: A retrospective study of the EBMT Autoimmune Disease Working Party. Blood 2011, 118, 1693–1698. [Google Scholar] [CrossRef]
- He, J.; Li, Z. Dilemma of immunosuppression and infection risk in systemic lupus erythematosus. Rheumatology 2023, 62 (Suppl. 1), i22–i29. [Google Scholar] [CrossRef] [PubMed]
- Burt, R.K.; Han, X.; Gozdziak, P.; Yaung, K.; Morgan, A.; Clendenan, A.M.; Henry, J.; Calvario, M.A.; Datta, S.K.; Helenowski, I.; et al. Five year follow-up after autologous peripheral blood hematopoietic stem cell transplantation for refractory, chronic, corticosteroid-dependent systemic lupus erythematosus: Effect of conditioning regimen on outcome. Bone Marrow Transplant. 2018, 53, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Tyndall, A. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus 2004, 13, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, W.; Ren, G.; Zhao, L.; Guo, J.; Gong, D.; Zeng, C.; Hu, W.; Liu, Z. Autologous Hematopoietic Stem Cell Transplantation for Refractory Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2019, 14, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Daikeler, T.; Tichelli, A.; Passweg, J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr. Res. 2012, 71 Pt 2, 439–444. [Google Scholar] [CrossRef]
- Jasim, S.A.; Yumashev, A.V.; Abdelbasset, W.K.; Margiana, R.; Markov, A.; Suksatan, W.; Pineda, B.; Thangavelu, L.; Ahmadi, S.H. Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases. Stem Cell Res. Ther. 2022, 13, 101. [Google Scholar] [CrossRef]
- Sun, L.; Akiyama, K.; Zhang, H.; Yamaza, T.; Hou, Y.; Zhao, S.; Xu, T.; Le, A.; Shi, S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009, 27, 1421–1432. [Google Scholar] [CrossRef]
- LLiang, J.; Zhang, H.; Hua, B.; Wang, H.; Lu, L.; Shi, S.; Hou, Y.; Zeng, X.; Gilkeson, G.S.; Sun, L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann. Rheum. Dis. 2010, 69, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, D.; Liang, J.; Zhang, H.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Ye, S.; Hu, X.; et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Guo, F.; Pan, Q.; Chen, S.; Chen, J.; Liu, H.F. Mesenchymal Stem Cell Therapy: Hope for Patients with Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 728190. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ye, H.; Li, K.; Shi, B.; Sun, X.; Wu, J. Efficacy of Mesenchymal Stem Cell Therapy on Lupus Nephritis and Renal Function in Systemic Lupus Erythematosus: A Meta-Analysis. Clin. Investig. Med. 2023, 46, E24–E35. [Google Scholar] [CrossRef]
- Wang, D.; Niu, L.; Feng, X.; Yuan, X.; Zhao, S.; Zhang, H.; Liang, J.; Zhao, C.; Wang, H.; Hua, B.; et al. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: A 6-year follow-up study. Clin. Exp. Med. 2017, 17, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Kong, W.; Deng, W.; Wang, D.; Feng, X.; Zhao, C.; Hua, B.; Wang, H.; Sun, L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: A long-term retrospective study. Stem Cell Res. Ther. 2018, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Payne, A.S. Engineering Cell Therapies for Autoimmune Diseases: From Preclinical to Clinical Proof of Concept. Immune Netw. 2022, 22, e37. [Google Scholar] [CrossRef] [PubMed]
- Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 2017, 17, 281–294. [Google Scholar] [CrossRef]
- Cornaby, C.; Gibbons, L.; Mayhew, V.; Sloan, C.S.; Welling, A.; Poole, B.D. B cell epitope spreading: Mechanisms and contribution to autoimmune diseases. Immunol. Lett. 2015, 163, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Carl, P.L.; Temple, B.R.; Cohen, P.L. Most nuclear systemic autoantigens are extremely disordered proteins: Implications for the etiology of systemic autoimmunity. Arthritis Res. Ther. 2005, 7, R1360–R1374. [Google Scholar] [CrossRef] [PubMed]
- Monneaux, F.; Muller, S. Epitope spreading in systemic lupus erythematosus: Identification of triggering peptide sequences. Arthritis Rheum. 2002, 46, 1430–1438. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Rosenberg, S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013, 10, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Draborg, A.H.; Duus, K.; Houen, G. Epstein-Barr virus and systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 2012, 370516. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J. Clin. Pathol. 2019, 72, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Nashi, E.; Wang, Y.; Diamond, B. The role of B cells in lupus pathogenesis. Int. J. Biochem. Cell Biol. 2010, 42, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Samy, E.; Wax, S.; Huard, B.; Hess, H.; Schneider, P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int. Rev. Immunol. 2017, 36, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Amengual, O. B cells targeting therapy in the management of systemic lupus erythematosus. Immunol. Med. 2020, 43, 16–35. [Google Scholar] [CrossRef]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nat. Rev. Drug Discov. 2021, 20, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Weiner, G.J. Rituximab: Mechanism of action. Semin. Hematol. 2010, 47, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Anolik, J.H.; Barnard, J.; Owen, T.; Zheng, B.; Kemshetti, S.; Looney, R.J.; Sanz, I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007, 56, 3044–3056. [Google Scholar] [CrossRef] [PubMed]
- Forsthuber, T.G.; Cimbora, D.M.; Ratchford, J.N.; Katz, E.; Stüve, O. B cell-based therapies in CNS autoimmunity: Differentiating CD19 and CD20 as therapeutic targets. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418761697. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.T.; Neuwelt, C.M.; Wallace, D.J.; Shanahan, J.C.; Latinis, K.M.; Oates, J.C.; Utset, T.O.; Gordon, C.; Isenberg, D.A.; Hsieh, H.J.; et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010, 62, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Mysler, E.F.; Spindler, A.J.; Guzman, R.; Bijl, M.; Jayne, D.; Furie, R.A.; Houssiau, F.A.; Drappa, J.; Close, D.; Maciuca, R.; et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: Results from a randomized, double-blind, phase III study. Arthritis Rheum. 2013, 65, 2368–2379. [Google Scholar] [CrossRef]
- Arnold, J.; Dass, S.; Twigg, S.; Jones, C.H.; Rhodes, B.; Hewins, P.; Chakravorty, M.; Courtney, P.; Ehrenstein, M.; Yusof, Y.M.; et al. Efficacy and safety of obinutuzumab in systemic lupus erythematosus patients with secondary non-response to rituximab. Rheumatology 2022, 61, 4905–4909. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.A.; Aroca, G.; Cascino, M.D.; Garg, J.P.; Rovin, B.H.; Alvarez, A.; Fragoso-Loyo, H.; Zuta-Santillan, E.; Schindler, T.; Brunetta, P.; et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: A randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2022, 81, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Clowse, M.E.B.; Wallace, D.J.; Furie, R.A.; Petri, M.A.; Pike, M.C.; Leszczyński, P.; Neuwelt, C.M.; Hobbs, K.; Keiserman, M.; Duca, L.; et al. Efficacy and Safety of Epratuzumab in Moderately to Severely Active Systemic Lupus Erythematosus: Results From Two Phase III Randomized, Double-Blind, Placebo-Controlled Trials. Arthritis Rheumatol. 2017, 69, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Handu, S.S.; Dubey, S.; Sharma, P.; Sharma, K.K.; Ahmed, Q.M. Belimumab: First targeted biological treatment for systemic lupus erythematosus. J. Pharmacol. Pharmacother. 2011, 2, 317–319. [Google Scholar] [CrossRef]
- Wallace, D.J.; Ginzler, E.M.; Merrill, J.T.; Furie, R.A.; Stohl, W.; Chatham, W.W.; Weinstein, A.; McKay, J.D.; McCune, W.J.; Petri, M.; et al. Safety and Efficacy of Belimumab Plus Standard Therapy for Up to Thirteen Years in Patients with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Manzi, S.; Sánchez-Guerrero, J.; Merrill, J.T.; Furie, R.; Gladman, D.; Navarra, S.V.; Ginzler, E.M.; D’Cruz, D.P.; Doria, A.; Cooper, S.; et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: Combined results from two phase III trials. Ann. Rheum. Dis. 2012, 71, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef]
- Merrill, J.T.; van Vollenhoven, R.F.; Buyon, J.P.; Furie, R.A.; Stohl, W.; Morgan-Cox, M.; Dickson, C.; Anderson, P.W.; Lee, C.; Berclaz, P.-Y.; et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: Results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2016, 75, 332–340. [Google Scholar]
- Merrill, J.T.; Shanahan, W.R.; Scheinberg, M.; Kalunian, K.C.; Wofsy, D.; Martin, R.S. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): Results from a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2018, 77, 883–889. [Google Scholar] [CrossRef]
- Petri, M.A.; Martin, R.S.; Scheinberg, M.A.; Furie, R.A. Assessments of fatigue and disease activity in patients with systemic lupus erythematosus enrolled in the Phase 2 clinical trial with blisibimod. Lupus 2017, 26, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, D.; Gordon, C.; Licu, D.; Copt, S.; Rossi, C.P.; Wofsy, D. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann. Rheum. Dis. 2015, 74, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.T.; Wallace, D.J.; Wax, S.; Kao, A.; Fraser, P.A.; Chang, P.; Isenberg, D.; ADDRESS II Investigators. Efficacy and Safety of Atacicept in Patients with Systemic Lupus Erythematosus: Results of a Twenty-Four-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm, Phase IIb Study. Arthritis Rheumatol. 2018, 70, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J.; Isenberg, D.A.; Morand, E.F.; Vazquez–Mateo, C.; Kao, A.H.; Aydemir, A.; Pudota, K.; Ona, V.; Aranow, C.; Merrill, J.T. Safety and clinical activity of atacicept in the long-term extension of the phase 2b ADDRESS II study in systemic lupus erythematosus. Rheumatology 2021, 60, 5379–5389. [Google Scholar] [CrossRef]
- Bachanova, V.; Nachman, P.H. Two for one? CAR-T therapy for lymphoma benefits concurrent autoimmune disorders. Bone Marrow Transplant. 2023, 58, 1175–1176. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Y.; Song, Q.; Jiang, X.; Cao, W.; Li, L.; Yi, H.; Weng, X.; Chen, S.; Wang, Z.; et al. Concurrent remission of lymphoma and Sjögren’s disease following anti-CD19 chimeric antigen receptor-T cell therapy for diffuse large B-cell lymphoma: A case report. Front. Immunol. 2023, 14, 1298815. [Google Scholar] [CrossRef]
- Wang, J.; Alkrekshi, A.; Dasari, S.; Lin, H.C.; Elantably, D.; Armashi, A.R.A. CD19-targeted chimeric antigen receptor T-cell therapy in patients with concurrent B-cell Non-Hodgkin lymphoma and rheumatic autoimmune diseases: A propensity score matching study. Bone Marrow Transplant. 2023, 58, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, Q.; Pu, C.; Zhu, K.; Lu, C.; Jiang, Y.; Xiao, L.; Han, Y.; Lu, L. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol. Immunol. 2021, 18, 1896–1903. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Krönke, G.; Völkl, S.; Kretschmann, S.; Aigner, M.; Kharboutli, S.; Böltz, S.; Manger, B.; Mackensen, A.; Schett, G. CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus. N. Engl. J. Med. 2021, 385, 567–569. [Google Scholar] [CrossRef]
- Taubmann, J.; Müller, F.; Mutlu, M.Y.; Völkl, S.; Aigner, M.; Bozec, A.; Mackensen, A.; Grieshaber-Bouyer, R.; Schett, G. CD19 Chimeric Antigen Receptor T Cell Treatment: Unraveling the Role of B Cells in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2024, 76, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 2022, 28, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Taubmann, J.; Bucci, L.; Wilhelm, A.; Bergmann, C.; Völkl, S.; Aigner, M.; Rothe, T.; Minopoulou, I.; Tur, C.; et al. CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up. N. Engl. J. Med. 2024, 390, 687–700. [Google Scholar] [CrossRef]
- Krickau, T.; Naumann-Bartsch, N.; Aigner, M.; Kharboutli, S.; Kretschmann, S.; Spoerl, S.; Vasova, I.; Völkl, S.; Woelfle, J.; Mackensen, A.; et al. CAR T-cell therapy rescues adolescent with rapidly progressive lupus nephritis from haemodialysis. Lancet. 2024, 403, 1627–1630. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; Zhang, W.; Zhang, H.; DeStefano, V.M.; Wada, M.; Pinz, K.; Deener, G.; Shah, D.; Hagag, N.; et al. BCMA-CD19 compound CAR T cells for systemic lupus erythematosus: A phase 1 open-label clinical trial. Ann. Rheum. Dis. 2024, 83, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Camarena, D.C.; Palafox-Sánchez, C.A.; Cruz, A.; Marín-Rosales, M.; Muñoz-Valle, J.F. Analysis of the receptor BCMA as a biomarker in systemic lupus erythematosus patients. Sci. Rep. 2020, 10, 6236. [Google Scholar] [CrossRef]
- Martin, J.; Cheng, Q.; Laurent, S.A.; Thaler, F.S.; Beenken, A.E.; Meinl, E.; Krönke, G.; Hiepe, F.; Alexander, T. B-Cell Maturation Antigen (BCMA) as a Biomarker and Potential Treatment Target in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2024, 25, 10845. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, A.; Chambers, D.; Müller, F.; Bozec, A.; Grieshaber-Bouyer, R.; Winkler, T.; Mougiakakos, D.; Mackensen, A.; Schett, G.; Krönke, G. Selective CAR T cell-mediated B cell depletion suppresses IFN signature in SLE. JCI Insight 2024, 9, e179433. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Schett, G.; Mougiakakos, D. B cell-targeting chimeric antigen receptor T cells as an emerging therapy in neuroimmunological diseases. Lancet Neurol. 2024, 23, 615–624. [Google Scholar] [CrossRef]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-cell therapy in the era of solid tumor treatment: Current challenges and emerging therapeutic advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Farrukh, H.; Chittepu, V.; Xu, H.; Pan, C.X.; Zhu, Z. CAR race to cancer immunotherapy: From CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef]
- Denlinger, N.; Bond, D.; Jaglowski, S. CAR T-cell therapy for B-cell lymphoma. Curr. Probl. Cancer 2022, 46, 100826. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.; Sehn, L.H. CAR T cells as a second-line therapy for large B-cell lymphoma: A paradigm shift? Blood 2022, 139, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Haslauer, T.; Greil, R.; Zaborsky, N.; Geisberger, R. CAR T-Cell Therapy in Hematological Malignancies. Int. J. Mol. Sci. 2021, 22, 8996. [Google Scholar] [CrossRef]
- Cook, M.R.; Dorris, C.S.; Makambi, K.H.; Luo, Y.; Munshi, P.N.; Donato, M.; Rowley, S.; Saad, A.; Goy, A.; Dunleavy, K.; et al. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: A meta-analysis of 128 patients. Blood Adv. 2023, 7, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Barrett, D.; Teachey, D.T.; Grupp, S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.D.; Smith, M.; Shah, N.N. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood 2023, 141, 2430–2442. [Google Scholar] [PubMed]
- Sun, S.; Hao, H.; Yang, G.; Zhang, Y.; Fu, Y. Immunotherapy with CAR-Modified T Cells: Toxicities and Overcoming Strategies. J. Immunol. Res. 2018, 2018, 2386187. [Google Scholar] [CrossRef]
- Maus, M.V.; Haas, A.R.; Beatty, G.L.; Albelda, S.M.; Levine, B.L.; Liu, X.; Zhao, Y.; Kalos, M.; June, C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013, 1, 26–31. [Google Scholar] [CrossRef]
- Verdun, N.; Marks, P. Secondary Cancers after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2024, 390, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Hay, K.A. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br. J. Haematol. 2018, 183, 364–374. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Ma, C.; O’connell, R.M.; Mehta, A.; DiLoreto, R.; Heath, J.R.; Baltimore, D. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell. 2014, 14, 445–459. [Google Scholar] [CrossRef]
- Brentjens, R.; Yeh, R.; Bernal, Y.; Riviere, I.; Sadelain, M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: Case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 2010, 18, 666–668. [Google Scholar] [CrossRef]
- Kotch, C.; Barrett, D.; Teachey, D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert. Rev. Clin. Immunol. 2019, 15, 813–822. [Google Scholar] [CrossRef]
- Frey, N.; Porter, D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2019, 25, e123–e127. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C.H.J.; Willemsen, R.; van Elzakker, P.; van Steenbergen-Langeveld, S.; Broertjes, M.; Oosterwijk-Wakka, J.; Oosterwijk, E.; Sleijfer, S.; Debets, R.; Gratama, J.W. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 2011, 117, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.L.; Pasquini, M.C.; Connolly, J.E.; Porter, D.L.; Gustafson, M.P.; Boelens, J.J.; Horwitz, E.M.; Grupp, S.A.; Maus, M.V.; Locke, F.L.; et al. Unanswered questions following reports of secondary malignancies after CAR-T cell therapy. Nat. Med. 2024, 30, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, V.; Yazdanpanah, N.; Rezaei, N. The immunologic aspects of cytokine release syndrome and graft versus host disease following CAR T cell therapy. Int. Rev. Immunol. 2022, 41, 649–668. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, S.; Durgin, J.S.; Nunez-Cruz, S.; Patel, J.; Leferovich, J.; Pinzone, M.; Shen, F.; Cummins, K.D.; Plesa, G.; Cantu, V.A.; et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 2022, 6, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.J.; Barba, P.; Jäger, U.; Shah, N.N.; Blaise, D.; Briones, J.; Shune, L.; Boissel, N.; Bondanza, A.; Mariconti, L.; et al. A Novel Autologous CAR-T Therapy, YTB323, with Preserved T-cell Stemness Shows Enhanced CAR T-cell Efficacy in Preclinical and Early Clinical Development. Cancer Discov. 2023, 13, 1982–1997. [Google Scholar] [CrossRef] [PubMed]
- Tur, C.; Eckstein, M.; Velden, J.; Rauber, S.; Bergmann, C.; Auth, J.; Bucci, L.; Corte, G.; Hagen, M.; Wirsching, A.; et al. CD19-CAR T-cell therapy induces deep tissue depletion of B cells. Ann. Rheum. Dis. 2024, 84, 106–114. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Sengupta, R.; Gold, R.; Schroers, R.; Haghikia, A.; Lorente, M.; Pendleton, M.; Register, A.; Heesen, C.; Kröger, N.; et al. Successful generation of fully human, second generation, anti-CD19 CAR T cells for clinical use in patients with diverse autoimmune disorders. Cytotherapy 2024, 27, 236–246. [Google Scholar] [CrossRef] [PubMed]
- van Leuven, S.I.; Duivenvoorden, R. CAR-T cell therapy in systemic lupus erythematosus and beyond: A brave new world? Rheumatology 2024, 63, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Kambayana, G.; Surya Rini, S. Autologous CD19-Targeted Chimeric Antigen Receptor (CAR)T-Cells as the Future of Systemic Lupus Erythematosus Treatment. Curr. Rheumatol. Rev. 2023, 19, 260–269. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanassiou, P.; Athanassiou, L.; Kostoglou-Athanassiou, I.; Shoenfeld, Y. Targeted Cellular Treatment of Systemic Lupus Erythematosus. Cells 2025, 14, 210. https://doi.org/10.3390/cells14030210
Athanassiou P, Athanassiou L, Kostoglou-Athanassiou I, Shoenfeld Y. Targeted Cellular Treatment of Systemic Lupus Erythematosus. Cells. 2025; 14(3):210. https://doi.org/10.3390/cells14030210
Chicago/Turabian StyleAthanassiou, Panagiotis, Lambros Athanassiou, Ifigenia Kostoglou-Athanassiou, and Yehuda Shoenfeld. 2025. "Targeted Cellular Treatment of Systemic Lupus Erythematosus" Cells 14, no. 3: 210. https://doi.org/10.3390/cells14030210
APA StyleAthanassiou, P., Athanassiou, L., Kostoglou-Athanassiou, I., & Shoenfeld, Y. (2025). Targeted Cellular Treatment of Systemic Lupus Erythematosus. Cells, 14(3), 210. https://doi.org/10.3390/cells14030210







