Therapeutic Targeting of the Galectin-1/miR-22-3p Axis Regulates Cell Cycle and EMT Depending on the Molecular Subtype of Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Cell Culture
2.3. miR-22-3p Mimics (miR-22-3p), miRNA-22-3p Inhibitor (Anti-miR-22-3p), and Short Interfering RNA (siRNA) Transfection
2.4. Establishing a Stable Knockdown Cell for Galectin-1 Using Short-Hairpin RNA (shRNA) and Stable Overexpressing Cell for miR-22-3p
2.5. Luciferase Reporter Assay
2.6. RNA Immunoprecipitation Assay
2.7. Cell Proliferation Assay
2.8. Colony Forming Assay
2.9. Wound Healing Assay
2.10. Invasion Assay
2.11. Western Blotting
2.12. RNA Extraction and qRT-PCR
2.13. Animal Experiments
2.14. Flow Cytometry
2.15. Statistical Analysis
3. Results
3.1. Clinical Relevance of Galectin-1 Expression in Breast Cancer
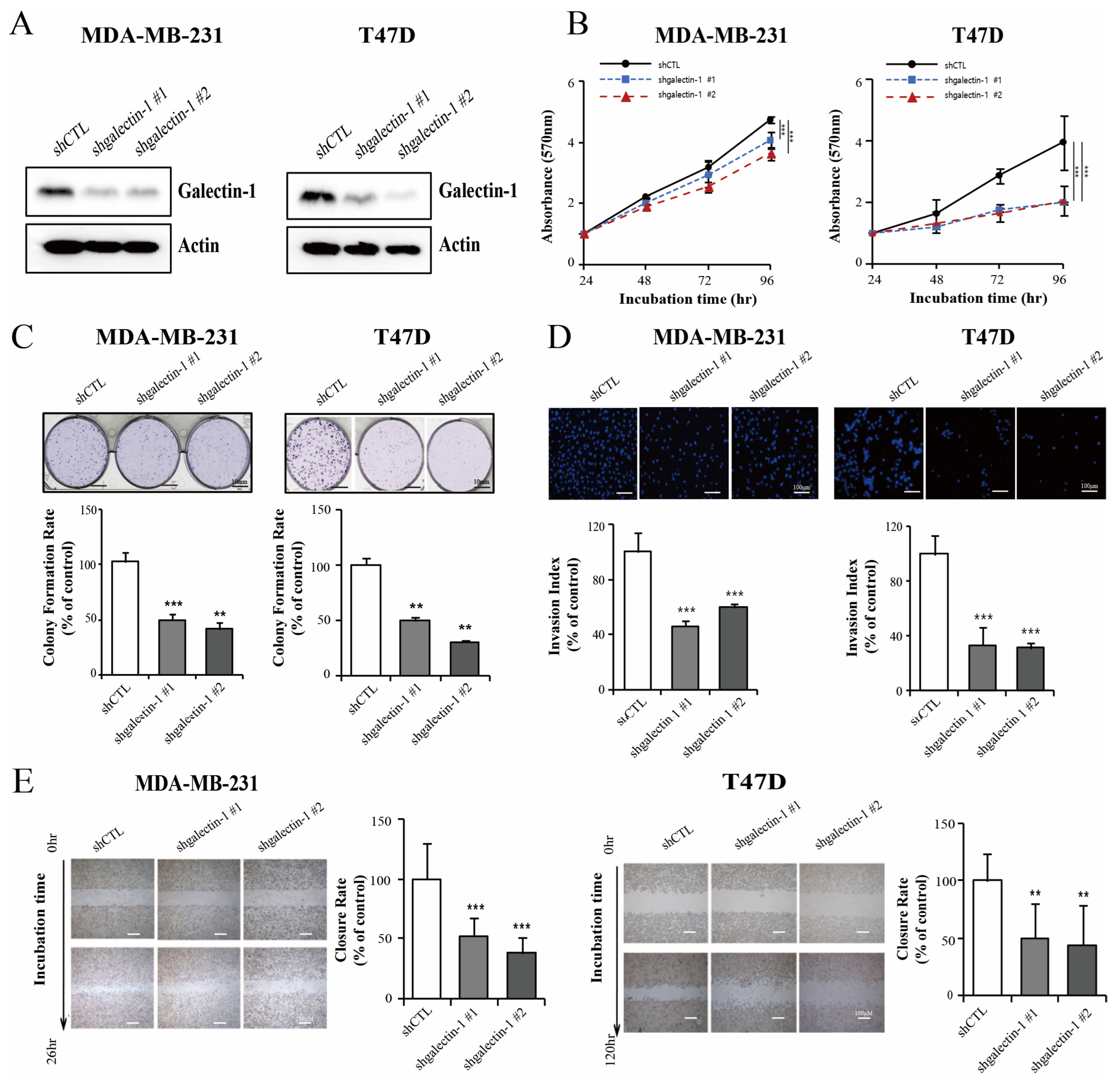
3.2. Galectin-1 Knockdown Inhibits Breast Cancer Proliferation and Invasiveness
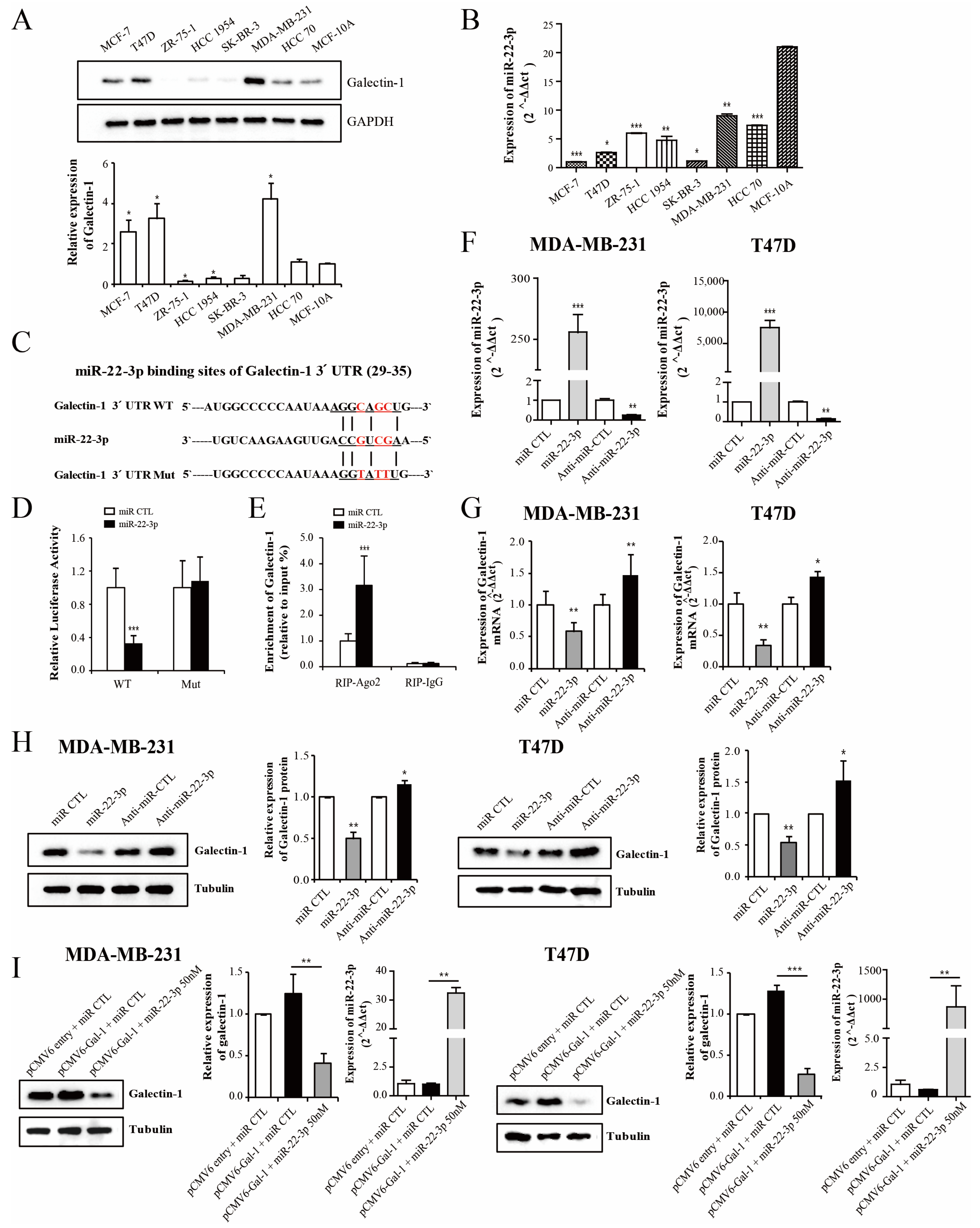
3.3. Galectin-1 Is the Direct Target Gene for miR-22-3p
3.4. miR-22-3p Expression Is Associated with the Prognosis in Patients with Breast Cancer
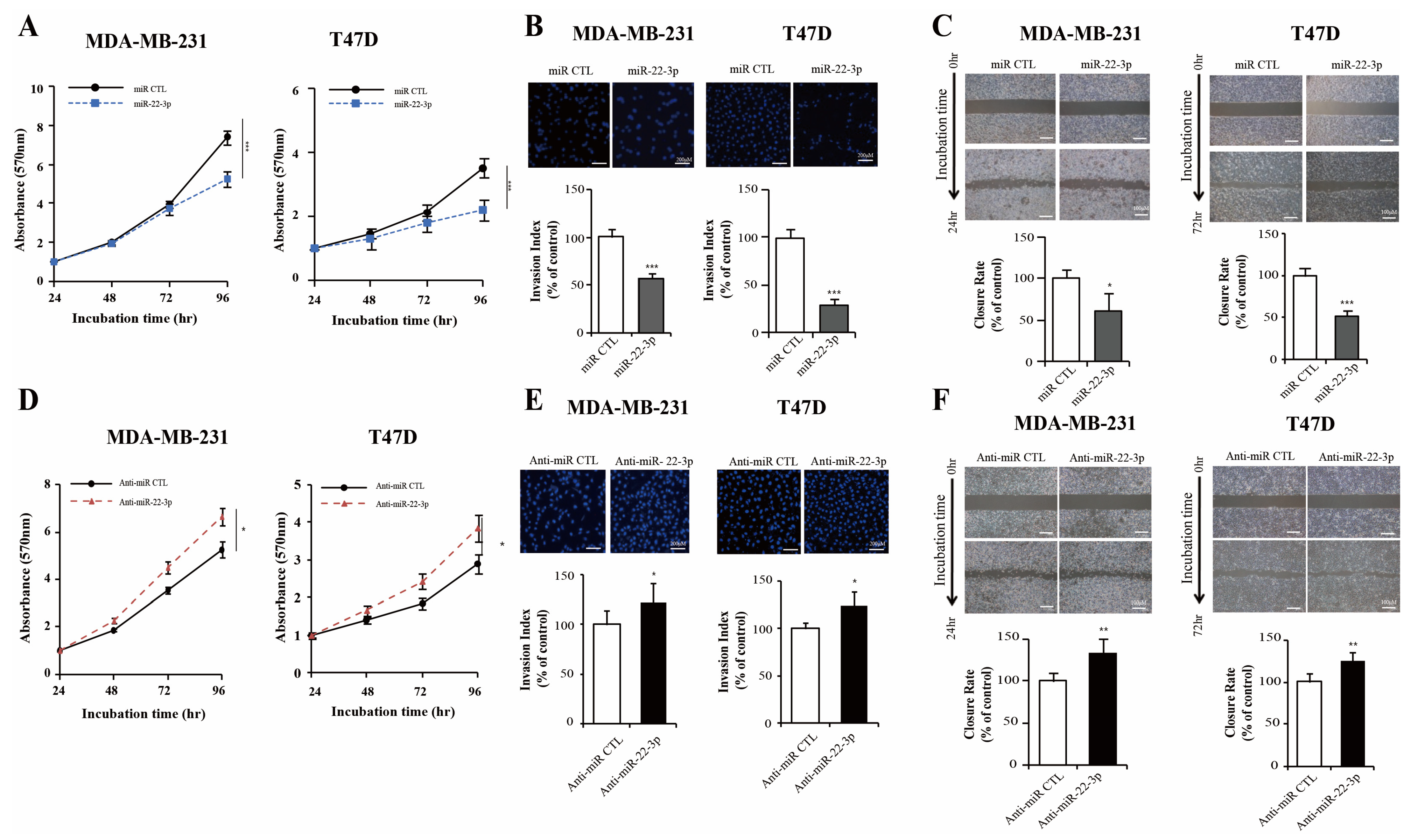
3.5. miR-22-3p Inhibits Breast Cancer Cell Proliferation and Invasiveness
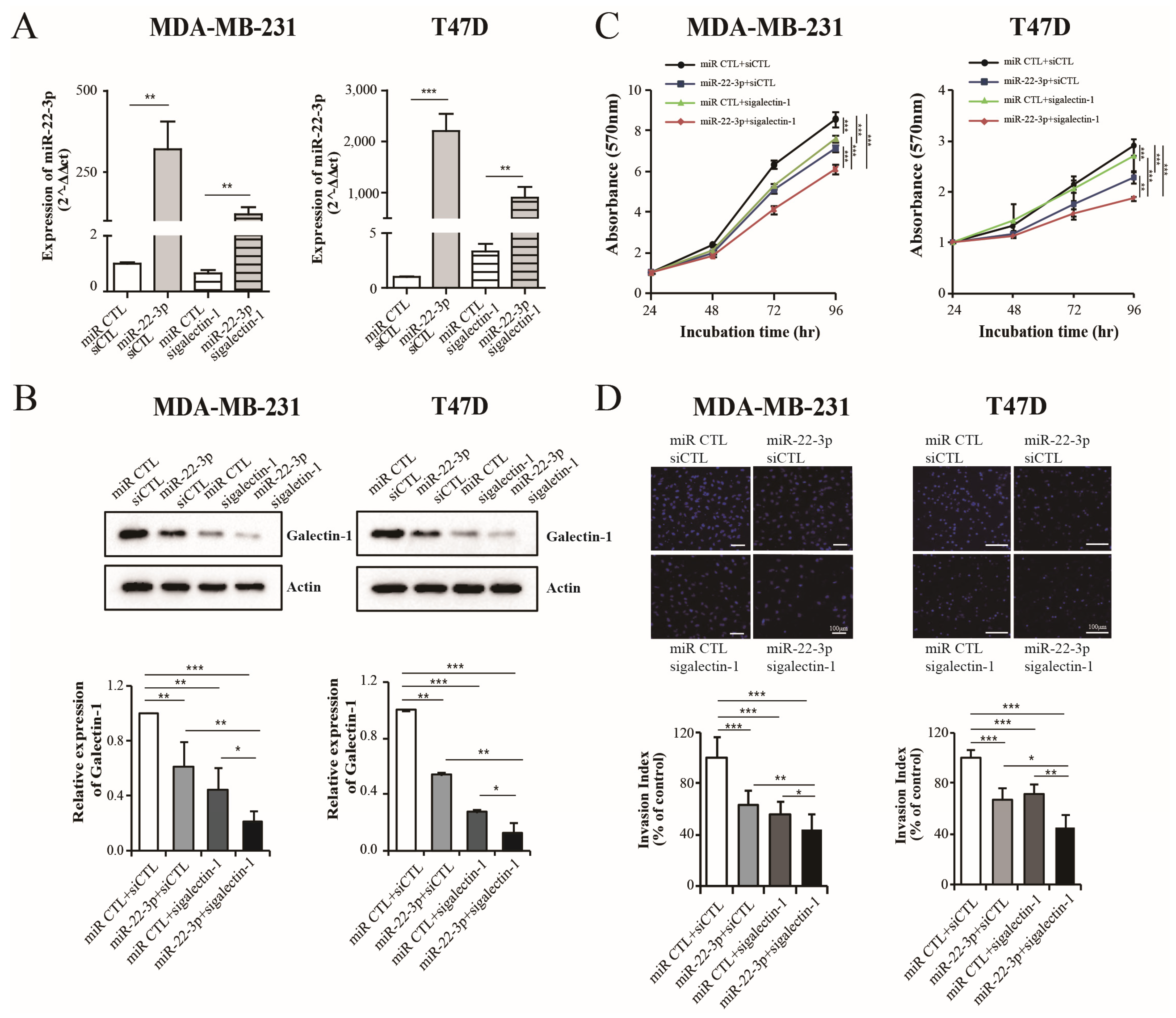
3.6. Dual Inhibition of Galectin-1 by siRNA and miR-22-3p Showed an Enhanced Antitumor Effect
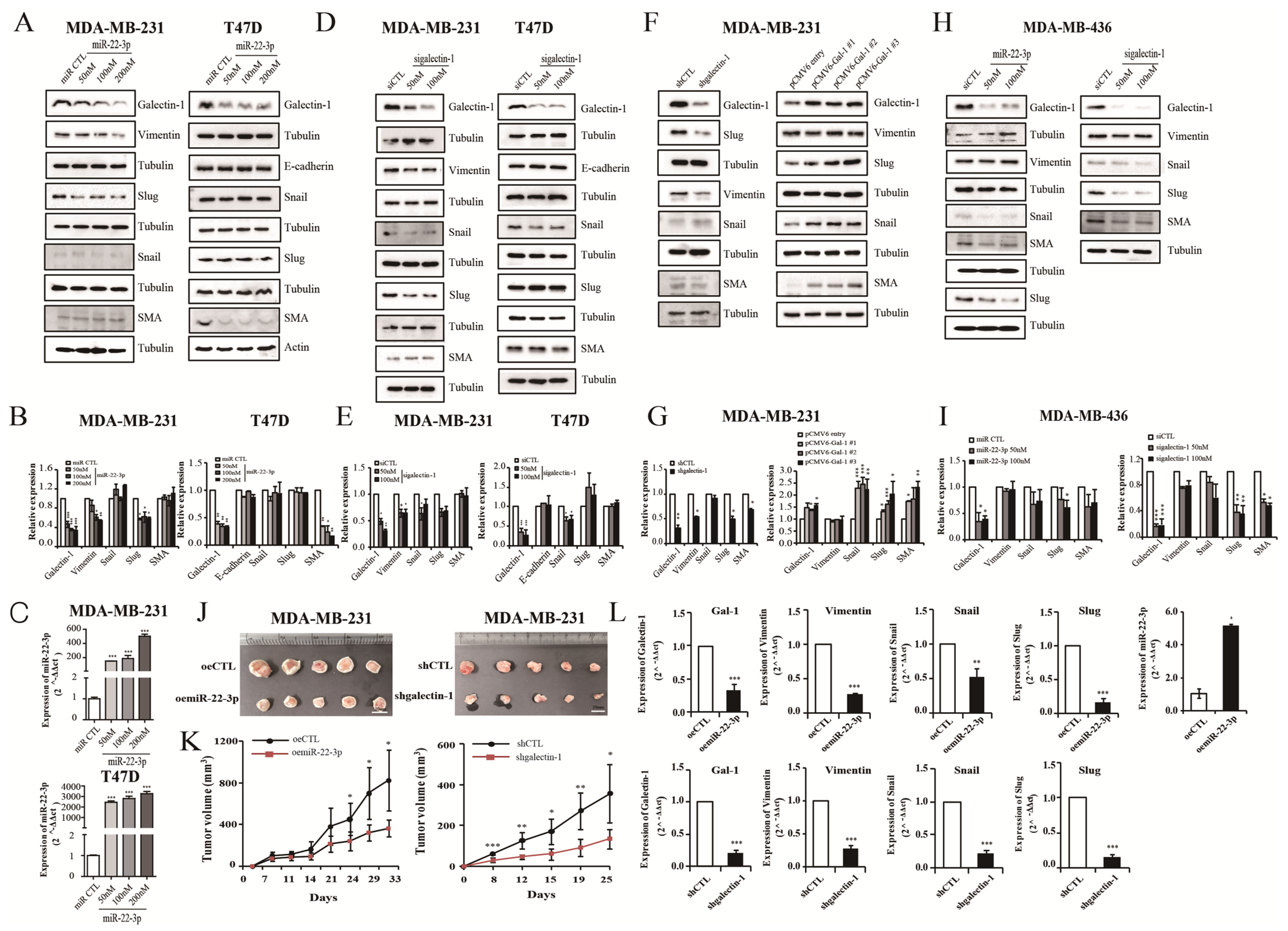
3.7. miR-22-3p Overexpression and Galectin-1 Knockdown Regulate Epithelial-to-Mesenchymal Transition (EMT)
3.8. miR-22-3p Overexpression and Galectin-1 Knockdown Inhibit Tumor Growth and EMT Pathway In Vivo
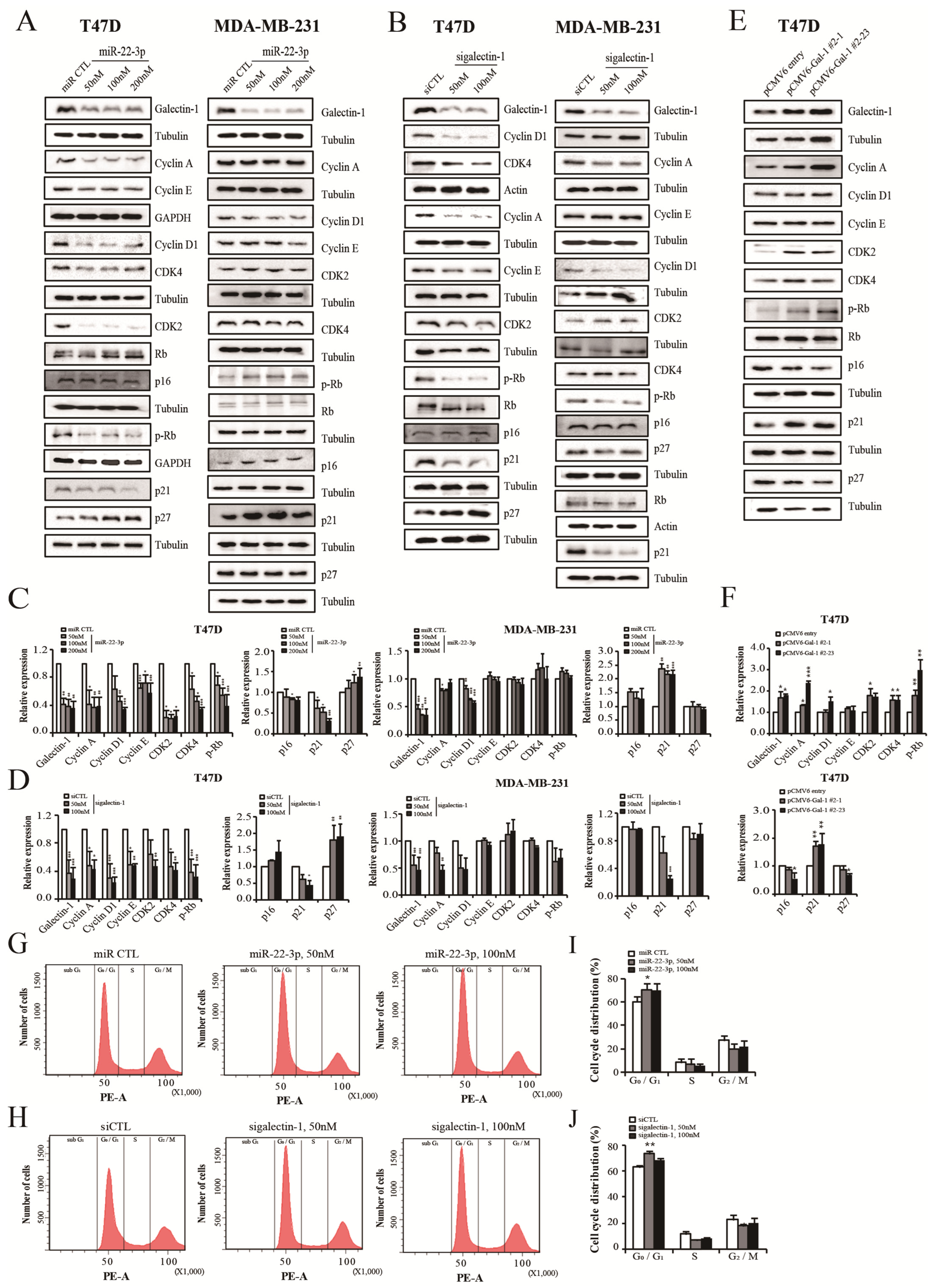
3.9. miR-22-3p Overexpression and Galectin-1 Knockdown Control Cell Cycle
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plevritis, S.K.; Munoz, D.; Kurian, A.W.; Stout, N.K.; Alagoz, O.; Near, A.M.; Lee, S.J.; Broek, J.J.v.D.; Huang, X.; Schechter, C.B.; et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012. JAMA 2018, 319, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Harbeck, N.; Barrios, C.H.; Bergh, J.; Cortés, J.; El Saghir, N.; Francis, P.A.; Hudis, C.A.; Ohno, S.; Partridge, A.H.; et al. Research needs in breast cancer. Ann. Oncol. 2017, 28, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.A.; Vandenberghe, M.E.; Marchiò, C.; Ellis, I.O.; Sapino, A.; Rakha, E.A. Tumour heterogeneity of breast cancer: From morphology to personalised medicine. Pathobiology 2018, 85, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Su, B. Small but influential: The role of microRNAs on gene regulatory network and 3′UTR evolution. J. Genet. Genom. 2009, 36, 1–6. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Lowery, A.J.; Miller, N.; McNeill, R.E.; Kerin, M.J. MicroRNAs as prognostic indicators and therapeutic targets: Potential effect on breast cancer management. Clin. Cancer Res. 2008, 14, 360–365. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Pérez-Moreno, P.; Pérez, Y.; Calaf, G.M. The role of microRNAs in breast cancer and the challenges of their clinical application. Diagnostics 2023, 13, 3072. [Google Scholar] [CrossRef]
- Karkhane, M.; Lashgarian, H.E.; Hormozi, M.; Fallahi, S.; Cheraghipour, K.; Marzban, A. Oncogenesis and tumor inhibition by microRNAs and its potential therapeutic applications: A systematic review. MicroRNA 2020, 9, 198–215. [Google Scholar] [CrossRef]
- Shah, M.Y.; Ferrajoli, A.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. microRNA Therapeutics in Cancer—An Emerging Concept. eBioMedicine 2016, 12, 34–42. [Google Scholar] [CrossRef]
- Sharma, G.; Agarwal, S.M. Identification of critical microRNA gene targets in cervical cancer using network properties. MicroRNA 2014, 3, 37–44. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Sebastiani, P.; de las Morenas, A.; Lu, J.; Rosenberg, C.L. Expression of microRNA and their gene targets are dysregulated in preinvasive breast cancer. Breast Cancer Res. 2011, 13, R24. [Google Scholar] [CrossRef] [PubMed]
- Chipman, L.B.; Pasquinelli, A.E. miRNA targeting: Growing beyond the seed. Trends Genet. 2019, 35, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, S.; Zeng, J.; Zhang, Y.; Ruan, X.; Han, W.; Yin, B.; Yuan, J.; Qiang, B.; Ying, W.; et al. Proteomic screening and identification of microRNA-128 targets in glioma cells. Proteomics 2015, 15, 2602–2617. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Moon, H.; Cho, B.I.; Jeong, C.; Joo, Y.; Lee, Y.; Hong, S.; Choi, S.; Ha, W.; Kim, J.W.; et al. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int. J. Cancer 2007, 120, 2331–2338. [Google Scholar] [CrossRef]
- Moon, H.-G.; Jeong, S.-H.; Ju, Y.-T.; Jeong, C.-Y.; Lee, J.S.; Lee, Y.-J.; Hong, S.-C.; Choi, S.-K.; Ha, W.-S.; Park, S.-T.; et al. Up-regulation of RhoGDI2 in human breast cancer and its prognostic implications. Cancer Res. Treat. 2010, 42, 151–156. [Google Scholar] [CrossRef][Green Version]
- Goud, N.S.; Bhattacharya, A. Human galectin-1 in multiple cancers: A privileged molecular target in oncology. Mini-Rev. Med. Chem. 2021, 21, 2169–2186. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Rabinovich, G.A.; Griffioen, A.W. Vascular galectins: Regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev. 2013, 24, 547–558. [Google Scholar] [CrossRef]
- Salajegheh, A.; Dolan-Evans, E.; Sullivan, E.; Irani, S.; Rahman, M.A.; Vosgha, H.; Gopalan, V.; Smith, R.A.; Lam, A.K. The expression profiles of the galectin gene family in primary and metastatic papillary thyroid carcinoma with particular emphasis on galectin-1 and galectin-3 expression. Exp. Mol. Pathol. 2014, 96, 212–218. [Google Scholar] [CrossRef]
- Gopalan, V.; Saremi, N.; Sullivan, E.; Kabir, S.; Lu, C.T.; Salajegheh, A.; Leung, M.; Smith, R.A.; Lam, A.K. The expression profiles of the galectin gene family in colorectal adenocarcinomas. Hum. Pathol. 2016, 53, 105–113. [Google Scholar] [CrossRef]
- Hittelet, A.; Legendre, H.; Nagy, N.; Bronckart, Y.; Pector, J.C.; Salmon, I.; Yeaton, P.; Gabius, H.J.; Kiss, R.; Camby, I. Upregulation of galectins-1 and -3 in human colon cancer and their role in regulating cell migration. Int. J. Cancer 2003, 103, 370–379. [Google Scholar] [CrossRef]
- Laderach, D.J.; Gentilini, L.D.; Giribaldi, L.; Delgado, V.C.; Nugnes, L.; Croci, D.O.; Al Nakouzi, N.; Sacca, P.; Casas, G.; Mazza, O.; et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013, 73, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, L.G., 3rd; Nilson, A.E.; Goble, J.M.; Ballman, K.V.; James, C.D.; Lefranc, F.; Kiss, R.; Uhm, J.H. Galectin-1, a gene preferentially expressed at the tumor margin, promotes glioblastoma cell invasion. Mol. Cancer 2012, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Shimonishi, T.; Miyazaki, K.; Kono, N.; Sabit, H.; Tuneyama, K.; Harada, K.; Hirabayashi, J.; Kasai, K.; Nakanuma, Y. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum. Pathol. 2001, 32, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhang, J.; Yuan, Z.; Gao, J.; Wang, S.; Ye, N.; Li, P.; Gao, S.; Miao, Y.; Wang, D.; et al. Pancreatic satellite cells derived galectin-1 increase the progression and less survival of pancreatic ductal adenocarcinoma. PLoS ONE 2014, 9, e90476. [Google Scholar] [CrossRef]
- van den Brûle, F.; Califice, S.; Garnier, F.; Fernandez, P.L.; Berchuck, A.; Castronovo, V. Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Lab. Investig. 2003, 83, 377–386. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Zhang, K.; Xu, F.; Yin, Y.; Zhu, L.; Zhou, F. Galectin-1 knockdown in carcinoma-associated fibroblasts inhibits migration and invasion of human MDA-MB-231 breast cancer cells by modulating MMP-9 expression. Acta Biochim. Biophys. Sin. 2016, 48, 462–467. [Google Scholar] [CrossRef]
- Lai, J.; Lu, D.; Zhang, C.; Zhu, H.; Gao, L.; Wang, Y.; Bao, R.; Zhao, Y.; Jia, B.; Wang, F.; et al. Noninvasive small-animal imaging of galectin-1 upregulation for predicting tumor resistance to radiotherapy. Biomaterials 2018, 158, 1–9. [Google Scholar] [CrossRef]
- Nam, K.; Son, S.H.; Oh, S.; Jeon, D.; Kim, H.; Noh, D.Y.; Kim, S.; Shin, I. Binding of galectin-1 to integrin β1 potentiates drug resistance by promoting survivin expression in breast cancer cells. Oncotarget 2017, 8, 35804–35823. [Google Scholar] [CrossRef]
- Wang, F.; Lv, P.; Gu, Y.; Li, L.; Ge, X.; Guo, G. Galectin-1 knockdown improves drug sensitivity of breast cancer by reducing P-glycoprotein expression through inhibiting the Raf-1/AP-1 signaling pathway. Oncotarget 2017, 8, 25097–25106. [Google Scholar] [CrossRef]
- Huang, Z.P.; Chen, J.; Seok, H.Y.; Zhang, Z.; Kataoka, M.; Hu, X.; Wang, D.Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 2013, 112, 1234–1243. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Yang, T.; Liu, Y.; Li, A.; Fu, S.; Wu, M.; Pan, Z.; Zhou, W. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br. J. Cancer 2010, 103, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xia, S.; Tian, H.; Liu, Z.; Zhou, T. Clinical significance of miR-22 expression in patients with colorectal cancer. Med. Oncol. 2012, 29, 3108–3112. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.M.; Hu, L.H.; Wang, Y.Q.; Chen, P.; Huang, J.G.; Lu, N.; He, J.H.; Liao, C.G. miR-22 is down-regulated in gastric cancer, and its overexpression inhibits cell migration and invasion via targeting transcription factor Sp1. Med. Oncol. 2013, 30, 542. [Google Scholar] [CrossRef]
- Chen, B.; Tang, H.; Liu, X.; Liu, P.; Yang, L.; Xie, X.; Ye, F.; Song, C.; Xie, X.; Wei, W. miR-22 as a prognostic factor targets glucose transporter protein type 1 in breast cancer. Cancer Lett. 2015, 356, 410–417. [Google Scholar] [CrossRef]
- Xiong, J.; Du, Q.; Liang, Z. Tumor-suppressive microRNA-22 inhibits the transcription of E-box-containing c-Myc target genes by silencing c-Myc binding protein. Oncogene 2010, 29, 4980–4988. [Google Scholar] [CrossRef]
- Bar, N.; Dikstein, R. miR-22 forms a regulatory loop in PTEN/AKT pathway and modulates signaling kinetics. PLoS ONE 2010, 5, e10859. [Google Scholar] [CrossRef]
- Ting, Y.; Medina, D.J.; Strair, R.K.; Schaar, D.G. Differentiation-associated miR-22 represses Max expression and inhibits cell cycle progression. Biochem. Biophys. Res. Commun. 2010, 394, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.P.; Picard, D. miR-22 inhibits estrogen signaling by directly targeting the estrogen receptor alpha mRNA. Mol. Cell. Biol. 2009, 29, 3783–3790. [Google Scholar] [CrossRef]
- Xu, D.; Takeshita, F.; Hino, Y.; Fukunaga, S.; Kudo, Y.; Tamaki, A.; Matsunaga, J.; Takahashi, R.U.; Takata, T.; Shimamoto, A.; et al. miR-22 represses cancer progression by inducing cellular senescence. J. Cell. Biol. 2011, 193, 409–424. [Google Scholar] [CrossRef]
- Chen, J.; Tang, D.; Wang, S.; Li, Q.G.; Zhang, J.R.; Li, P.; Lu, Q.; Niu, G.; Gao, J.; Ye, N.Y.; et al. High expressions of galectin-1 and VEGF are associated with poor prognosis in gastric cancer patients. Tumor Biol. 2014, 35, 2513–2519. [Google Scholar] [CrossRef]
- Schulz, H.; Schmoeckel, E.; Kuhn, C.; Hofmann, S.; Mayr, D.; Mahner, S.; Jeschke, U. Galectins-1, -3, and -7 Are Prognostic Markers for Survival of Ovarian Cancer Patients. Int. J. Mol. Sci. 2017, 18, 1230. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, J.; Yang, T.; Zhang, W.; Liu, M. MiR-22 suppresses the growth and metastasis of bladder cancer cells by targeting E2F3. Int. J. Clin. Exp. Pathol. 2020, 13, 587–596. [Google Scholar] [PubMed]
- Budd, W.T.; Seashols-Williams, S.J.; Clark, G.C.; Weaver, D.; Calvert, V.; Petricoin, E.; Dragoescu, E.A.; O’Hanlon, K.; Zehner, Z.E. Dual action of miR-125b as a tumor suppressor and OncomiR-22 promotes prostate cancer tumorigenesis. PLoS ONE 2015, 10, e0142373. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tan, J.X.; Dai, H.S.; Chen, H.W.; Xu, X.J.; Yang, A.G.; Zhang, Y.J.; Bai, L.H.; Bie, P. MiRNA-22 inhibits oncogene galectin-1 in hepatocellular carcinoma. Oncotarget 2016, 7, 57099–57116. [Google Scholar] [CrossRef]
- Nishimura, M.; Jung, E.-J.; Shah, M.Y.; Lu, C.; Spizzo, R.; Shimizu, M.; Han, H.D.; Ivan, C.; Rossi, S.; Zhang, X.; et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013, 3, 1302–1315. [Google Scholar] [CrossRef]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef]
- Ito, K.; Ralph, S.J. Inhibiting galectin-1 reduces murine lung metastasis with increased CD4(+) and CD8 (+) T cells and reduced cancer cell adherence. Clin. Exp. Metastasis 2012, 29, 561–572. [Google Scholar] [CrossRef]
- Wu, M.H.; Hong, H.C.; Hong, T.M.; Chiang, W.F.; Jin, Y.T.; Chen, Y.L. Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression. Clin. Cancer Res. 2011, 17, 1306–1316. [Google Scholar] [CrossRef]
- Banh, A.; Zhang, J.; Cao, H.; Bouley, D.M.; Kwok, S.; Kong, C.; Giaccia, A.J.; Koong, A.C.; Le, Q.T. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 2011, 71, 4423–4431. [Google Scholar] [CrossRef]
- Chung, L.Y.; Tang, S.J.; Sun, G.H.; Chou, T.Y.; Yeh, T.S.; Yu, S.L.; Sun, K.H. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin. Cancer Res. 2012, 18, 4037–4047. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhou, F. Effect of ex vivo-expanded γδ-T cells combined with galectin-1 antibody on the growth of human cervical cancer xenografts in SCID mice. Clin. Investig. Med. 2010, 33, E280–E289. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jeon, H.K.; Cho, Y.J.; Park, Y.A.; Choi, J.J.; Do, I.G.; Song, S.Y.; Lee, Y.Y.; Choi, C.H.; Kim, T.J.; et al. High galectin-1 expression correlates with poor prognosis and is involved in epithelial ovarian cancer proliferation and invasion. Eur. J. Cancer 2012, 48, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Lu, Z.; Tang, D.; Yao, J.; An, Y.; Wu, J.; Li, Q.; Gao, W.; Xu, Z.; Qian, Z.; et al. Galectin-1 secreted by activated stellate cells in pancreatic ductal adenocarcinoma stroma promotes proliferation and invasion of pancreatic cancer cells: An in vitro study on the microenvironment of pancreatic ductal adenocarcinoma. Pancreas 2011, 40, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, H.; Yan, Y.; Li, Y.; Che, G.; Zhou, C.; Nicot, C.; Ma, H. NCAPG promotes the oncogenesis and progression of non-small cell lung cancer cells through upregulating LGALS1 expression. Mol. Cancer 2022, 21, 55. [Google Scholar] [CrossRef]
- Fischer, C.; Sanchez-Ruderisch, H.; Welzel, M.; Wiedenmann, B.; Sakai, T.; André, S.; Gabius, H.J.; Khachigian, L.; Detjen, K.M.; Rosewicz, S. Galectin-1 interacts with the α5β1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J. Biol. Chem. 2005, 280, 37266–37277. [Google Scholar] [CrossRef]
- Kopitz, J.; von Reitzenstein, C.; Burchert, M.; Cantz, M.; Gabius, H.J. Galectin-1 is a major receptor for ganglioside GM1, a product of the growth-controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J. Biol. Chem. 1998, 73, 11205–11211. [Google Scholar] [CrossRef]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Méndez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107–1117. [Google Scholar] [CrossRef]
- Geiger, P.; Mayer, B.; Wiest, I.; Schulze, S.; Jeschke, U.; Weissenbacher, T. Binding of galectin-1 to breast cancer cells MCF7 induces apoptosis and inhibition of proliferation in vitro in a 2D- and 3D- cell culture model. BMC Cancer 2016, 16, 870. [Google Scholar] [CrossRef]
- Wu, M.H.; Hong, T.M.; Cheng, H.W.; Pan, S.H.; Liang, Y.R.; Hong, H.C.; Chiang, W.F.; Wong, T.Y.; Shieh, D.B.; Shiau, A.L.; et al. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol. Cancer Res. 2009, 7, 311–318. [Google Scholar] [CrossRef]
- Su, Y.L.; Luo, H.L.; Huang, C.C.; Liu, T.T.; Huang, E.Y.; Sung, M.T.; Lin, J.J.; Chiang, P.H.; Chen, Y.T.; Kang, C.H.; et al. Galectin-1 Overexpression Activates the FAK/PI3K/AKT/mTOR Pathway and Is Correlated with Upper Urinary Urothelial Carcinoma Progression and Survival. Cells 2020, 9, 806. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, Y.; Zhang, H.; Liu, Y.; Sun, H.; Zhang, P. Galectin-1 induces metastasis and epithelial-mesenchymal transition (EMT) in human ovarian cancer cells via activation of the MAPK JNK/p38 signalling pathway. Am. J. Transl. Res. 2019, 11, 3862–3878. [Google Scholar]


| Tumor Characteristics | Number | Mean ± SD | p Value | |
|---|---|---|---|---|
| 54 | 0.0427 ± 0.0337 | |||
| T stage | 1 | 25 | 0.0409 ± 0.0314 | 0.554 |
| 2–3 | 29 | 0.0464 ± 0.0366 | ||
| N stage | 0 | 31 | 0.0553 ± 0.0396 | 0.001 |
| 1–3 | 23 | 0.0284 ± 0.0147 | ||
| TNM stage | 1 | 16 | 0.0471 ± 0.0357 | 0.110 |
| 2 | 21 | 0.0526 ± 0.0409 | ||
| 3 | 17 | 0.0299 ± 0.0341 | ||
| Estrogen receptor | Negative | 22 | 0.0370 ± 0.0209 | 0.178 |
| Positive | 32 | 0.0485 ± 0.0404 | ||
| Progesterone receptor | Negative | 17 | 0.0405 ± 0.0208 | 0.545 |
| Positive | 37 | 0.0454 ± 0.0388 | ||
| HER-2/neu status | Negative | 44 | 0.0451 ± 0.0371 | 0.376 |
| Positive | 10 | 0.0385 ± 0.0149 | ||
| Tumor Characteristics | Mean MFS (m, 95% CI) | Log Rank | |
|---|---|---|---|
| T stage | 1 | 114.50 (103.47–125.53) | 0.428 |
| 2–4 | 108.74 (91.79–125.70) | ||
| N stage | 0 | 124.14 (117.71–130.58) | 0.005 |
| 1–3 | 96.0 (75.85–116.19) | ||
| Estrogen receptor | Negative | 106.27 (86.67–125.87) | 0.328 |
| Positive | 114.98 (103.68–126.30) | ||
| Progesterone receptor | Negative | 100.57 (78.10–123.04) | 0.070 |
| Positive | 116.21 (105.65–126.78) | ||
| HER-2/neu status | Negative | 119.10 (108.26–129.95) | 0.030 |
| Positive | 84.60 (54.66–114.55) | ||
| miRNA-22-3p level | Low | 105.37 (89.98–120.78) | 0.040 |
| High | 123.33 (116.39–130.28) | ||
| Galectin-1 level | Low | 118.09 (104.45–131.74) | 0.253 |
| High | 92.60 (79.30–105.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Lee, J.H.; Jung, E.J.; Son, Y.S.; Park, H.J.; Kim, J.M.; Park, T.; Jeong, S.-H.; Lee, J.; Kim, T.H.; et al. Therapeutic Targeting of the Galectin-1/miR-22-3p Axis Regulates Cell Cycle and EMT Depending on the Molecular Subtype of Breast Cancer. Cells 2025, 14, 310. https://doi.org/10.3390/cells14040310
Kim JY, Lee JH, Jung EJ, Son YS, Park HJ, Kim JM, Park T, Jeong S-H, Lee J, Kim TH, et al. Therapeutic Targeting of the Galectin-1/miR-22-3p Axis Regulates Cell Cycle and EMT Depending on the Molecular Subtype of Breast Cancer. Cells. 2025; 14(4):310. https://doi.org/10.3390/cells14040310
Chicago/Turabian StyleKim, Ju Yeon, Jun Ho Lee, Eun Jung Jung, Young Sim Son, Hee Jin Park, Jae Myung Kim, Taejin Park, Sang-Ho Jeong, Jinkwon Lee, Tae Han Kim, and et al. 2025. "Therapeutic Targeting of the Galectin-1/miR-22-3p Axis Regulates Cell Cycle and EMT Depending on the Molecular Subtype of Breast Cancer" Cells 14, no. 4: 310. https://doi.org/10.3390/cells14040310
APA StyleKim, J. Y., Lee, J. H., Jung, E. J., Son, Y. S., Park, H. J., Kim, J. M., Park, T., Jeong, S.-H., Lee, J., Kim, T. H., Lee, S. M., & Heo, J. D. (2025). Therapeutic Targeting of the Galectin-1/miR-22-3p Axis Regulates Cell Cycle and EMT Depending on the Molecular Subtype of Breast Cancer. Cells, 14(4), 310. https://doi.org/10.3390/cells14040310







