Abstract
Fibromyalgia represents a chronic pain pathology characterized by severe musculoskeletal pain, fatigue, disturbances in sleep, and cognitive issues. Despite its presence, the underlying mechanisms of fibromyalgia remain inadequately understood; however, recent investigations have suggested that inflammation could play a fundamental role in the pathophysiology of this condition. Several studies highlight elevated concentrations of pro-inflammatory cytokines, dysregulation of immune responses, and neuroinflammation in fibromyalgia patients. Furthermore, chronic low-grade inflammation has been proposed as a potential catalyst for the sensitization of pain pathways, which exacerbates the symptoms of fibromyalgia. Understanding the role of inflammation in this disease might open new avenues for therapeutic interventions while providing a more profound insight into the complex nature of this debilitating disorder. Although progress has been made, further research is needed to uncover the complexities involved. This review investigates the intricate relationship between inflammation and fibromyalgia, analyzing the evidence that supports the involvement of both peripheral and central inflammatory processes in the onset and persistence of the disorder.
1. Introduction
Fibromyalgia represents a chronic condition characterized by widespread musculoskeletal pain, frequently accompanied by a plethora of symptoms that profoundly reduce the overall quality of life [1]. This disorder is estimated to impact between 0.2 and 6.6% of the global population, with a noticeable predominance among women [2,3]. The hallmark manifestations of fibromyalgia encompass persistent discomfort throughout the body, debilitating fatigue, sleep disturbances, and cognitive impairments (known as “fibro-fog”) [4]. Patients frequently report heightened sensitivity to pain (allodynia and hyperalgesia), stiffness in muscles and joints, headaches, and irritable bowel syndrome (IBS), along with mood disorders such as anxiety and depression [4]. Although the exact cause of fibromyalgia remains poorly understood, researchers postulate that it might stem from aberrant pain processing within the central nervous system (CNS), potentially prompted by several factors such as physical trauma, infections, stress, and genetic predispositions [5]. The diagnosis is especially challenging due to the lack of definitive tests and the significant overlap of symptoms with those of other conditions [6]. Although a definitive treatment for fibromyalgia has not been established, treatment often necessitates a multifaceted approach that includes lifestyle changes and pharmacological agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), to manage symptoms [7]. Despite its challenges, effective management strategies enable many individuals with fibromyalgia to maintain their fulfilling lives and continue their daily activities.
Inflammation in the CNS (known as central inflammation) has been identified as a contributing factor in the development of fibromyalgia [8]. Several studies have shown elevated levels of pro-inflammatory cytokines in the cerebrospinal fluid (CSF) of fibromyalgia patients [9,10]. Moreover, brain imaging techniques have revealed strong activation of the glial cells in large parts of the CNS in fibromyalgia patients [11,12]. The degree of glial cell activation has been highly correlated with the severity of fatigue reported by the patients, suggesting a potential link between neuroinflammation and symptom manifestation [13]. In addition to central inflammation, peripheral mechanisms (known as peripheral inflammation) also participate in fibromyalgia pathogenesis [14]. Evidence suggests that nociceptors placed in the skin and muscles experience changes, such as sensitization of vanilloid receptors (TRPVs), acid-sensing ion channel receptors (ASICs), and purinergic receptors [15,16]. These changes in peripheral pain processing contribute to pain sensitivity typical of the symptomatology associated with fibromyalgia.
The interplay between peripheral and central mechanisms is very complex, with peripheral stimulation potentially initiating and maintaining central sensitization. However, most researchers believe that the CNS plays essential roles in modulating peripheral sensory inputs, leading to amplification of pain signals in fibromyalgia [16]. The central sensitization process results in neurogenic inflammation, which contributes to several hallmark features of fibromyalgia, including abnormal tenderness, pain, peripheral swelling, and cognitive dysfunction [17,18].
This article examines the complex relationship between inflammation and fibromyalgia, investigating the potential role of inflammatory mechanisms in the onset and progression of this multifaceted condition. The article emphasizes substantial evidence from studies showing increased levels of pro-inflammatory markers in individuals with fibromyalgia, indicating a significant link between inflammation and the development of pain, fatigue, and other debilitating symptoms. By elucidating these associations, this review underscores the promise of pioneering therapeutic approaches designed to target inflammatory pathways. Such advancements could change the management of symptoms and substantially improve the overall quality of life for individuals with fibromyalgia.
2. Fibromyalgia: A Comprehensive Overview
In this section, the general characteristics of fibromyalgia will be thoroughly examined, focusing on its symptomatology, diagnostic criteria, and epidemiology. It examines the clinical manifestations of the condition, the approaches and challenges in diagnosing it, and the prevalence trends across various populations. Through the exploration of these aspects, this section aims to provide a clearer understanding of the complexity of fibromyalgia and its impact on both individuals and public health.
2.1. Signs and Symptoms
Fibromyalgia constitutes a complex disorder characterized by persistent, widespread musculoskeletal pain, particularly affecting lumbar, gluteal, cervical, scapular, and dorsal regions [2,4,19]. Pain can also extend to the head and occasionally to the limbs; however, peripheral pain is less common [2,4,19]. Diagnosing fibromyalgia requires consideration of the interplay of symptoms, as any misinterpretation could lead to improper treatments [20]. Pain exhibits variability over time, influenced by factors including seasonal changes and physical activity levels; in fact, inactivity has been found to intensify symptoms [20]. Moreover, fibromyalgia usually evokes severe fatigue, sleep disturbances, and cognitive dysfunctions, which can significantly disrupt daily functioning, highlighting the need for treatments that target both musculoskeletal and systemic pain-related symptoms [21,22,23]. Finally, stress, humidity, and low temperatures increase the sensation of pain [7].
2.2. Diagnosis
Prior to 2010, the diagnosis was established based on the American College of Rheumatology (ACR) 1990 criteria, which required documentation of widespread pain lasting at least three months and the detection of tender points [24]. Although tender points remain a vital component of a comprehensive clinical evaluation, these criteria were comprehensively revised in 2016 to reduce the likelihood of misdiagnosing fibromyalgia [25]. However, differences in individual phenotypes and the presence of concurrent pathologies can result in incomplete clinical evaluations, which might compromise diagnostic accuracy. In this respect, the probability of defining an accepted diagnostic criterion is problematic [26].
In 2020, the ACR revised the 2016 criteria to highlight two key parameters focusing on somatic and cognitive symptoms [27]: the Widespread Pain Index (WPI) and the Symptom Severity (SS) score. The WPI evaluates pain across 19 anatomical regions [28], while the SS score assesses severity of symptoms such as fatigue, sleep disturbances, and cognitive impairments [29]. Although these revisions improve diagnostic accuracy, they require a comprehensive understanding of individual presentations [27].
Currently, no specific biomarkers have been identified for diagnosing fibromyalgia. As a result, most ongoing research studies are focused on the search for diagnostic markers by exploring genetic, environmental, and epigenetic variables that influence the pathophysiology of fibromyalgia [30].
2.3. Epidemiology and Socioeconomic Impact
Understanding the epidemiology of fibromyalgia offers clinical and financial benefits [31]. Prevalence rates exhibit significant variability, originating from differences in methodology, diagnostic criteria, and geographic location [32]. Most studies are primarily focused on specific cities and regions, resulting in a lack of comprehensive data on nationwide prevalence [33].
Global estimates range from 0.2% to 6.6% [3], with Europe reporting a prevalence of 2.31% [34]. In specific countries, prevalence rates are 1.6% in France [35], 2.1% in Germany [36], and 2.4% in Spain [37]. Other countries, such as the United States, Canada, and Japan, report prevalence rates of 6.4%, 1.5%, and 2.1%, respectively [38,39,40]. This disease is more prevalent among females and tends to escalate with age [41,42]. Additionally, additional factors associated with an increased likelihood of developing fibromyalgia include diagnoses of major depressive disorder, IBS, and restless legs syndrome (RLS) [43,44].
On the other hand, fibromyalgia significantly affects society in both clinical and economic costs. Individuals with lower socioeconomic status frequently encounter more pronounced symptoms, reduced productivity, and elevated absenteeism, all contributing to the economic burden of ill patients [45,46,47]. The healthcare costs associated with this condition are considerable; patients usually necessitate many treatments and consultations to manage their symptoms [48]. The nature of this condition leads to accumulating costs over time, creating a substantial financial strain on patients, their corresponding families, and healthcare systems [48]. Addressing these problems remains challenging because the interplay between health and socioeconomic factors is complex. Although interventions can mitigate numerous effects, the overarching impact of fibromyalgia on economic stability cannot be overlooked.
The nature of fibromyalgia results in delays in both diagnosis and treatments, consequently intensifying its socioeconomic repercussions [49]. The psychological impact of fibromyalgia can substantially decrease the quality of life and social participation, which, in turn, may indirectly influence economic outcomes [50]. Consequently, the diverse socioeconomic consequences of fibromyalgia outline the importance of complex treatment approaches to cover medical and social aspects of the disease [7]. Still, further research has an urgent need to enhance the knowledge of such interrelations.
2.4. Current Pharmacological Treatments
A significant body of research has clarified a variety of pharmacological approaches used as supportive therapies in the management of fibromyalgia. These interventions include antidepressants, monoamine reuptake inhibitors, serotonin and dopamine receptor antagonists, gabapentinoids, opioids, and cannabinoids. The main goal of these pharmacological agents is to improve sleep quality, alleviate symptoms of depression and anxiety, and decrease fatigue. While each intervention functions through distinct mechanisms of action, their aim is to improve the overall quality of life for patients. The pharmacological agents employed in the management of fibromyalgia are outlined as follows (Table 1):

Table 1.
A list of drugs used in the management of pain-related symptoms of fibromyalgia. Abbreviations: TCAs (tricyclic antidepressants); 5-HT (serotonin); NA (noradrenaline); NMDA (N-Methyl-D-aspartate); SNRIs (serotonin-noradrenaline reuptake inhibitors); CNS (central nervous system); NRIs (selective noradrenaline reuptake inhibitors); SSRIs (selective serotonin reuptake inhibitors); 5-HT2A (serotonin 5-HT2A receptor); 5-HT2C (serotonin 5-HT2C receptor); 5-HT3 (serotonin 5-HT3 receptor); D2 (dopamine D2 receptor); VGCCs (voltage-gated calcium channels); MOR (μ-opioid receptor); CB1 (cannabinoid receptor 1); CB1 (cannabinoid receptor 2).
3. The Relationship Between Fibromyalgia and Inflammation
The relationship between fibromyalgia and inflammation represents a multifaceted and dynamic area of investigation, highlighting the sophisticated interplay between the immune system and the nervous system [120]. While fibromyalgia has traditionally been classified as a disorder primarily characterized by CNS sensitization, recent evidence increasingly emphasizes the role of inflammation in its pathophysiology [121,122]. The inflammatory milieu is thought to exacerbate the increased sensitivity to pain that typifies this condition, because it promotes peripheral nociceptor sensitization while amplifying central sensitization processes [121]. Furthermore, neuroinflammation (the interplay between immune cells and the nervous system) may worsen symptoms, creating a feedback loop that perpetuates pain [122].
Although the complexity of this relationship is evident, it underscores the need for a multidisciplinary approach to fully understand this connection. Given these findings, researchers and clinicians must work together to uncover the underlying mechanisms.
3.1. Peripheral Inflammation and Fibromyalgia
The recent evidence underscores the pivotal role of peripheral inflammation in the pathophysiology of fibromyalgia, indicating that inflammation is not simply a result of fibromyalgia but a fundamental factor driving its development and ongoing persistence [121]. Some studies, employing the ELISA method, have demonstrated that fibromyalgia patients often exhibit elevated levels of inflammatory markers (Table 2), including C-reactive protein (CRP), which serves as a good indicator of low-grade systemic inflammation [123,124,125]. However, not all individuals with fibromyalgia manifest elevated CRP levels; in fact, the extent of elevation can differ markedly across patients. Some studies have indicated that CRP concentrations in individuals with fibromyalgia may be influenced by comorbid conditions, such as obesity or concurrent inflammatory disorders, highly prevalent within this cohort [126,127]. This inflammatory state can begin a catalytic effect, amplifying the painful symptoms that characterize fibromyalgia and potentially exacerbating the overall burden of the condition [128]. Such complexity requires further investigation, as understanding these relationships is vital for developing effective treatment strategies. CCL2, a chemokine involved in the inflammation process [129], has been identified in the plasma of patients with fibromyalgia using the ELISA method [9].

Table 2.
List of biomarkers with significant differences in patients with fibromyalgia. Abbreviations: CRP (C-reactive protein), CCL2 (chemokine (C-C motif) ligand 2), S100A8 (S100 calcium-binding protein A8), S100A9 (S100 calcium-binding protein A9), VCAM (vascular cell adhesion molecule), CD163 (cluster of differentiation 163), SERPINA1 (serpin family A member 1), ANXA1 (annexin A1), PGAM1 (phosphoglycerate mutase 1), C4A (complement component 4), C1QC (complement C1q C chain), IL-8 (interleukin 8), IL-37 (interleukin 37), AXIN 1 (axis inhibition protein 1), SIRT2 (NAD-dependent deacetylase sirtuin 2), IL-4 (interleukin 4), IL-6 (interleukin 6), IL-10 (interleukin 10), IL-13 (interleukin 13), TNF-α (tumor necrosis factor alpha), IGF-1 (insulin-like growth factor 1), SP (substance P), NPY (neuropeptide Y), MMP-3 (matrix metalloproteinase-3), CX3CL1 (chemokine (C-X3-C motif) ligand 1), and CSF (cerebrospinal fluid).
On the other hand, some studies have identified the interferon gene signature in fibromyalgia patients. This signature is characterized by the increased expression of interferon-regulated genes in peripheral B cells, as assessed by RNA sequencing and RT-qPCR. These genes, including S100A8, S100A9, VCAM, CD163, SERPINA1, and ANXA1, are involved in immune response and inflammation [14]. The elevated expression of these genes demonstrates that fibromyalgia might involve an autoimmune-like aspect, with the immune system targeting the body’s own tissues [139].
A recent publication showed the proteomes of plasma, serum, and saliva in healthy individuals and fibromyalgia patients. The most significant proteins identified in patients with fibromyalgia included transferrin; α-, β-, and γ-fibrinogen chains; profilin-1; transaldolase; PGAM1; apolipoprotein-C3; complement C4A and C1QC; immunoglobulin components; and acute phase reactants [130]. Some of these are implicated in the maintenance of chronic low-grade inflammation [140,141,142,143]. Moreover, increased levels of inflammatory serum proteins, including IL-8, IL-37, AXIN1, and SIRT2, have been identified through proteomics and ELISA as correlates of fibromyalgia symptom severity [14,144]. IL-8 and IL-37 participate in the recruitment of some immune cells to inflammation sites. Consequently, its elevated levels show a highly active inflammatory response in patients with fibromyalgia [145,146]. Similarly, AXIN1 and SIRT2 are linked to immune regulation and cellular stress and have been linked to the severity of pain, fatigue, and some other symptoms in fibromyalgia [147,148]. Other investigations that analyzed plasma proteins using ELISA have reported elevated levels (pro-inflammatory cytokines) alongside reductions in IL-4 and IL-13 (anti-inflammatory cytokines), thereby inducing the activation of various immune cells, including mast cells [131,132,133,134,135]. In this regard, fibromyalgia patients have displayed, as assessed by flow cytometry, an increased neutrophil/lymphocyte ratio and alterations in several T lymphocyte subpopulations, including CD4+ T cells and NKT cells [149,150]. All the aforementioned biomarkers provide important insights into the inflammatory processes involved in fibromyalgia, thereby serving as potential targets for the development of future diagnostic tools [151,152].
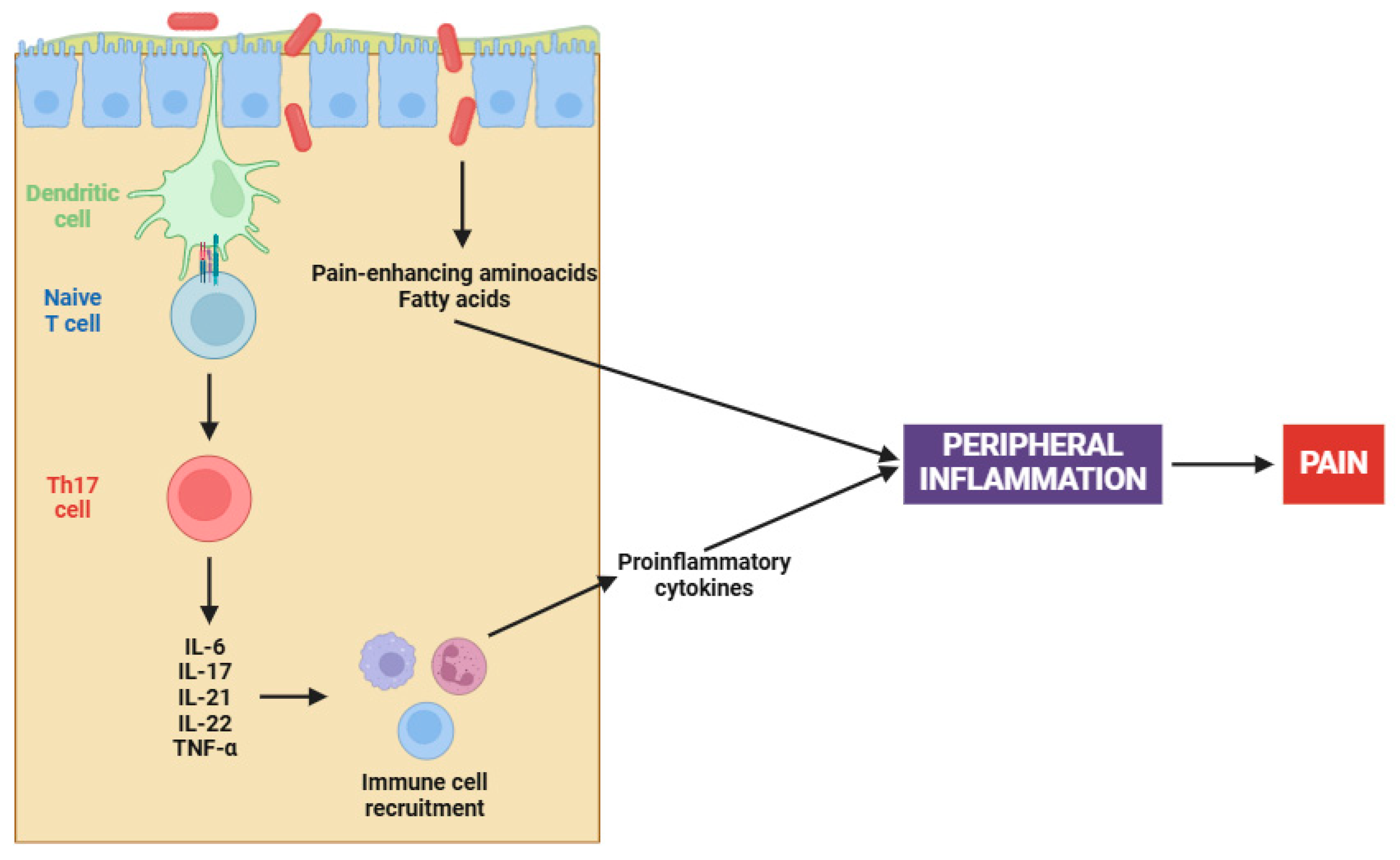
On the other hand, the complex interaction between gut microbiota and fibromyalgia has emerged as a central focus in elucidating the pathophysiology of this chronic condition [153,154]. Recent studies have illuminated a compelling causal nexus between altered gut microbiota and fibromyalgia symptoms, particularly in relation to peripheral sensitization [155]. Pioneering research has demonstrated that fecal microbiota transplantation (FMT) from patients with fibromyalgia into germ-free mice induces pain hypersensitivity; however, transplantation from healthy individuals has been ineffective in reproducing this phenomenon [156,157]. Certainly, this involves an increase in pro-inflammatory cytokines (e.g., IL-17 and TNF-α) and the activation of monocytes and lymphocytes, both contributing to the peripheral sensitization process (Figure 1) [156,157].
Figure 1.
Gut dysbiosis in fibromyalgia patients. Abbreviations: IL-6 (interleukin 6), IL-17 (interleukin 17), IL-21 (interleukin 21), IL-22 (interleukin 22), and TNF-α (tumor necrosis factor alpha).
The altered microbiota in fibromyalgia patients is characterized by imbalances in certain bacterial species, such as Flavonifractor plautii, Parabacteroides merdae, and Faecalibacterium prausnitzii [158]. Apart from cytokine participation, microbial imbalances contribute to peripheral sensitization through several mechanisms, including the synthesis of pain-enhancing amino acids (e.g., glutamate), alterations in fat metabolism, and changes in bile acid production [159]. Chronic immune activation, when coupled with an altered gut microbiota, results in increased intestinal permeability. This ultimately leads to further activation of the immune system and initiates an inflammatory cascade that stimulates nociceptive signals [156,157].
The connection between peripheral inflammation and fibromyalgia extends beyond pain perception; it also notably influences the comorbid conditions commonly seen in individuals with fibromyalgia [160,161]. Numerous conditions, including inflammatory arthritis, chronic spontaneous urticaria (CSU), and functional bowel disorders (FBD), are usually encountered alongside fibromyalgia [160,161]. These conditions suggest the presence of an underlying inflammatory pathway may be involved, impacting various organ systems and intensifying the overall symptom burden [162,163,164]. Continuous activation of the immune system in these conditions perpetuates the inflammatory state, therefore underscoring the complex nature of fibromyalgia [160,161].
In conclusion, the interplay between peripheral inflammation and fibromyalgia underscores the importance of inflammation as a key factor in the onset and progression of the disease. Inflammatory cytokines and activation of the immune cells in the periphery can directly affect the function of the nervous system, enhancing excitability of pain pathways and leading to an exaggerated pain response. Consequently, a self-sustaining feedback loop is established, where pain, inflammation, and immune dysfunction reinforce each other. However, these interconnections complicate the understanding of the underlying mechanisms.
3.2. Central Inflammation and Fibromyalgia
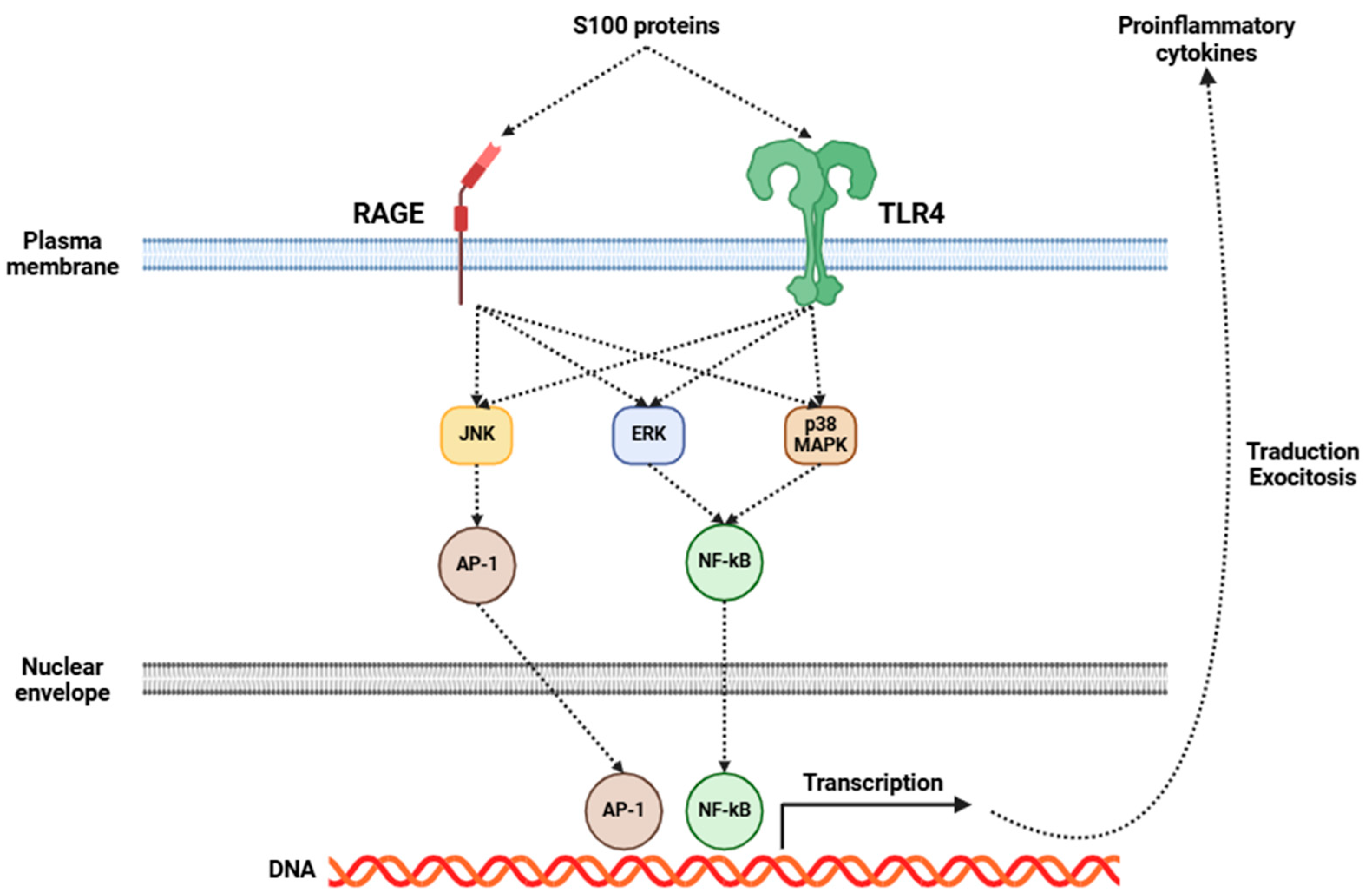
Fibromyalgia has been increasingly recognized as a disorder intricately connected to central inflammation, with neuroinflammation emerging as an essential component of its pathophysiology [5,165,166]. Central to this process is the activation of glial cells, particularly microglia and astrocytes. Upon activation, these cells initiate a series of inflammatory events, resulting in the release of many pro-inflammatory cytokines, including IL-1β, IL-6, IL-8, IL-10, TNF-α, BDNF, and GDNF, among others [167,168]. Several investigations have shown elevated levels of some of the aforementioned cytokines in the CSF of fibromyalgia patients (Table 2), indicating a pervasive state of central inflammation [136,137,138,169]. Moreover, recent studies have emphasized the significance of S100 proteins in the field of fibromyalgia research [170]. These proteins are involved in many inflammatory processes, and they may exert a considerable influence on fibromyalgia’s development and progression. The role of S100 proteins in fibromyalgia may be mediated through RAGE and TLR4, which, in turn, activate signaling pathways that promote the release of several pro-inflammatory cytokines (Figure 2), as previously mentioned [170].
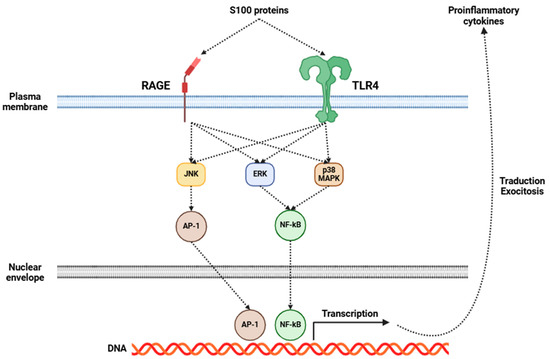
Figure 2.
Mechanisms of action triggered by the activation of RAGE and TLR4 receptors in glial cells by S100 proteins. This activation leads to the release of pro-inflammatory cytokines from these cells. Abbreviations: RAGE (receptor for advanced glycation endproducts), TLR4 (Toll-like receptor 4), JNK (c-Jun N-terminal kinase), ERK (extracellular-signal-regulated kinase), p38 MAPK (p38 mitogen-activated protein kinase), AP-1 (activator protein 1), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), and DNA (deoxyribonucleic acid).
Central sensitization enhances pain signaling, converting otherwise innocuous stimuli into significant sources of discomfort and contributing to the chronic and widespread pain characteristic of fibromyalgia. In addition to pain amplification, dysregulation of inflammatory processes leads to an imbalance between pro-inflammatory and anti-inflammatory cytokines [171]. As a result, the neuroinflammatory state is sustained, establishing a feedback loop that further worsens the condition [8]. Neuroimaging studies have further corroborated these findings, revealing microglial activation in patients with fibromyalgia [8,11]. The consequences of this persistent neuroinflammatory state extend beyond pain hypersensitivity, playing a key role in the development of a broad spectrum of debilitating symptoms commonly associated with fibromyalgia [172]. Cognitive dysfunction, often referred to as “fibro fog”, serves as an additional manifestation of this inflammatory state, likely stemming from the detrimental effects of cytokines on neural connectivity and synaptic function [173]. Sleep disturbances, which are both a symptom and a contributing factor to fibromyalgia, may be intricately linked to the inflammatory process, as cytokines have the ability to affect sleep regulation and disrupt restorative sleep cycles [174,175].
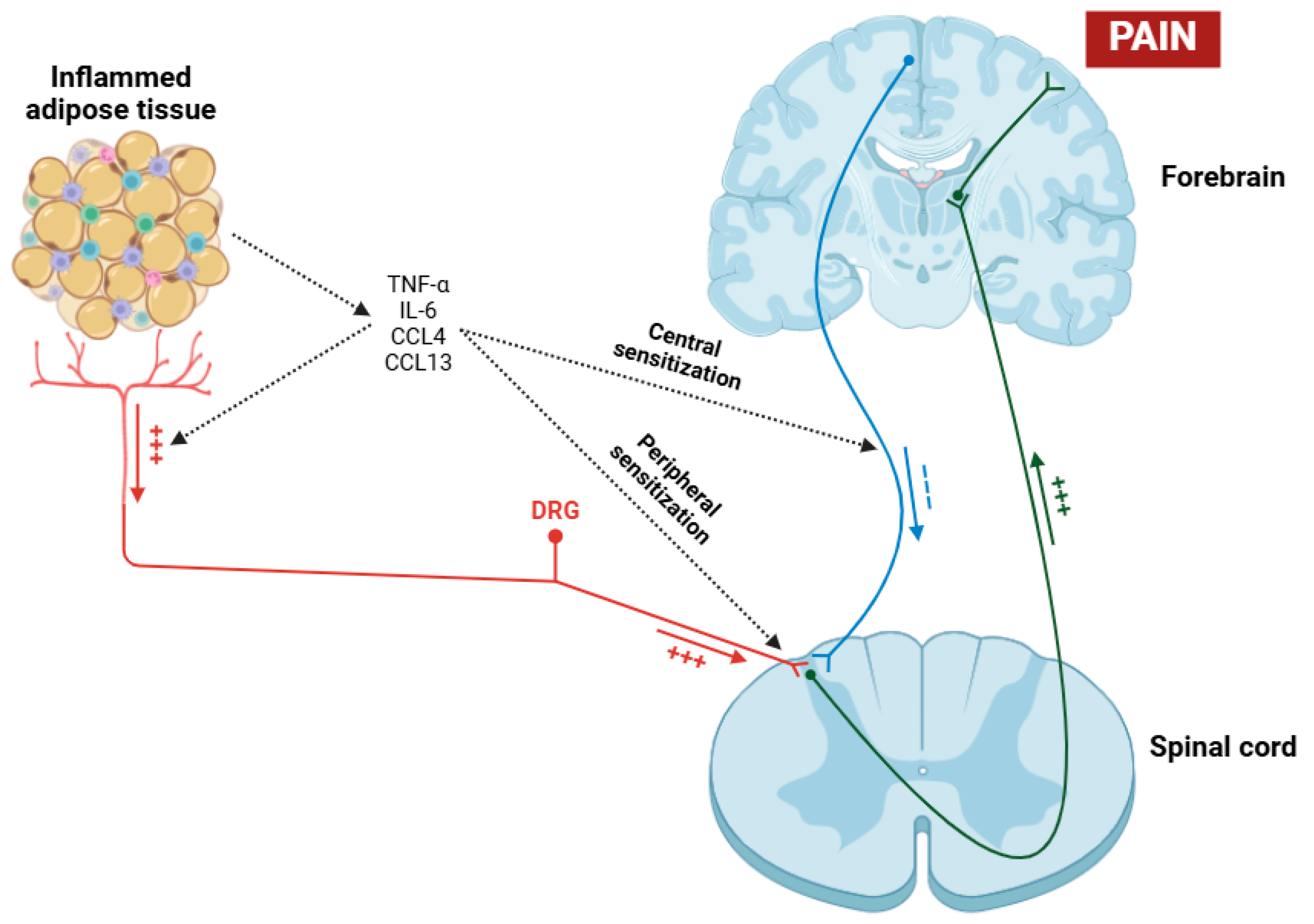
On the other hand, obesity exerts a profound and multifaceted influence on central inflammation in fibromyalgia patients [176,177]. The relationship between obesity and fibromyalgia is complex and bidirectional, with obesity potentially serving as a risk factor and an aggravating factor for this condition [178]. Obesity is involved in central inflammation through some mechanisms, primarily the secretion of pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, CCL4, and CCL13 by adipose tissue, primarily due to macrophages [179,180,181,182]. These cytokines, in addition to exerting their effects on the sensitization of nociceptors and dorsal root ganglia (DRGs) [183], can cross the blood–brain barrier (BBB) and have the potential to activate microglial cells placed in the CNS, thereby maintaining a state of neuroinflammation (Figure 3) [184,185]. Moreover, a recent study has shown that obesity in fibromyalgia patients acts as a disruptor of the descending pain pathway, thereby exacerbating symptoms associated with this condition [186].
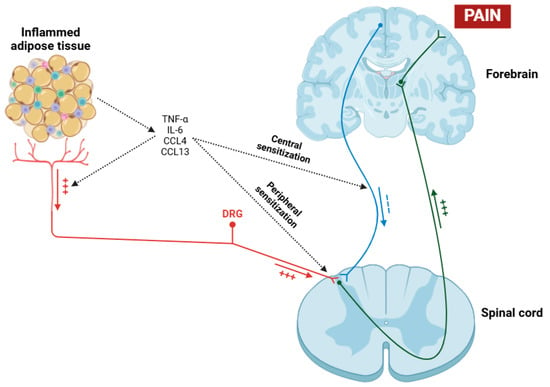
Figure 3.
Pathological process in which obesity acts as a contributing factor to hyperalgesia in patients with fibromyalgia. Through the mechanisms of peripheral and central sensitization induced by the action of various cytokines (TNF-α, IL-6, CCL4, and CCL13), nociceptive signal transmission to the cerebral cortex is facilitated, while descending inhibitory signals are suppressed. Abbreviations: TNF-α (tumor necrosis factor alpha), IL-6 (interleukin 6), CCL4 (chemokine (C-C motif) ligand 4), CCL13 (chemokine (C-C motif) ligand 13), and DRG (dorsal root ganglia).
Obesity is closely linked to metabolic dysregulation, including insulin resistance and leptin resistance, both of which play a role in the modulation of pain perception and neuroinflammation [187]. Higher levels of leptin have been found to correlate with increased pain sensitivity and inflammatory markers in fibromyalgia patients, suggesting a direct relationship between adiposity and central pain processing [188,189]. Furthermore, obesity exacerbates systemic low-grade inflammation, which amplifies central sensitization processes characteristic of fibromyalgia, establishing a vicious cycle in which pain and inflammation sustain each other [190]. The comorbidity of obesity and fibromyalgia is also linked to worsened clinical outcomes (such as higher pain intensity) and reduced physical function; however, increased fatigue can further compromise quality of life [191]. In addition to these effects, obesity usually results in sleep disturbances and obstructive sleep apnea, both of which are prevalent in fibromyalgia and contribute to the further disruption of inflammatory and neuroendocrine pathways [176].
Common behavioral and lifestyle factors, including physical inactivity and poor dietary habits, also contribute to both conditions, perpetuating the inflammatory state and hindering effective management [190]. Given this interplay, addressing obesity in fibromyalgia patients through weight management, anti-inflammatory interventions, and lifestyle modifications may offer a promising therapeutic approach to mitigate central inflammation, reduce symptom severity, and improve overall health outcomes. Nevertheless, it is very important to recognize the complexity of these interactions, as they have a significant impact on treatment effectiveness. Although these factors are crucial, additional underlying mechanisms must also be explored to obtain a thorough understanding of their impact on patient health.
4. Current Anti-Inflammatory Strategies for Fibromyalgia
Contemporary anti-inflammatory interventions for fibromyalgia condense a diverse array of pharmacological and non-pharmacological therapies. Clinical guidelines recommend low-dose acetaminophen and NSAIDs for managing some chronic pain conditions linked to fibromyalgia [192,193,194]. Although typically used, no Cochrane review supports the effectiveness of acetaminophen for fibromyalgia. In contrast, a Cochrane review of six randomized controlled trials (RCTs) found that NSAIDs did not provide a significant pain-relief benefit compared to placebo [195]. Moreover, the European League Against Rheumatism (EULAR) does not recommend the use of NSAIDs [196].
Fibromyalgia is classified as a condition linked to low-grade inflammation. This level of inflammation is likely too mild for NSAID treatments to be effective on their own [197]. However, NSAIDs are more effective in patients with fibromyalgia who also have comorbid inflammatory conditions, like osteoarthritis [192], migraine [193], and rheumatoid arthritis [194]. This is due to the presence of a higher degree of inflammation, which is substantial enough for NSAIDs to be highly effective. In such cases, the NSAIDs can target the more pronounced inflammatory processes, leading to greater symptom relief [198]. As a result, this enhanced efficacy not only helps reduce the pain associated with the comorbid condition but also contributes to an overall improvement in the symptoms of fibromyalgia.
Several NSAIDs, including ibuprofen, naproxen, and aspirin, are usually advocated for pain management, although their prolonged administration poses inherent risks, such as gastrointestinal hemorrhaging and cardiovascular problems [199]. Administration of NSAIDs, for example, celecoxib, is within the context of combination therapies that synergize with the antiviral agent famciclovir. This strategy has demonstrated potential in some clinical studies aimed at reducing fibromyalgia-related pain and fatigue [200].
Non-pharmacological therapies, including dietary modifications, play a crucial role in managing fibromyalgia and are typically regarded as first-line treatments due to their positive safety profiles and potential for long-term benefits [201]. Among these therapies, dietary modifications are gaining recognition as vital components of an anti-inflammatory strategy for managing fibromyalgia [202,203]. Dietary changes (e.g., anti-inflammatory diets) have yielded prominent results in the management of fibromyalgia symptoms [204,205]. Novel investigations have examined many dietary strategies, including an anti-inflammatory FODMAP diet, which resulted in better patient-reported outcomes, including reduced pain, fatigue, and gastrointestinal challenges. This diet excludes gluten, dairy, sugar, and ultra-processed foods [206]. A personalized Mediterranean diet has also shown affirmative effects on pain reduction and quality of life enhancement in fibromyalgia patients [207]. Furthermore, a gluten-free (FODMAP) and low histamine diet (IGUBAC-Diet®), when combined with an olive-tree-based supplement, exhibited advantageous effects on the severity of fibromyalgia symptoms [208]. On the other hand, while many research have studied the potential benefits of supplementation with antioxidants, magnesium, CoQ10, and vitamins C and D, the management of symptomatology remains a complex domain [209,210,211,212,213,214]. In fact, the Dietary Inflammatory Index (DII) has been correlated with pressure pain thresholds in fibromyalgia patients; suggesting that a diet with a reduced inflammatory profile may help mitigate pain hypersensitivity [204].
These dietary interventions, aimed at reducing inflammation, are designed to modulate the gut microbiome and address potential nutrient deficiencies that may contribute to fibromyalgia symptoms [215]. Although further research is required to establish definitive dietary guidelines for fibromyalgia management, the available evidence suggests that an anti-inflammatory diet approach may constitute a valuable component of comprehensive treatment plans for individuals with fibromyalgia [216]. However, it is crucial to recognize that this approach is not universally applicable, because individual responses to dietary changes can vary significantly [217].
Other significant non-pharmacological therapies include physical exercise, particularly aerobic and strength training programs, which have been proven effective in reducing symptoms such as pain, sleep disturbances, fatigue, and depression [218]. Aerobic exercise enhances pain relief and functionality by stimulating endogenous opioid pathways and promoting the activation of opioid receptors in the CNS [219]. Regular exercise also promotes an increase in β-END synthesis, which exerts an anti-inflammatory effect and contributes to increase pain thresholds [220,221,222].
Although these interventions are supported by a growing body of evidence, the quality of studies varies significantly, underscoring the necessity for further rigorous research [223]. However, the existing evidence underscores the great importance of incorporating non-pharmacological treatments into a multidisciplinary approach to fibromyalgia care, as they can notably improve patient outcomes [224].
5. Conclusions
Fibromyalgia is a chronic condition marked by severe musculoskeletal pain, fatigue, sleep disturbances, and cognitive impairments. Numerous investigations suggest that inflammation plays a fundamental role in the onset and development of this condition. On the other hand, fibromyalgia is characterized by increased contents of pro-inflammatory cytokines in some biological fluids (e.g., plasma and serum), immune dysregulation, and neuroinflammation processes. Chronic low-grade inflammation appears to contribute significantly to the sensitization of pain pathways, thereby exacerbating fibromyalgia symptoms.
This review offers a comprehensive analysis of the role of inflammation in the pathophysiology of fibromyalgia, clarifying its significant contribution to chronic pain experienced by affected individuals. In addition to explaining several characteristics of this pathology, this paper highlights that both peripheral and central inflammation processes are crucial factors in the initiation and progression of fibromyalgia symptoms. Specifically, it emphasizes the influence of pro-inflammatory cytokines in the development of neuroinflammation and how this biological phenomenon contributes significantly to the dysregulation of pain processing pathways, amplifying the obstacles experienced by individuals with this disease. Although the complexity of the mechanisms involved seems formidable, the relationship between inflammation and pain is defined by a strong interaction: chronic pain exacerbates inflammatory responses, and these persistent inflammatory processes, in turn, intensify pain sensation. This vicious cycle significantly contributes to the debilitating symptoms of fibromyalgia; however, understanding it is crucial for developing effective therapeutic strategies. In summary, future research could investigate the function of inflammatory markers in patients with fibromyalgia, aiming to determine whether they contribute to the peripheral and central sensitization that defines this condition. Additionally, investigating the impact of many environmental factors, genetic predispositions, and comorbid conditions on the inflammatory response in fibromyalgia will be essential for advancing personalized treatment approaches.
On the other hand, the utilization of NSAIDs in fibromyalgia has been a subject of continuous research and clinical investigation. Although fibromyalgia is associated with an inflammatory component, studies indicate that NSAIDs alone are not significantly effective in managing the condition. This limited efficacy implies that the inflammation associated with fibromyalgia may differ in both complexity and nature from the type typically targeted by NSAIDs. However, the identification of a potential inflammatory component in fibromyalgia has driven the advancement of new therapeutic strategies. While NSAIDs might not offer a definitive solution, the focus on inflammation has motivated researchers to explore more targeted approaches to modulate the inflammatory response in fibromyalgia patients.
Finally, future research will focus on identifying specific subgroups of fibromyalgia patients who may derive greater benefits from NSAIDs while also exploring the potential integration of these drugs with other therapies. Additionally, investigations are expected to examine how NSAIDs may influence neuroinflammatory processes that are believed to play a fundamental role in the development of fibromyalgia symptoms. However, current research on the efficacy of NSAIDs for fibromyalgia is limited, particularly due to the lack of long-term studies. Longitudinal research is crucial to understanding the long-term effects of NSAIDs on fibromyalgia, aiding informed treatment decisions.
Funding
This research received no external funding.
Data Availability Statement
No new data were generated.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-HT | Serotonin |
| 5-HT2A | Serotonin 5-HT2A receptor |
| 5-HT2C | Serotonin 5-HT2C receptor |
| 5-HT3 | Serotonin 5-HT3 receptor |
| ACR | American College of Rheumatology |
| ANXA1 | Annexin A1 |
| AP-1 | Activator protein 1 |
| ASIC | Acid-sensing ion channel |
| AXIN1 | Axis inhibition protein 1 |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| C1QC | Complement C1q C chain |
| C4A | Complement component 4 |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| CCL13 | Chemokine (C-C motif) ligand 13 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCL4 | Chemokine (C-C motif) ligand 4 |
| CD163 | Cluster of differentiation 163 |
| CNS | Central nervous system |
| CoQ10 | Coenzyme Q10 |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| CSU | Chronic spontaneous urticaria |
| CX3CL1 | Chemokine (C-X3-C motif) ligand 1 |
| D2 | Dopamine D2 receptor |
| DII | Dietary Inflammatory Index |
| DNA | Deoxyribonucleic acid |
| DRG | Dorsal root ganglia |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinase |
| EULAR | European Alliance of Associations for Rheumatology |
| FBD | Functional bowel disorder |
| FMT | Fecal microbiota transplantation |
| FODMAP | Fermentable oligo-, di-, and mono-saccharides and polyols |
| GDNF | Glial-cell-line-derived neurotrophic factor |
| JNK | c-Jun N-terminal kinase |
| IBS | Irritable bowel syndrome |
| IGF-1 | Insulin-like growth factor 1 |
| IL-10 | Interleukin 10 |
| IL-13 | Interleukin 13 |
| IL-17 | Interleukin 17 |
| IL-1β | Interleukin 1 beta |
| IL-21 | Interleukin 21 |
| IL-22 | Interleukin 22 |
| IL-37 | Interleukin 37 |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IGUBAC-Diet® | Inflammatory gut–brain axis control diet |
| MMP-3 | Matrix metalloproteinase-3 |
| MOR | μ-opioid receptor |
| NA | Noradrenaline |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NMDA | N-methyl-D-aspartate |
| NPY | Neuropeptide Y |
| NRI | Selective noradrenaline reuptake inhibitor |
| NSAID | Non-steroidal anti-inflammatory drug |
| p38 MAPK | p38 mitogen-activated protein kinase |
| PGAM1 | Phosphoglycerate mutase 1 |
| RAGE | Receptor for advanced glycation end products |
| RCT | Randomized controlled trial |
| RLS | Restless leg syndrome |
| RNA | Ribonucleic acid |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| S100 | S100 protein |
| S100A8 | S100 calcium-binding protein A8 |
| S100A9 | S100 calcium-binding protein A9 |
| SERPINA1 | Serpin family A member 1 |
| SIRT2 | NAD-dependent deacetylase sirtuin 2 |
| SNRI | Serotonin-noradrenaline reuptake inhibitor |
| SP | Substance P |
| SS | Symptom Severity |
| SSRI | Selective serotonin reuptake inhibitor |
| TCA | Tricyclic antidepressant |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| TRPV | Transient receptor potential vanilloid |
| VCAM | Vascular cell adhesion molecule |
| VGCC | Voltage-gated calcium channel |
| WPI | Widespread Pain Index |
References
- Jurado-Priego, L.N.; Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Fibromyalgia: A Review of the Pathophysiological Mechanisms and Multidisciplinary Treatment Strategies. Biomedicines 2024, 12, 1543. [Google Scholar] [CrossRef] [PubMed]
- Ruschak, I.; Montesó-Curto, P.; Rosselló, L.; Aguilar Martín, C.; Sánchez-Montesó, L.; Toussaint, L. Fibromyalgia Syndrome Pain in Men and Women: A Scoping Review. Healthcare 2023, 11, 223. [Google Scholar] [CrossRef]
- Marques, A.P.; Santo, A.S.D.E.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of fibromyalgia: Literature review update. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Mezhov, V.; Guymer, E.; Littlejohn, G. Central sensitivity and fibromyalgia. Intern. Med. J. 2021, 51, 1990–1998. [Google Scholar] [CrossRef]
- Qureshi, A.G.; Jha, S.K.; Iskander, J.; Avanthika, C.; Jhaveri, S.; Patel, V.H.; Rasagna Potini, B.; Talha Azam, A. Diagnostic Challenges and Management of Fibromyalgia. Cureus 2021, 13, e18692. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.R.; Jeyaraman, M.; Jeyaraman, N.; Nallakumarasamy, A.; Khanna, M.; Gupta, A.; Yadav, S. Beyond the Pain: A Systematic Narrative Review of the Latest Advancements in Fibromyalgia Treatment. Cureus 2023, 15, e48032. [Google Scholar] [CrossRef]
- Mueller, C.; Fang, Y.D.; Jones, C.; McConathy, J.E.; Raman, F.; Lapi, S.E.; Younger, J.W. Evidence of neuroinflammation in fibromyalgia syndrome: A [18F]DPA-714 positron emission tomography study. Pain 2023, 164, 2285–2295. [Google Scholar] [CrossRef]
- Ang, D.C.; Moore, M.N.; Hilligoss, J.; Tabbey, R. MCP-1 and IL-8 as pain biomarkers in fibromyalgia: A pilot study. Pain Med. 2011, 12, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Altawil, R.; Kadetoff, D.; Finn, A.; Westman, M.; Le Maître, E.; Andersson, M.; Jensen-Urstad, M.; Lampa, J. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain--interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J. Neuroimmunol. 2015, 280, 49–55. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Höglund, C.O.; Catana, C.; et al. Brain glial activation in fibromyalgia—A multi-site positron emission tomography investigation. Brain Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Loggia, M.L. “Neuroinflammation”: Does it have a role in chronic pain? Evidence from human imaging. Pain 2024, 165, S58–S67. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Ifuku, M.; Hossain, M.S.; Katafuchi, T. Glial Activation and Expression of the Serotonin Transporter in Chronic Fatigue Syndrome. Front. Psychiatry. 2018, 9, 589. [Google Scholar] [CrossRef]
- Fineschi, S.; Klar, J.; Gustafsson, K.A.; Jonsson, K.; Karlsson, B.; Dahl, N. Inflammation and Interferon Signatures in Peripheral B-Lymphocytes and Sera of Individuals with Fibromyalgia. Front. Immunol. 2022, 13, 874490. [Google Scholar] [CrossRef]
- Serra, J.; Collado, A.; Solà, R.; Antonelli, F.; Torres, X.; Salgueiro, M.; Quiles, C.; Bostock, H. Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 2014, 75, 196–208. [Google Scholar] [CrossRef]
- Chen, W.N.; Lee, C.H.; Lin, S.H.; Wong, C.W.; Sun, W.H.; Wood, J.N.; Chen, C.C. Roles of ASIC3, TRPV1, and NaV1.8 in the transition from acute to chronic pain in a mouse model of fibromyalgia. Mol. Pain 2014, 10, 40. [Google Scholar] [CrossRef]
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef]
- Arnold, L.M.; Gebke, K.B.; Choy, E.H. Fibromyalgia: Management strategies for primary care providers. Int. J. Clin. Pract. 2016, 70, 99–112. [Google Scholar] [CrossRef]
- Culpepper, L. Evaluating the patient with fibromyalgia. J. Clin. Psychiatry 2010, 71, 27684. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.T.; Vincent, A. A historical and clinical perspective endorsing person-centered management of fibromyalgia syndrome. Curr. Rheumatol. Rev. 2015, 11, 86–95. [Google Scholar] [CrossRef]
- Vincent, A.; Benzo, R.P.; Whipple, M.O.; McAllister, S.J.; Erwin, P.J.; Saligan, L.N. Beyond pain in fibromyalgia: Insights into the symptom of fatigue. Arthritis Res. Ther. 2013, 15, 221. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Reyes Del Paso, G.A.; Duschek, S. Cognitive Impairments in Fibromyalgia Syndrome: Associations with Positive and Negative Affect, Alexithymia, Pain Catastrophizing and Self-Esteem. Front. Psychol. 2018, 9, 377. [Google Scholar] [CrossRef]
- Kratz, A.L.; Whibley, D.; Kim, S.; Sliwinski, M.; Clauw, D.; Williams, D.A. Fibrofog in Daily Life: An Examination of Ambulatory Subjective and Objective Cognitive Function in Fibromyalgia. Arthritis Care. Res. 2020, 72, 1669–1677. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Berwick, R.; Barker, C.; Goebel, A.; guideline development group. The diagnosis of fibromyalgia syndrome. Clin. Med. 2022, 22, 570–574. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care. Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Wolfe, F.; Egloff, N.; Häuser, W. Widespread Pain and Low Widespread Pain Index Scores among Fibromyalgia-positive Cases Assessed with the 2010/2011 Fibromyalgia Criteria. J. Rheumatol. 2016, 43, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Huang, C.J.; Fang, S.C.; Ko, L.H.; Tsai, P.S. Cognitive Impairment in Fibromyalgia: A Meta-Analysis of Case-Control Studies. Psychosom. Med. 2018, 80, 432–438. [Google Scholar] [CrossRef]
- Hackshaw, K.V. The Search for Biomarkers in Fibromyalgia. Diagnostics 2021, 11, 156. [Google Scholar] [CrossRef]
- Palacio, A.; Uribe, C.L.; Li, H.; Hanna, J.; Deminski, M.; Alvir, J.; Chandran, A.; Sanchez, R. Financial and clinical characteristics of fibromyalgia: A case-control comparison. Am. J. Manag. Care 2010, 16, S118–S125. [Google Scholar]
- Kocyigit, B.F.; Akyol, A. Fibromyalgia syndrome: Epidemiology, diagnosis and treatment. Reumatologia 2022, 60, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Ghavidel-Parsa, B.; Bidari, A.; Amir Maafi, A.; Ghalebaghi, B. The Iceberg Nature of Fibromyalgia Burden: The Clinical and Economic Aspects. Korean J. Pain 2015, 28, 169–176. [Google Scholar] [CrossRef]
- Cabo-Meseguer, A.; Cerdá-Olmedo, G.; Trillo-Mata, J.L. Fibromyalgia: Prevalence, epidemiologic profiles and economic costs. Med. Clin. 2017, 149, 441–448. [Google Scholar] [CrossRef]
- Perrot, S.; Vicaut, E.; Servant, D.; Ravaud, P. Prevalence of fibromyalgia in France: A multi-step study research combining national screening and clinical confirmation: The DEFI study (Determination of Epidemiology of FIbromyalgia). BMC Musculoskelet. Disord. 2011, 12, 224. [Google Scholar] [CrossRef]
- Wolfe, F.; Brähler, E.; Hinz, A.; Häuser, W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: Results from a survey of the general population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Loza, E.; Abásolo, L.; Jover, J.A.; Carmona, L.; EPISER Study Group. Burden of disease across chronic diseases: A health survey that measured prevalence, function, and quality of life. J. Rheumatol. 2008, 35, 159–165. [Google Scholar]
- Vincent, A.; Lahr, B.D.; Wolfe, F.; Clauw, D.J.; Whipple, M.O.; Oh, T.H.; Barton, D.L.; St Sauver, J. Prevalence of fibromyalgia: A population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res. 2013, 65, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Rusu, C.; Gee, M.E.; Lagacé, C.; Parlor, M. Chronic fatigue syndrome and fibromyalgia in Canada: Prevalence and associations with six health status indicators. Health Promot. Chronic Dis. Prev. Can. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Lee, L.K.; Ebata, N.; Hlavacek, P.; DiBonaventura, M.; Cappelleri, J.C.; Sadosky, A. Humanistic and economic burden of fibromyalgia in Japan. J. Pain Res. 2016, 9, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Cronan, T.A.; Serber, E.R.; Walen, H.R.; Jaffe, M. The influence of age on fibromyalgia symptoms. J. Aging Health 2002, 14, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Arout, C.A.; Sofuoglu, M.; Bastian, L.A.; Rosenheck, R.A. Gender Differences in the Prevalence of Fibromyalgia and in Concomitant Medical and Psychiatric Disorders: A National Veterans Health Administration Study. J. Womens Health 2018, 27, 1035–1044. [Google Scholar] [CrossRef]
- Kleykamp, B.A.; Ferguson, M.C.; McNicol, E.; Bixho, I.; Arnold, L.M.; Edwards, R.R.; Fillingim, R.; Grol-Prokopczyk, H.; Turk, D.C.; Dworkin, R.H. The Prevalence of Psychiatric and Chronic Pain Comorbidities in Fibromyalgia: An ACTTION systematic review. Semin. Arthritis Rheum. 2021, 51, 166–174. [Google Scholar]
- Fitzcharles, M.A.; Perrot, S.; Häuser, W. Comorbid fibromyalgia: A qualitative review of prevalence and importance. Eur. J. Pain 2018, 22, 1565–1576. [Google Scholar] [CrossRef]
- Assumpção, A.; Cavalcante, A.B.; Capela, C.E.; Sauer, J.F.; Chalot, S.D.; Pereira, C.A.; Marques, A.P. Prevalence of fibromyalgia in a low socioeconomic status population. BMC Musculoskelet. Disord. 2009, 10, 64. [Google Scholar]
- McDonald, M.; DiBonaventura, M.d.; Ullman, S. Musculoskeletal pain in the workforce: The effects of back, arthritis, and fibromyalgia pain on quality of life and work productivity. J. Occup. Environ. Med. 2011, 53, 765–770. [Google Scholar]
- Palstam, A.; Mannerkorpi, K. Work Ability in Fibromyalgia: An Update in the 21st Century. Curr. Rheumatol. Rev. 2017, 13, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Dukes, E.; Martin, S.; Edelsberg, J.; Oster, G. Characteristics and healthcare costs of patients with fibromyalgia syndrome. Int. J. Clin. Pract. 2007, 61, 1498–1508. [Google Scholar] [CrossRef]
- Amris, K.; Ibsen, R.; Duhn, P.H.; Olsen, J.; Lolk, K.; Kjellberg, J.; Kristensen, L.E. Health inequities and societal costs for patients with fibromyalgia and their spouses: A Danish cohort study. RMD Open 2024, 10, e003904. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Duschek, S.; Reyes Del Paso, G.A. Psychological impact of fibromyalgia: Current perspectives. Psychol. Res. Behav. Manag. 2019, 12, 117–127. [Google Scholar]
- Lawson, K. Tricyclic antidepressants and fibromyalgia: What is the mechanism of action? Expert. Opin. Investig. Drugs 2002, 11, 1437–1445. [Google Scholar] [CrossRef]
- Heymann, R.E.; Helfenstein, M.; Feldman, D. A double-blind, randomized, controlled study of amitriptyline, nortriptyline and placebo in patients with fibromyalgia. An analysis of outcome measures. Clin. Exp. Rheumatol. 2001, 19, 697–702. [Google Scholar] [PubMed]
- Lawson, K. A Brief Review of the Pharmacology of Amitriptyline and Clinical Outcomes in Treating Fibromyalgia. Biomedicines 2017, 5, 24. [Google Scholar] [CrossRef]
- Godfrey, R.G. A guide to the understanding and use of tricyclic antidepressants in the overall management of fibromyalgia and other chronic pain syndromes. Arch. Intern. Med. 1996, 156, 1047–1052. [Google Scholar] [CrossRef]
- Thiwan, S.; Drossman, D.A.; Morris, C.B.; Dalton, C.; Toner, B.B.; Diamant, N.E.; Hu, J.B.; Whitehead, W.E.; Leserman, J.; Bangdiwala, S.I. Not all side effects associated with tricyclic antidepressant therapy are true side effects. Clin. Gastroenterol. Hepatol. 2009, 7, 446–451. [Google Scholar] [CrossRef][Green Version]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Baroncini, A.; Bell, A.; Colarossi, G. Duloxetine for fibromyalgia syndrome: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2023, 18, 504. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Girma, B.; Jenkins, J.S.; Kaufman, S.E.; Lee, C.A.; Kaye, A.D. Milnacipran for the Treatment of Fibromyalgia. Health Psychol. Res. 2021, 9, 25532. [Google Scholar] [CrossRef]
- Welsch, P.; Üçeyler, N.; Klose, P.; Walitt, B.; Häuser, W. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst. Rev. 2018, 2, CD010292. [Google Scholar]
- Shelton, R.C. Serotonin and Norepinephrine Reuptake Inhibitors. Handb. Exp. Pharmacol. 2019, 250, 145–180. [Google Scholar]
- Hayashida, K.I.; Obata, H. Strategies to Treat Chronic Pain and Strengthen Impaired Descending Noradrenergic Inhibitory System. Int. J. Mol. Sci. 2019, 20, 822. [Google Scholar] [CrossRef] [PubMed]
- Krell, H.V.; Leuchter, A.F.; Cook, I.A.; Abrams, M. Evaluation of reboxetine, a noradrenergic antidepressant, for the treatment of fibromyalgia and chronic low back pain. Psychosomatics 2005, 46, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Hirsch, I.; Sanders, P.; Ellis, A.; Hughes, B. Safety and efficacy of esreboxetine in patients with fibromyalgia: A fourteen-week, randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis Rheum. 2012, 64, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Nikirk, J.; Chiu, J.; Brown, A.; Woolford, M. Symptomatic Improvement of Fibromyalgia Symptoms with Atomoxetine (P5-13.008). Neurology 2024, 102, 3690. [Google Scholar] [CrossRef]
- Walitt, B.; Urrútia, G.; Nishishinya, M.B.; Cantrell, S.E.; Häuser, W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst. Rev. 2015, 2015, CD011735. [Google Scholar]
- Anderberg, U.M.; Marteinsdottir, I.; von Knorring, L. Citalopram in patients with fibromyalgia--a randomized, double-blind, placebo-controlled study. Eur. J. Pain 2000, 4, 27–35. [Google Scholar] [CrossRef]
- Arnold, L.M.; Hess, E.V.; Hudson, J.I.; Welge, J.A.; Berno, S.E.; Keck, P.E., Jr. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am. J. Med. 2002, 112, 191–197. [Google Scholar] [CrossRef]
- Patkar, A.A.; Masand, P.S.; Krulewicz, S.; Mannelli, P.; Peindl, K.; Beebe, K.L.; Jiang, W. A randomized, controlled, trial of controlled release paroxetine in fibromyalgia. Am. J. Med. 2007, 120, 448–454. [Google Scholar] [CrossRef] [PubMed]
- González-Viejo, M.A.; Avellanet, M.; Hernández-Morcuende, M.I. A comparative study of fibromyalgia treatment: Ultrasonography and physiotherapy versus sertraline treatment. Ann. Readapt. Med. Phys. 2005, 48, 610–615. [Google Scholar] [CrossRef]
- Albunayyan, R.F. A meta-analysis of the effect of selective serotonin reuptake inhibitors on pain in fibromyalgia. J. Pharm. Res. Int. 2022, 34, 54–65. [Google Scholar] [CrossRef]
- Häuser, W.; Wolfe, F.; Tölle, T.; Uçeyler, N.; Sommer, C. The role of antidepressants in the management of fibromyalgia syndrome: A systematic review and meta-analysis. CNS Drugs 2012, 26, 297–307. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, R.; Ablin, J.N. Current and Emerging Pharmacotherapy for Fibromyalgia. Pain. Res. Manag. 2020, 2020, 6541798. [Google Scholar] [CrossRef]
- Lederman, S.; Arnold, L.M.; Vaughn, B.; Kelley, M.; Sullivan, G.M. Efficacy and Safety of Sublingual Cyclobenzaprine for the Treatment of Fibromyalgia: Results from a Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care. Res. 2023, 75, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Späth, M.; Stratz, T.; Färber, L.; Haus, U.; Pongratz, D. Treatment of fibromyalgia with tropisetron—Dose and efficacy correlations. Scand. J. Rheumatol. Suppl. 2004, 119, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Morillas-Arques, P.; Rodriguez-Lopez, C.M.; Molina-Barea, R.; Rico-Villademoros, F.; Calandre, E.P. Trazodone for the treatment of fibromyalgia: An open-label, 12-week study. BMC Musculoskelet. Disord. 2010, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D. Targeting the 5-HT system: Potential side effects. Neuropharmacology 2020, 179, 108233. [Google Scholar] [CrossRef]
- Derry, S.; Cording, M.; Wiffen, P.J.; Law, S.; Phillips, T.; Moore, R.A. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst. Rev. 2016, 9, CD011790. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Knobe, M.; Tenze, G.; Aljalloud, A.; Colarossi, G. Pregabalin administration in patients with fibromyalgia: A Bayesian network meta-analysis. Sci. Rep. 2022, 12, 12148. [Google Scholar] [CrossRef]
- Häuser, W.; Bernardy, K.; Uçeyler, N.; Sommer, C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—A meta-analysis of randomized controlled trials. Pain 2009, 145, 69–81. [Google Scholar] [CrossRef]
- Athavale, A.; Murnion, B. Gabapentinoids: A therapeutic review. Aust. Prescr. 2023, 46, 80–85. [Google Scholar] [CrossRef]
- Arnold, L.M.; Whitaker, S.; Hsu, C.; Jacobs, D.; Merante, D. Efficacy and safety of mirogabalin for the treatment of fibromyalgia: Results from three 13-week randomized, double-blind, placebo- and active-controlled, parallel-group studies and a 52-week open-label extension study. Curr. Med. Res. Opin. 2019, 35, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, A.; Koyyalagunta, D.; Huh, B.; D’Souza, R.S.; Javed, S. A comprehensive review of the typical and atypical side effects of gabapentin. Pain Pract. 2024, 24, 1051–1058. [Google Scholar] [CrossRef]
- Calandre, E.P.; Rico-Villademoros, F. The role of antipsychotics in the management of fibromyalgia. CNS Drugs 2012, 26, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Morin, M.; Cloutier, C.; Gendron, A.; Bissonnette, A.; Marchand, S. Add-on treatment of quetiapine for fibromyalgia: A pilot, randomized, double-blind, placebo-controlled 12-week trial. J. Clin. Psychopharmacol. 2012, 32, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Freedenfeld, R.N.; Murray, M.; Fuchs, P.N.; Kiser, R.S. Decreased pain and improved quality of life in fibromyalgia patients treated with olanzapine, an atypical neuroleptic. Pain Pract. 2006, 6, 112–118. [Google Scholar] [CrossRef]
- Chow, R.T.S.; Whiting, D.; Favril, L.; Ostinelli, E.; Cipriani, A.; Fazel, S. An umbrella review of adverse effects associated with antipsychotic medications: The need for complementary study designs. Neurosci. Biobehav. Rev. 2023, 155, 105454. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; MacKie, P.J.; Kareken, D.A.; Hutchins, G.D.; Chumin, E.J.; Christian, B.T.; Yoder, K.K. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016, 10, 829–839. [Google Scholar] [CrossRef]
- Wood, P.B.; Schweinhardt, P.; Jaeger, E.; Dagher, A.; Hakyemez, H.; Rabiner, E.A.; Bushnell, M.C.; Chizh, B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007, 25, 3576–3582. [Google Scholar] [CrossRef]
- Holman, A.J.; Myers, R.R. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005, 52, 2495–2505. [Google Scholar] [CrossRef]
- Holman, A.J. Ropinirole, open preliminary observations of a dopamine agonist for refractory fibromyalgia. J. Clin. Rheumatol. 2003, 9, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Li, B.D.; Bi, Z.Y.; Liu, J.F.; Si, W.J.; Shi, Q.Q.; Xue, L.P.; Bai, J. Adverse effects produced by different drugs used in the treatment of Parkinson’s disease: A mixed treatment comparison. CNS Neurosci. Ther. 2017, 23, 827–842. [Google Scholar] [CrossRef]
- da Rocha, A.P.; Mizzaci, C.C.; Nunes Pinto, A.C.P.; da Silva Vieira, A.G.; Civile, V.; Trevisani, V.F.M. Tramadol for management of fibromyalgia pain and symptoms: Systematic review. Int. J. Clin. Pract. 2020, 74, e13455. [Google Scholar] [CrossRef]
- Rivera, J.; Molina-Collada, J.; Martínez-Barrio, J.; Serrano-Benavente, B.; Castrejón, I.; Vallejo, M.A.; Álvaro-Gracia, J.M. Opioids and fibromyalgia: Frequency of use and factors associated with increased consumption in patients remitted to a tertiary care center. BMC Musculoskelet. Disord. 2024, 25, 121. [Google Scholar] [CrossRef]
- MacLean, A.J.; Schwartz, T.L. Tramadol for the treatment of fibromyalgia. Expert. Rev. Neurother. 2015, 15, 469–475. [Google Scholar] [CrossRef]
- van de Donk, T.; van Velzen, M.; Dahan, A.; Niesters, M. Cornea nerve fibre state determines analgesic response to tapentadol in fibromyalgia patients without effective endogenous pain modulation. Eur. J. Pain 2019, 23, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Roulet, L.; Rollason, V.; Desmeules, J.; Piguet, V. Tapentadol Versus Tramadol: A Narrative and Comparative Review of Their Pharmacological, Efficacy and Safety Profiles in Adult Patients. Drugs 2021, 81, 1257–1272. [Google Scholar] [CrossRef]
- Yang, J.; Shin, K.M.; Do, A.; Bierle, D.M.; Abu Dabrh, A.M.; Yin, Z.; Bauer, B.A.; Mohabbat, A.B. The Safety and Efficacy of Low-Dose Naltrexone in Patients with Fibromyalgia: A Systematic Review. J. Pain Res. 2023, 16, 1017–1023. [Google Scholar] [CrossRef]
- Li, W.; McIntyre, R.L.; Schomakers, B.V.; Kamble, R.; Luesink, A.H.G.; van Weeghel, M.; Houtkooper, R.H.; Gao, A.W.; Janssens, G.E. Low-dose naltrexone extends healthspan and lifespan in C. elegans via SKN-1 activation. iScience 2024, 27, 109949. [Google Scholar] [CrossRef] [PubMed]
- Tempel, A.; Kessler, J.A.; Zukin, R.S. Chronic naltrexone treatment increases expression of preproenkephalin and preprotachykinin mRNA in discrete brain regions. J. Neurosci. 1990, 10, 741–747. [Google Scholar] [CrossRef]
- Li, Z.; You, Y.; Griffin, N.; Feng, J.; Shan, F. Low-dose naltrexone (LDN): A promising treatment in immune-related diseases and cancer therapy. Int. Immunopharmacol. 2018, 61, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet. Disord. 2007, 8, 27. [Google Scholar] [CrossRef]
- Gaskell, H.; Moore, R.A.; Derry, S.; Stannard, C. Oxycodone for pain in fibromyalgia in adults. Cochrane Database Syst. Rev. 2016, 9, CD012329. [Google Scholar] [CrossRef]
- Mullican, W.S.; Lacy, J.R.; TRAMAP-ANAG-006 Study Group. Tramadol/acetaminophen combination tablets and codeine/acetaminophen combination capsules for the management of chronic pain: A comparative trial. Clin. Ther. 2001, 23, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D.; Staud, R.; Robinson, M.E.; Mauderli, A.P.; Cannon, R.; Vierck, C.J. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002, 99, 49–59. [Google Scholar] [CrossRef]
- Comer, S.D.; Cahill, C.M. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci. Biobehav. Rev. 2019, 106, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, G.; Guymer, E. Modulation of NMDA Receptor Activity in Fibromyalgia. Biomedicines 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Pastrak, M.; Abd-Elsayed, A.; Ma, F.; Vrooman, B.; Visnjevac, O. Systematic Review of the Use of Intravenous Ketamine for Fibromyalgia. Ochsner J. 2021, 21, 387–394. [Google Scholar] [CrossRef]
- Olivan-Blázquez, B.; Herrera-Mercadal, P.; Puebla-Guedea, M.; Pérez-Yus, M.C.; Andrés, E.; Fayed, N.; López-Del-Hoyo, Y.; Magallon, R.; Roca, M.; Garcia-Campayo, J. Efficacy of memantine in the treatment of fibromyalgia: A double-blind, randomised, controlled trial with 6-month follow-up. Pain 2014, 155, 2517–2525. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Badraoui, R.; Jahan, S.; Alshahrani, M.M.; Siddiqui, M.A.; Khan, A.; Adnan, M. Targeting NMDA receptor in Alzheimer’s disease: Identifying novel inhibitors using computational approaches. Front. Pharmacol. 2023, 14, 1208968. [Google Scholar] [CrossRef]
- Kurlyandchik, I.; Lauche, R.; Tiralongo, E.; Warne, L.N.; Schloss, J. Plasma and interstitial levels of endocannabinoids and N-acylethanolamines in patients with chronic widespread pain and fibromyalgia: A systematic review and meta-analysis. Pain Rep. 2022, 7, e1045. [Google Scholar] [CrossRef]
- Bourke, S.L.; Schlag, A.K.; O’Sullivan, S.E.; Nutt, D.J.; Finn, D.P. Cannabinoids and the endocannabinoid system in fibromyalgia: A review of preclinical and clinical research. Pharmacol. Ther. 2022, 240, 108216. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, J.H.; Bunt, G.; Blum, K.; Maggirwar, S.B.; Galanter, M.; Potenza, M.N. Review: Cannabinoids as Medicinals. Curr. Addict. Rep. 2022, 9, 630–646. [Google Scholar] [CrossRef]
- Boehnke, K.F.; Gagnier, J.J.; Matallana, L.; Williams, D.A. Cannabidiol Use for Fibromyalgia: Prevalence of Use and Perceptions of Effectiveness in a Large Online Survey. J. Pain 2021, 22, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Wipfler, K.; Simon, T.A.; Katz, P.; Wolfe, F.; Michaud, K. Increase in Cannabis Use Among Adults with Rheumatic Diseases: Results from a 2014–2019 United States Observational Study. Arthritis Care Res. 2022, 74, 2091–2099. [Google Scholar] [CrossRef]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth. Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.H.; Karst, M.; Schneider, U.; Zurier, R.B. Ajulemic acid: A novel cannabinoid produces analgesia without a “high”. Life Sci. 2004, 75, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Klose, P.; Fitzcharles, M.A.; Phillips, T.; Häuser, W. Cannabinoids for fibromyalgia. Cochrane Database Syst. Rev. 2016, 7, CD011694. [Google Scholar] [PubMed]
- Jackson, M.A.; Brown, A.L.; Johnston, J.; Clancy, R.; McGregor, I.; Bruno, R.; Lintzeris, N.; Montebello, M.; Luksza, J.; Bowman, J.; et al. The use and effects of synthetic cannabinoid receptor agonists by New South Wales cannabis treatment clients. J. Cannabis Res. 2021, 3, 33. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Bains, A.; Kohrman, S.; Punko, D.; Fricchione, G. A Link Between Inflammatory Mechanisms and Fibromyalgia. Adv. Exp. Med. Biol. 2023, 1411, 357–378. [Google Scholar] [PubMed]
- Andrés-Rodríguez, L.; Borràs, X.; Feliu-Soler, A.; Pérez-Aranda, A.; Angarita-Osorio, N.; Moreno-Peral, P.; Montero-Marin, J.; García-Campayo, J.; Carvalho, A.F.; Maes, M.; et al. Peripheral immune aberrations in fibromyalgia: A systematic review, meta-analysis and meta-regression. Brain Behav. Immun. 2020, 87, 881–889. [Google Scholar] [CrossRef]
- Beiner, E.; Brenner Miguel, S.; Friederich, H.C.; Tesarz, J.; PerPAIN Consortium. Elevated high sensitive C-reactive protein in fibromyalgia. Front. Psychiatry 2023, 14, 1237518. [Google Scholar] [CrossRef]
- Zetterman, T.; Markkula, R.; Kalso, E. Elevated highly sensitive C-reactive protein in fibromyalgia associates with symptom severity. Rheumatol. Adv. Pract. 2022, 6, rkac053. [Google Scholar] [CrossRef]
- Groven, N.; Fors, E.A.; Reitan, S.K. Patients with Fibromyalgia and Chronic Fatigue Syndrome show increased hsCRP compared to healthy controls. Brain Behav. Immun. 2019, 81, 172–177. [Google Scholar] [CrossRef]
- Meresh, E.; Khieu, K.; Krupa, J.; Bull, M.; Shah, M.; Aijazi, S.; Jain, D.; Bae, J. Correlation of Psychological Factors, Obesity, Serum Cortisol, and C-Reactive Protein in Patients with Fibromyalgia Diagnosed with Obstructive Sleep Apnea and Other Comorbidities. Biomedicines 2024, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, E.A.; Abdul Hakim, M.M.; El-Zohiery, A.; Salama, S.M. Significance of inflammatory markers in primary Fibromyalgia syndrome and their relation in assessing the disease severity. Egypt J. Immunol. 2024, 31, 67–74. [Google Scholar]
- Coskun Benlidayi, I. Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatol. Int. 2019, 39, 781–791. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, J.L.; Chen, M.; Zheng, Z.M.; Li, M.Q.; Shao, J. CCL2: An important cytokine in normal and pathological pregnancies: A review. Front. Immunol. 2023, 13, 1053457. [Google Scholar] [CrossRef]
- Gkouvi, A.; Tsiogkas, S.G.; Bogdanos, D.P.; Gika, H.; Goulis, D.G.; Grammatikopoulou, M.G. Proteomics in Patients with Fibromyalgia Syndrome: A Systematic Review of Observational Studies. Curr. Pain Headache Rep. 2024, 28, 565–586. [Google Scholar] [CrossRef]
- Behm, F.G.; Gavin, I.M.; Karpenko, O.; Lindgren, V.; Gaitonde, S.; Gashkoff, P.A.; Gillis, B.S. Unique immunologic patterns in fibromyalgia. BMC Clin. Pathol. 2012, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Sturgill, J.; McGee, E.; Menzies, V. Unique cytokine signature in the plasma of patients with fibromyalgia. J. Immunol. Res. 2014, 2014, 938576. [Google Scholar] [CrossRef]
- Tsilioni, I.; Russell, I.J.; Stewart, J.M.; Gleason, R.M.; Theoharides, T.C. Neuropeptides CRH, SP, HK-1, and Inflammatory Cytokines IL-6 and TNF Are Increased in Serum of Patients with Fibromyalgia Syndrome, Implicating Mast Cells. J. Pharmacol. Exp. Ther. 2016, 356, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, D.; De la Cruz-Aguilera, D.L.; Barrera-Villalpando, M.I.; Becerril-Villanueva, E.; Arreola, R.; Hernández-Ferreira, E.; Pérez-Tapia, S.M.; Pérez-Sánchez, G.; Garcés-Alvarez, M.E.; Aguirre-Cruz, L.; et al. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J. Neuroimmunol. 2016, 290, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, S.; Han, Y.; Zhao, H.; Yin, Y.; Zhang, Y.; Zeng, X. Micro-inflammation related gene signatures are associated with clinical features and immune status of fibromyalgia. J. Transl. Med. 2023, 21, 594. [Google Scholar] [CrossRef] [PubMed]
- Bjersing, J.L.; Dehlin, M.; Erlandsson, M.; Bokarewa, M.I.; Mannerkorpi, K. Changes in pain and insulin-like growth factor 1 in fibromyalgia during exercise: The involvement of cerebrospinal inflammatory factors and neuropeptides. Arthritis Res. Ther. 2012, 14, R162. [Google Scholar] [CrossRef]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef]
- Bäckryd, E.; Tanum, L.; Lind, A.L.; Larsson, A.; Gordh, T. Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J. Pain Res. 2017, 10, 515–525. [Google Scholar] [CrossRef]
- Clauw, D.; Sarzi-Puttini, P.; Pellegrino, G.; Shoenfeld, Y. Is fibromyalgia an autoimmune disorder? Autoimmun. Rev. 2024, 23, 103424. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Tao, F.; Zhong, H.J.; Yang, C.; Chen, J. Targeting PGAM1 in cancer: An emerging therapeutic opportunity. Eur. J. Med. Chem. 2022, 244, 114798. [Google Scholar] [CrossRef]
- Trouw, L.A.; Pickering, M.C.; Blom, A.M. The complement system as a potential therapeutic target in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 538–547. [Google Scholar] [CrossRef]
- Kim, K.; Lee, K.; Lee, S.; Hong, B.; Yun, H.; Park, Y.; Yoo, S.; Kim, W. The acute phase reactant orosomucoid-2 directly promotes rheumatoid inflammation. Exp. Mol. Med. 2024, 56, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Ovrom, E.A.; Mostert, K.A.; Khakhkhar, S.; McKee, D.P.; Yang, P.; Her, Y.F. A Comprehensive Review of the Genetic and Epigenetic Contributions to the Development of Fibromyalgia. Biomedicines 2023, 11, 1119. [Google Scholar] [CrossRef]
- Tiwari, A.; Surendran, S.; Mithun, C.; Chandran, V.; Balan, S. Serum interleukin-6, interleukin-8, and interleukin-1 receptor antagonist levels in South Indian fibromyalgia patients and its correlation with disease severity. Indian J. Rheumatol. 2021, 16, 381. [Google Scholar] [CrossRef]
- Conti, P.; Gallenga, C.E.; Caraffa, A.; Ronconi, G.; Kritas, S.K. Impact of mast cells in fibromyalgia and low-grade chronic inflammation: Can IL-37 play a role? Dermatol. Ther. 2020, 33, e13191. [Google Scholar] [CrossRef] [PubMed]
- Sanson, R.; Lazzara, S.L.; Cune, D.; Pitasi, C.L.; Trentesaux, C.; Fraudeau, M.; Letourneur, F.; Saintpierre, B.; Le Gall, M.; Bossard, P.; et al. Axin1 Protects Colon Carcinogenesis by an Immune-Mediated Effect. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 689–715. [Google Scholar] [CrossRef]
- Warren, J.L.; MacIver, N.J. Regulation of Adaptive Immune Cells by Sirtuins. Front. Endocrinol. 2019, 10, 466. [Google Scholar] [CrossRef]
- Aktürk, S.; Büyükavcı, R. Evaluation of blood neutrophil-lymphocyte ratio and platelet distribution width as inflammatory markers in patients with fibromyalgia. Clin. Rheumatol. 2017, 36, 1885–1889. [Google Scholar] [CrossRef]
- Banfi, G.; Diani, M.; Pigatto, P.D.; Reali, E. T Cell Subpopulations in the Physiopathology of Fibromyalgia: Evidence and Perspectives. Int. J. Mol. Sci. 2020, 21, 1186. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J.; Gavin, I.M.; Karpenko, O.; Barkhordar, F.; Gillis, B.S. Cytokine and chemokine profiles in fibromyalgia, rheumatoid arthritis and systemic lupus erythematosus: A potentially useful tool in differential diagnosis. Rheumatol. Int. 2015, 35, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Favretti, M.; Iannuccelli, C.; Di Franco, M. Pain Biomarkers in Fibromyalgia Syndrome: Current Understanding and Future Directions. Int. J. Mol. Sci. 2023, 24, 10443. [Google Scholar] [CrossRef] [PubMed]
- Minerbi, A.; Fitzcharles, M.A. Gut microbiome: Pertinence in fibromyalgia. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S123), 99–104. [Google Scholar] [PubMed]
- Wang, Z.; Jiang, D.; Zhang, M.; Teng, Y.; Huang, Y. Causal association between gut microbiota and fibromyalgia: A Mendelian randomization study. Front. Microbiol. 2024, 14, 1305361. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Q.; Chen, Y.; Ren, H.; Zhang, Q.; Yang, H.; Zhang, W.; Ding, T.; Wang, S.; Zhang, Y.; et al. Gut microbiota in chronic pain: Novel insights into mechanisms and promising therapeutic strategies. Int. Immunopharmacol. 2023, 115, 109685. [Google Scholar] [CrossRef]
- Cai, W.; Haddad, M.; Haddad, R.; Kesten, I.; Hoffman, T.; Laan, R.; Wong, C.; Brown, N.; Tansley, S.; Lister, K.C.; et al. Gut microbiota promotes pain in fibromyalgia. BioRxiv 2023. [Google Scholar]
- Fang, H.; Hou, Q.; Zhang, W.; Su, Z.; Zhang, J.; Li, J.; Lin, J.; Wang, Z.; Yu, X.; Yang, Y.; et al. Fecal Microbiota Transplantation Improves Clinical Symptoms of Fibromyalgia: An Open-Label, Randomized, Nonplacebo-Controlled Study. J. Pain 2024, 25, 104535. [Google Scholar] [CrossRef]
- Goudman, L.; Demuyser, T.; Pilitsis, J.G.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Gut dysbiosis in patients with chronic pain: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1342833. [Google Scholar] [CrossRef] [PubMed]
- Palma-Ordóñez, J.F.; Moreno-Fernández, A.M.; Ramírez-Tejero, J.A.; Durán-González, E.; Martínez-Lara, A.; Cotán, D. Implication of intestinal microbiota in the etiopathogenesis of fibromyalgia: A systematic review. Int. J. Rheum. Dis. 2024, 27, e15021. [Google Scholar] [CrossRef]
- Creed, F. Psychiatric disorders and the onset of self-reported fibromyalgia and chronic fatigue syndrome: The lifelines cohort study. Front. Psychiatry 2023, 14, 1120250. [Google Scholar] [CrossRef]
- Zhao, S.S.; Duffield, S.J.; Goodson, N.J. The prevalence and impact of comorbid fibromyalgia in inflammatory arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101423. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Giacobazzi, G.; Di Carlo, M. Chronic Pain in Inflammatory Arthritis: Mechanisms, Metrology, and Emerging Targets-A Focus on the JAK-STAT Pathway. Pain Res. Manag. 2018, 2018, 8564215. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Bonnekoh, H.; Metz, M.; Maurer, M. Chronic Spontaneous Urticaria: A Review. JAMA 2024, 332, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.; Zarate-Lopez, N. Functional gastrointestinal disorders: History taking skills in practice. Clin. Med. 2021, 21, e480–e486. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Venkataraman, S.; Handa, G.; Yadav, S.L.; Wadhwa, S.; Singh, U.; Kochhar, K.P.; Deepak, K.K.; Sarkar, K. A Cross-Sectional Study on Central Sensitization and Autonomic Changes in Fibromyalgia. Front. Neurosci. 2020, 14, 788. [Google Scholar] [CrossRef]
- Dumolard, A.; Lefaucheur, J.P.; Hodaj, E.; Liateni, Z.; Payen, J.F.; Hodaj, H. Central Sensitization and Small-fiber Neuropathy Are Associated in Patients with Fibromyalgia. Clin. J. Pain 2023, 39, 8–14. [Google Scholar] [CrossRef]
- Puja, G.; Sonkodi, B.; Bardoni, R. Mechanisms of Peripheral and Central Pain Sensitization: Focus on Ocular Pain. Front. Pharmacol. 2021, 12, 764396. [Google Scholar] [CrossRef]
- Yang, J.X.; Wang, H.F.; Chen, J.Z.; Li, H.Y.; Hu, J.C.; Yu, A.A.; Wen, J.J.; Chen, S.J.; Lai, W.D.; Wang, S.; et al. Potential Neuroimmune Interaction in Chronic Pain: A Review on Immune Cells in Peripheral and Central Sensitization. Front. Pain Res. 2022, 3, 946846. [Google Scholar] [CrossRef]
- Rosenström, A.H.C.; Konsman, J.P.; Kosek, E. Cytokines in Cerebrospinal Fluid and Chronic Pain in Humans: Past, Present, and Future. Neuroimmunomodulation 2024, 31, 157–172. [Google Scholar] [CrossRef]
- O’Mahony, L.F.; Srivastava, A.; Mehta, P.; Ciurtin, C. Is fibromyalgia associated with a unique cytokine profile? A systematic review and meta-analysis. Rheumatology 2021, 60, 2602–2614. [Google Scholar] [CrossRef]
- Vega-Ramírez, M.T.; Becerril-Villanueva, E.; Maldonado-García, J.L.; Pavón, L.; Pérez-Sánchez, G. S100 proteins: A new frontier in fibromyalgia research. Mol. Brain 2024, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Junior, A.E.A.; Carbinatto, F.M.; Rodrigues, T.Z.; Garcia, V.; Canelada, A.C.N.; Bagnato, V.S. Outcomes of Non-Surgical Spinal Decompression Therapy in Patientswith a Herniated Disc Across Different Age Groups. J. Nov. Physiother. 2023, 13, 565. [Google Scholar]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, Y.; Liang, Z.; Lu, Y.; Wang, J.; Xing, F.; Mao, Y.; Wei, X.; Wang, Z.; Yang, J.; et al. A Narrative Review of the Reciprocal Relationship Between Sleep Deprivation and Chronic Pain: The Role of Oxidative Stress. J. Pain Res. 2024, 17, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- D’Onghia, M.; Ciaffi, J.; Lisi, L.; Mancarella, L.; Ricci, S.; Stefanelli, N.; Meliconi, R.; Ursini, F. Fibromyalgia and obesity: A comprehensive systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 51, 409–424. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Stewart, J.M.; Hatziagelaki, E.; Kolaitis, G. Brain “fog”, inflammation and obesity: Key aspects of neuropsychiatric disorders improved by luteolin. Front. Neurosci. 2015, 9, 225. [Google Scholar] [CrossRef]
- Mathkhor, A.J.; Ibraheem, N.M. Prevalence and Impact of obesity on fibromyalgia syndrome and its allied symptoms. J. Family Med. Prim. Care. 2023, 12, 123–127. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Ghafouri, B.; Edman, E.; Löf, M.; Lund, E.; Leinhard, O.D.; Lundberg, P.; Forsgren, M.F.; Gerdle, B.; Dong, H.J. Fibromyalgia in women: Association of inflammatory plasma proteins, muscle blood flow, and metabolism with body mass index and pain characteristics. Pain Rep. 2022, 7, e1042. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; Georgakopoulou, V.E. Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus. World J. Exp. Med. 2023, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, B.; Abolhasanzadeh, N.; Shademan, B.; Nourazarian, A. Deciphering pain: Molecular mechanisms and neurochemical pathways-challenges and future opportunities. Front. Mol. Biosci. 2024, 11, 1382555. [Google Scholar] [CrossRef]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef]
- Fabris-Moraes, W.; Lacerda, G.J.M.; Pacheco-Barrios, K.; Fregni, F. The Impact of Obesity as a Peripheral Disruptor of Brain Inhibitory Mechanisms in Fibromyalgia: A Cross-Sectional Study. J. Clin. Med. 2024, 13, 3878. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Shi, Y.; Yu, M. The association between obesity and chronic pain among community-dwelling older adults: A systematic review and meta-analysis. Geriatr. Nurs. 2021, 42, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.; Kapphahn, K.; Brennan, K.; Sullivan, S.D.; Stefanick, M.L. Association of Leptin with Body Pain in Women. J. Women’s Health 2016, 25, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.T.; Lin, Y.L.; Lin, C.T.; Hong, C.J.; Tsai, M.J.; Huang, W.C.; Shih, Y.H.; Lee, Y.Y.; Cheng, H.; Huang, M.C. Leptin is essential for microglial activation and neuropathic pain after preganglionic cervical root avulsion. Life Sci. 2017, 187, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Gianlorenço, A.C.; Daibes, M.; Queiroz, F.; Lacerda, G.; Martinez-Magallanes, D.; Camargo, L.; Alves, L.G.; Andrade, M.F.; Dodurgali, M.R.; et al. Physical Conditioning, Obesity and Fibromyalgia: Causal Relationship or Confounding? Princ. Pract. Clin. Res. 2023, 9, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Alciati, A.; Salaffi, F.; Di Carlo, M.; Bazzichi, L.; Govoni, M.; Biasi, G.; Di Franco, M.; Mozzani, F.; Gremese, E.; et al. The association between body mass index and fibromyalgia severity: Data from a cross-sectional survey of 2339 patients. Rheumatol. Adv. Pract. 2021, 5, rkab015. [Google Scholar] [CrossRef]
- Magni, A.; Agostoni, P.; Bonezzi, C.; Massazza, G.; Menè, P.; Savarino, V.; Fornasari, D. Management of Osteoarthritis: Expert Opinion on NSAIDs. Pain Ther. 2021, 10, 783–808. [Google Scholar] [CrossRef]
- Ong, J.J.Y.; De Felice, M. Migraine Treatment: Current Acute Medications and Their Potential Mechanisms of Action. Neurotherapeutics 2018, 15, 274–290. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15 (Suppl. S3), S2. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Wiffen, P.J.; Häuser, W.; Mücke, M.; Tölle, T.R.; Bell, R.F.; Moore, R.A. Oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults. Cochrane Database Syst. Rev. 2017, 3, CD012332. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Adler, U.C. Low-grade inflammation in chronic diseases: An integrative pathophysiology anticipated by homeopathy? Med. Hypotheses 2011, 76, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Lafforgue, P.; Pham, T. NSAID: Current limits to prescription. Joint Bone Spine. 2024, 91, 105685. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef] [PubMed]
- Pridgen, W.L.; Duffy, C.; Gendreau, J.F.; Gendreau, R.M. A famciclovir + celecoxib combination treatment is safe and efficacious in the treatment of fibromyalgia. J. Pain Res. 2017, 10, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Fisher, E.; Perrot, S.; Moore, R.A.; Makri, S.; Bidonde, J. Non-pharmacological interventions for fibromyalgia (fibromyalgia syndrome) in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2022, 2022, CD015074. [Google Scholar]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J. Fibromyalgia and nutrition: Therapeutic possibilities? Biomed. Pharmacother. 2018, 103, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Metyas, C.; Aung, T.T.; Cheung, J.; Joseph, M.; Ballester, A.M.; Metyas, S. Diet and Lifestyle Modifications for Fibromyalgia. Curr. Rheumatol. Rev. 2024, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Correa-Rodríguez, M.; Casas-Barragán, A.; González-Jiménez, E.; Schmidt-RioValle, J.; Molina, F.; Aguilar-Ferrándiz, M.E. Dietary Inflammatory Index Scores Are Associated with Pressure Pain Hypersensitivity in Women with Fibromyalgia. Pain Med. 2020, 21, 586–594. [Google Scholar] [CrossRef]
- Silva, A.R.; Bernardo, A.; de Mesquita, M.F.; Vaz Patto, J.; Moreira, P.; Silva, M.L.; Padrão, P. A study protocol for a randomized controlled trial of an anti-inflammatory nutritional intervention in patients with fibromyalgia. Trials 2021, 22, 198. [Google Scholar] [CrossRef] [PubMed]
- Marum, A.P.; Moreira, C.; Saraiva, F.; Tomas-Carus, P.; Sousa-Guerreiro, C. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand. J. Pain 2016, 13, 166–172. [Google Scholar] [CrossRef]
- Casini, I.; Ladisa, V.; Clemente, L.; Delussi, M.; Rostanzo, E.; Peparini, S.; Aloisi, A.M.; de Tommaso, M. A Personalized Mediterranean Diet Improves Pain and Quality of Life in Patients with Fibromyalgia. Pain Ther. 2024, 13, 609–620. [Google Scholar] [CrossRef]
- San, M.I.; Luis, C.; Sara, S.; Sara, L.; Raquel, C.M.P.A.; Elena, G. Short-Time strategy for fibromyalgia treatment based on olive Nutraceutical and Inflammatory Gut-Brain Axis Control Diet (IGUBAC) diet®. Curr. Top. Nutraceutical. Res. 2018, 17, 23–32. [Google Scholar]
- Fernández-Araque, A.; Verde, Z.; Torres-Ortega, C.; Sainz-Gil, M.; Velasco-Gonzalez, V.; González-Bernal, J.J.; Mielgo-Ayuso, J. Effects of Antioxidants on Pain Perception in Patients with Fibromyalgia-A Systematic Review. J. Clin. Med. 2022, 11, 2462. [Google Scholar] [CrossRef] [PubMed]
- Assavarittirong, C.; Samborski, W.; Grygiel-Górniak, B. Oxidative Stress in Fibromyalgia: From Pathology to Treatment. Oxid. Med. Cell. Longev. 2022, 2022, 1582432. [Google Scholar] [CrossRef]
- Boulis, M.; Boulis, M.; Clauw, D. Magnesium and Fibromyalgia: A Literature Review. J. Prim. Care. Community Health 2021, 12, 21501327211038433. [Google Scholar] [CrossRef] [PubMed]
- Sawaddiruk, P.; Apaijai, N.; Paiboonworachat, S.; Kaewchur, T.; Kasitanon, N.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, N.; Chattipakorn, S.C. Coenzyme Q10 supplementation alleviates pain in pregabalin-treated fibromyalgia patients via reducing brain activity and mitochondrial dysfunction. Free Radic. Res. 2019, 53, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Feraco, A.; Ottaviani, M.; Rizzo, G.; Camajani, E.; Caprio, M.; Armani, A. The Efficacy of Vitamin D Supplementation in the Treatment of Fibromyalgia Syndrome and Chronic Musculoskeletal Pain. Nutrients 2022, 14, 3010. [Google Scholar] [CrossRef] [PubMed]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Vuyisich, M.; Harnett, J.E. Investigating the association between the symptoms of women with Fibromyalgia, Digestive function, and markers of the microbiota of the Gastrointestinal Tract (The FIDGIT Study): Study protocol. BMC Musculoskelet. Disord. 2023, 24, 150. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Giangrandi, I.; Dinu, M.; Sofi, F.; Colombini, B. Nutritional Interventions in the Management of Fibromyalgia Syndrome. Nutrients 2020, 12, 2525. [Google Scholar] [CrossRef]
- Kadayifci, F.Z.; Bradley, M.J.; Onat, A.M.; Shi, H.N.; Zheng, S. Review of nutritional approaches to fibromyalgia. Nutr. Rev. 2022, 80, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Reina, M.D.; Nunez-Nagy, S.; Gallego-Izquierdo, T.; Pecos-Martín, D.; Monserrat, J.; Álvarez-Mon, M. Effectiveness of Therapeutic Exercise in Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Biomed. Res. Int. 2017, 2017, 2356346. [Google Scholar] [CrossRef]
- Berardi, G.; Senefeld, J.W.; Hunter, S.K.; Bement, M.K.H. Impact of isometric and concentric resistance exercise on pain and fatigue in fibromyalgia. Eur. J. Appl. Physiol. 2021, 121, 1389–1404. [Google Scholar] [CrossRef] [PubMed]
- Manojlović, D.; Kopše, E.I. The effectiveness of aerobic exercise for pain management in patients with fibromyalgia. Eur. J. Transl. Myol. 2023, 33, 11423. [Google Scholar] [CrossRef]
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. Int. J. Mol. Sci. 2020, 22, 338. [Google Scholar] [CrossRef]
- García-Domínguez, M. A comprehensive analysis of fibromyalgia and the role of the endogenous opioid system. Biomedicines 2025, 13, 165. [Google Scholar] [CrossRef]
- Mascarenhas, R.O.; Souza, M.B.; Oliveira, M.X.; Lacerda, A.C.; Mendonça, V.A.; Henschke, N.; Oliveira, V.C. Association of Therapies with Reduced Pain and Improved Quality of Life in Patients with Fibromyalgia: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2021, 181, 104–112. [Google Scholar] [CrossRef]
- Adams, N.; McVeigh, J.G.; Cuesta-Vargas, A.; Abokdeer, S. Evidence-based approaches for the management of fibromyalgia syndrome: A scoping review. Phys. Ther. Rev. 2023, 28, 1–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).