Inflammatory Stimuli and Fecal Microbiota Transplantation Accelerate Pancreatic Carcinogenesis in Transgenic Mice, Accompanied by Changes in the Microbiota Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objects and Protocol
2.2. Process of Pancreatic Carcinogenesis
2.3. Fecal Microbiota Transplantation
2.4. Sample Processing and DNA Extraction for Microbiota Analysis
2.5. 16S RNA Sequencing and Bioinformatic Analysis
2.6. Statistical Analysis
3. Results
3.1. Inflammation in the Presence of Oncogenic Kras Mutation Promotes Pancreatic Carcinogenesis
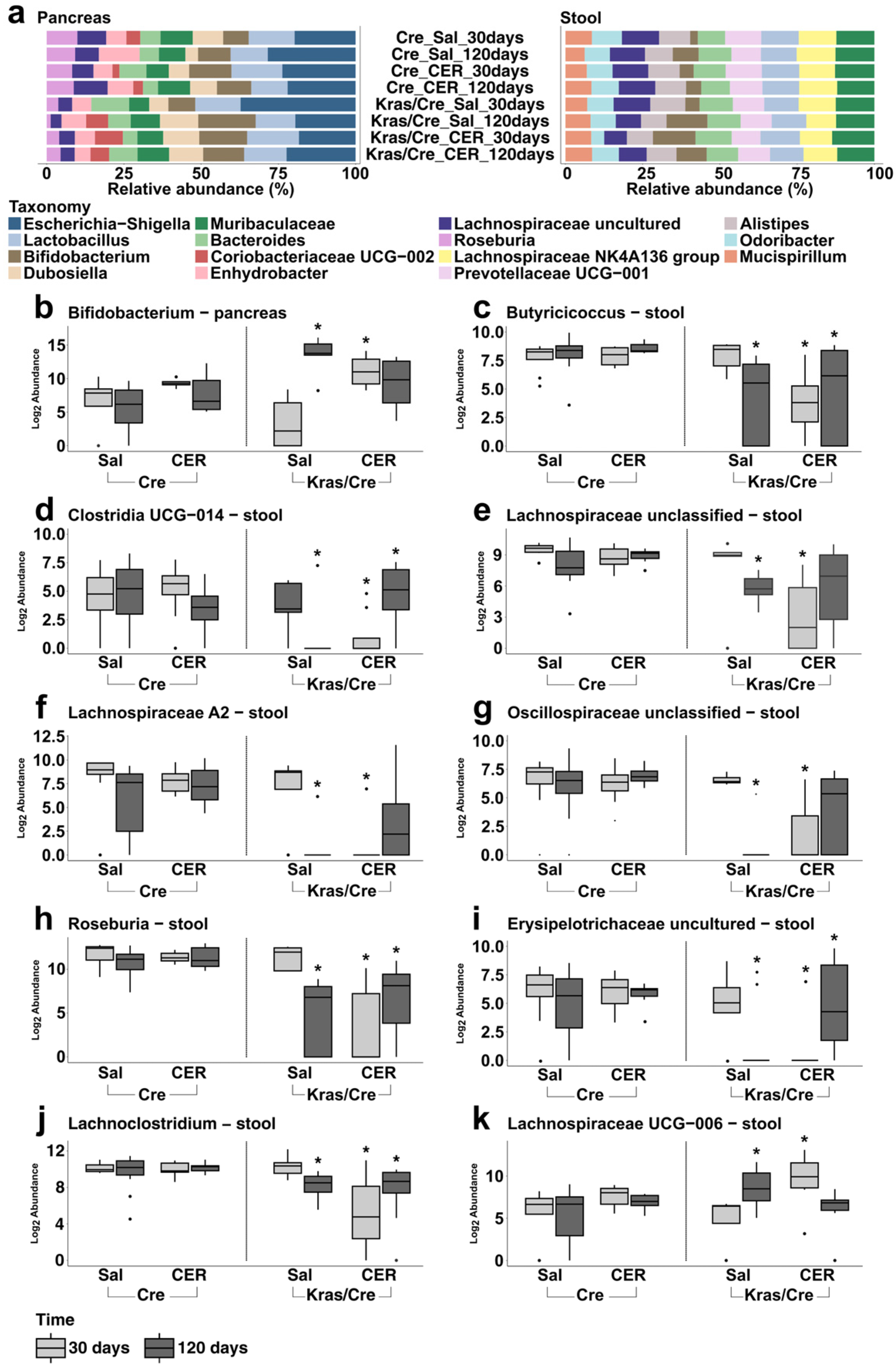
3.2. Characteristics of the Bacterial Community at the Phyla Level in Inflammation-Induced Pancreatic Carcinogenesis
3.2.1. Pancreatic Bacterial Phyla Abundance Change in the Course of Inflammation-Induced Carcinogenesis in Mice Genetically Predisposed to PDAC
3.2.2. Stool Bacterial Phyla Abundance Significantly Changes in the Course of Inflammation-Induced Carcinogenesis in Mice Genetically Predisposed to PDAC
3.3. Characteristics of the Bacterial Community at the Genera Level in Inflammation-Induced Pancreatic Carcinogenesis
3.3.1. Pancreatic Bacterial Genera Composition and Abundance Dynamically Change in the Course of Inflammation-Induced Carcinogenesis in Mice Genetically Predisposed to PDAC
3.3.2. Stool Bacterial Genera Abundance Dynamically Change in the Course of Inflammation-Induced Carcinogenesis in Mice Genetically Predisposed to PDAC
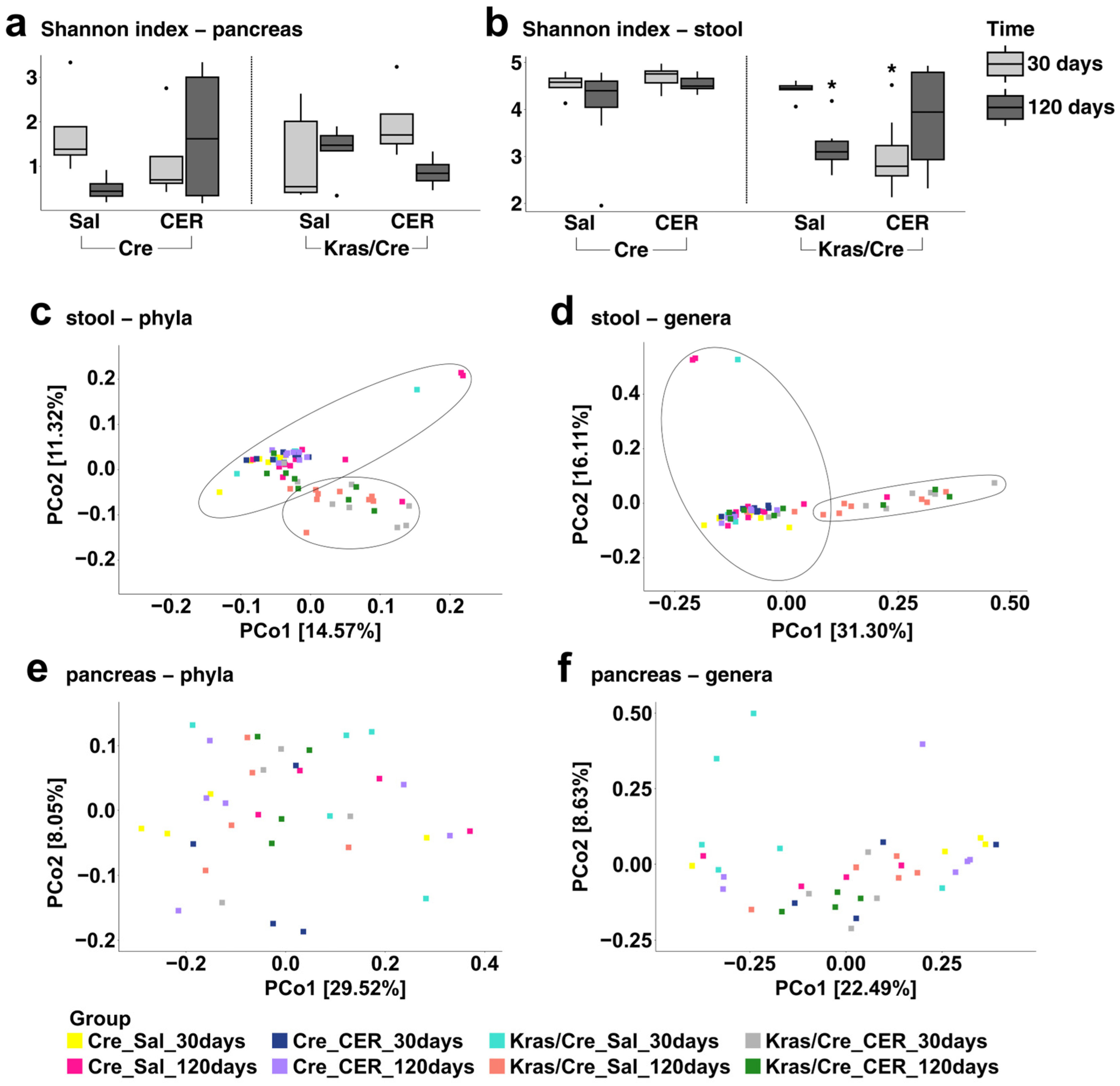
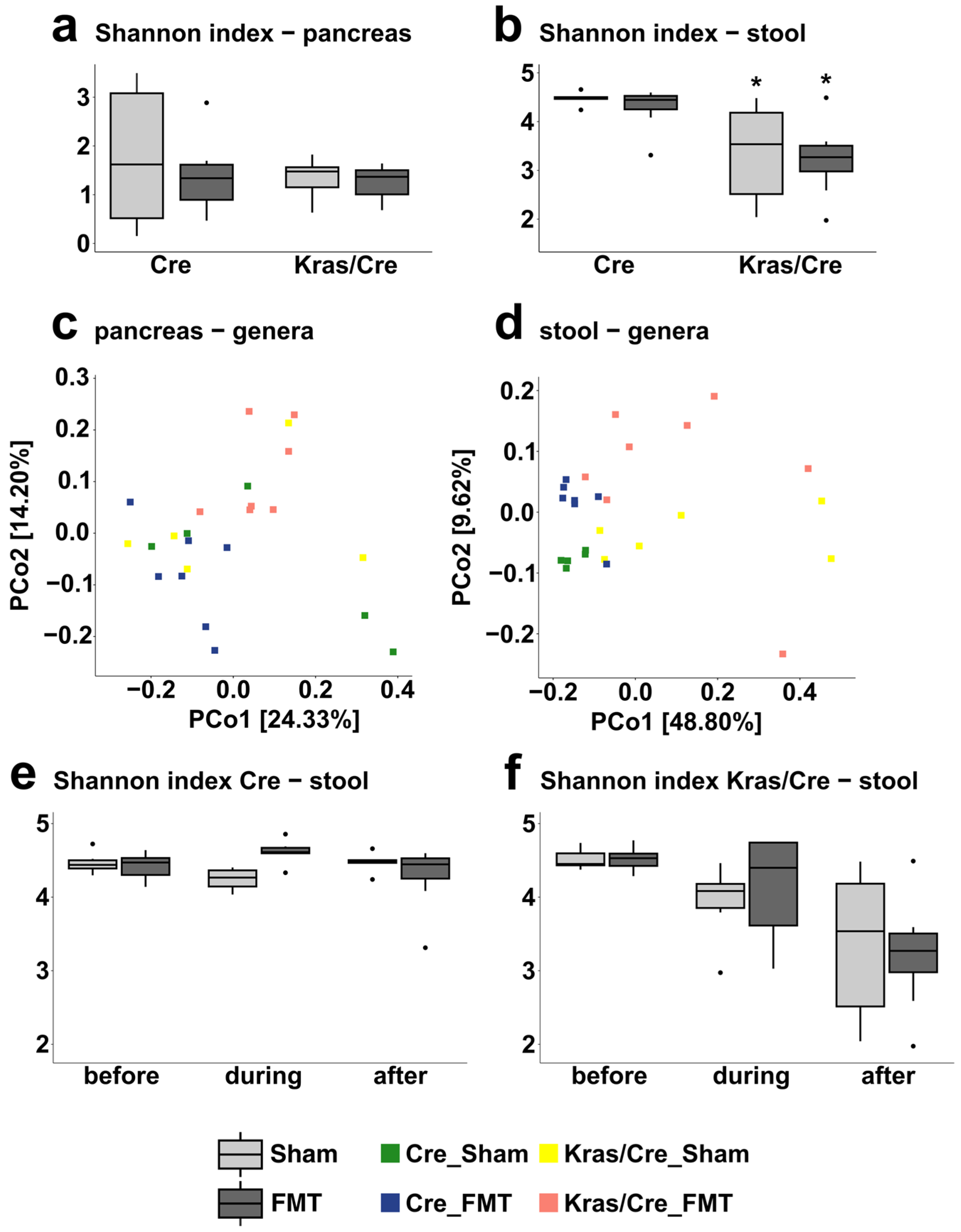
3.4. The Microbiota Alpha and Beta Diversities in Inflammation-Induced Pancreatic Carcinogenesis
3.5. Fecal Microbiota Transplantation Influences Pancreatic Carcinogenesis in Mice Genetically Predisposed to PDAC
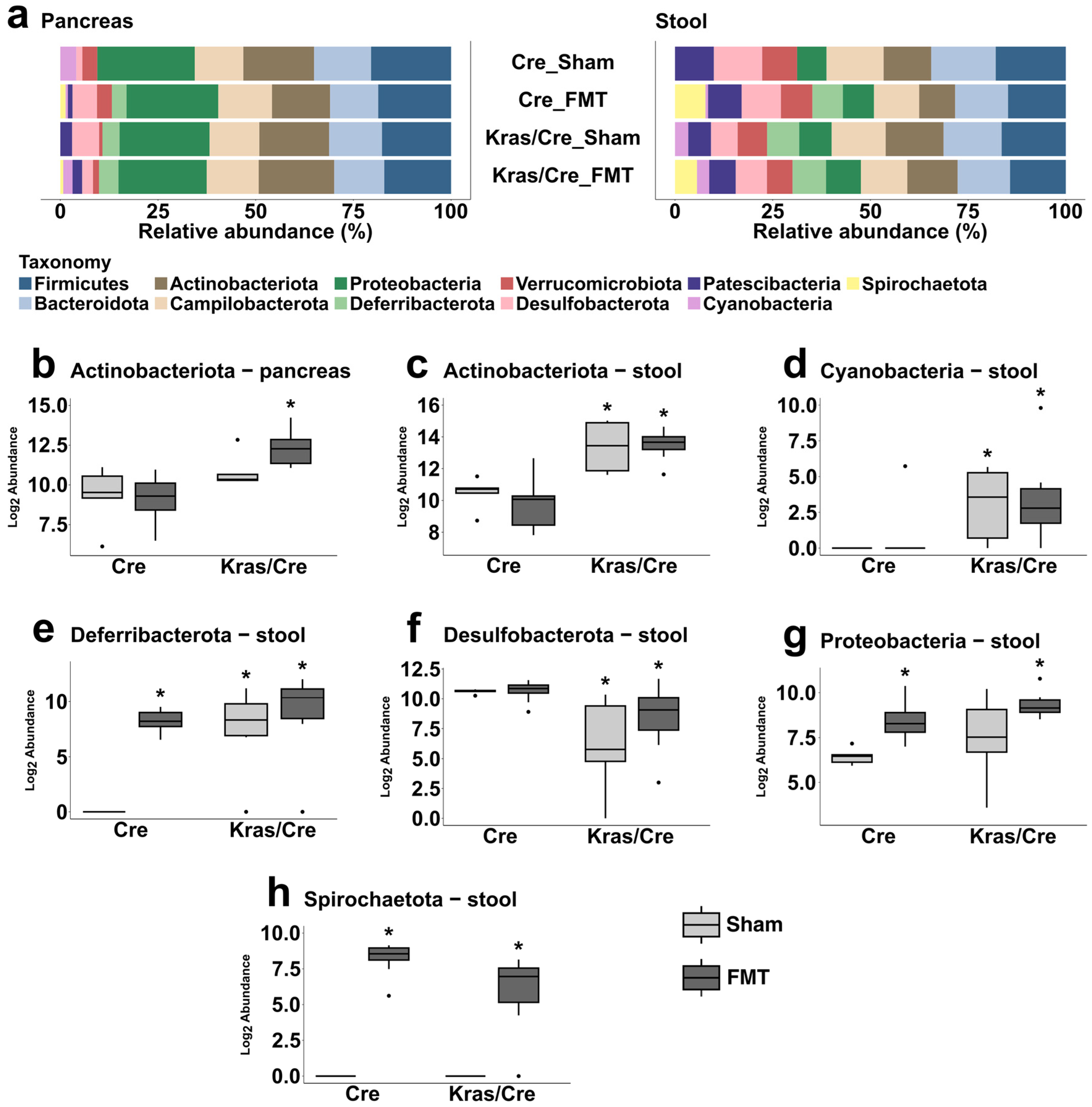
3.6. Characteristics of the Bacterial Community at the Phyla Level After Fecal Microbiota Transplantation
3.6.1. Fecal Microbiota Transplantation Alters the Pancreatic Bacterial Phyla Abundance in Mice Genetically Predisposed to PDAC
3.6.2. Fecal Microbiota Transplantation Alters the Stool Bacterial Phyla Abundance in Mice Genetically Predisposed to PDAC
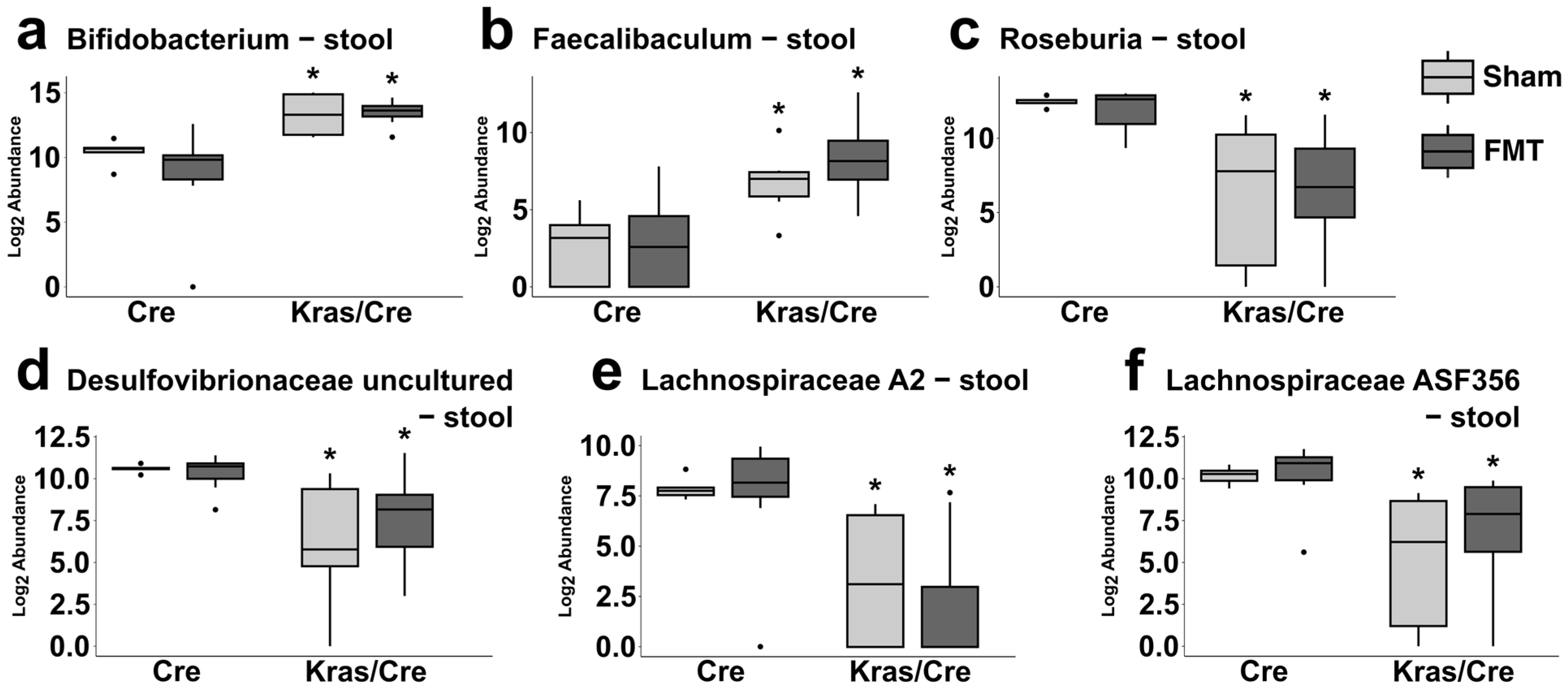
3.7. Characteristics of the Bacterial Community at the Genera Level After Fecal Microbiota Transplantation
3.7.1. Fecal Microbiota Transplantation Alters the Pancreatic Bacterial Genera Abundance in Mice Genetically Predisposed to PDAC
3.7.2. Fecal Microbiota Transplantation Alters the Stool Bacterial Genera Abundance in Mice Genetically Predisposed to PDAC
3.8. The Microbiota Alpha and Beta Diversities in Fecal Microbiota Transplantation-Associated Pancreatic Carcinogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| FMT | Fecal microbiota transplantation |
| PanIN | Pancreatic intraepithelial neoplasia |
| Kras/Cre | Transgenic mice with Kras G12D mutation |
| Cre | Transgenic mice without Kras G12D mutation |
| CER | Cerulein |
| SAL | Saline |
References
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 1, 17–48. [Google Scholar] [CrossRef]
- Hu, H.F.; Ye, Z.; Qin, Y.; Xu, X.W.; Yu, X.J.; Zhuo, Q.F.; Ji, S.R. Mutations in key driver genes of pancreatic cancer: Molecularly targeted therapies and other clinical implications. Acta Pharmacol. Sin. 2021, 11, 1725–1741. [Google Scholar] [CrossRef]
- Nouri, Z.; Choi, S.W.; Choi, J.I.; Ryu, K.W.; Woo, S.M.; Park, S.J.; Lee, W.J.; Choi, W.; Jung, Y.S.; Myung, S.K.; et al. Exploring Connections between Oral Microbiota, Short-Chain Fatty Acids, and Specific Cancer Types: A Study of Oral Cancer, Head and Neck Cancer, Pancreatic Cancer, and Gastric Cancer. Cancers 2023, 11, 2898. [Google Scholar] [CrossRef]
- Pourali, G.; Kazemi, D.; Chadeganipour, A.S.; Arastonejad, M.; Kashani, S.N.; Pourali, R.; Maftooh, M.; Akbarzade, H.; Fiuji, H.; Hassanian, S.M.; et al. Microbiome as a biomarker and therapeutic target in pancreatic cancer. BMC Microbiol. 2024, 1, 16. [Google Scholar] [CrossRef]
- Khan, S.; Banerjee, G.; Setua, S.; Jones, D.H.; Chauhan, B.V.; Dhasmana, A.; Banerjee, P.; Yallapu, M.M.; Behrman, S.; Chauhan, S.C. Metagenomic analysis unveils the microbial landscape of pancreatic tumors. Front. Microbiol. 2023, 14, 1275374. [Google Scholar] [CrossRef]
- Cruz, M.S.; Tintelnot, J.; Gagliani, N. Roles of microbiota in pancreatic cancer development and treatment. Gut Microbes 2024, 1, 2320280. [Google Scholar] [CrossRef]
- de Castilhos, J.; Tillmanns, K.; Blessing, J.; Laraño, A.; Borisov, V.; Stein-Thoeringer, C.K. Microbiome and pancreatic cancer: Time to think about chemotherapy. Gut Microbes 2024, 1, 2374596. [Google Scholar] [CrossRef]
- Daniluk, J.; Daniluk, U.; Rogalski, P.; Dabrowski, A.; Swidnicka-Siergiejko, A. Microbiome-Friend or Foe of Pancreatic Cancer? J. Clin. Med. 2021, 23, 5624. [Google Scholar] [CrossRef]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 56, 95176–95191. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 4, 403–416. [Google Scholar] [CrossRef]
- Nagata, N.; Nishijima, S.; Kojima, Y.; Hisada, Y.; Imbe, K.; Miyoshi-Akiyama, T.; Suda, W.; Kimura, M.; Aoki, R.; Sekine, K.; et al. Metagenomic Identification of Microbial Signatures Predicting Pancreatic Cancer From a Multinational Study. Gastroenterology 2022, 1, 222–238. [Google Scholar] [CrossRef]
- Kartal, E.; Schmidt, T.S.B.; Molina-Montes, E.; Rodríguez-Perales, S.; Wirbel, J.; Maistrenko, O.M.; Akanni, W.A.; Alashkar Alhamwe, B.; Alves, R.J.; Carrato, A.; et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut 2022, 7, 1359–1372. [Google Scholar] [CrossRef]
- Half, E.; Keren, N.; Reshef, L.; Dorfman, T.; Lachter, I.; Kluger, Y.; Reshef, N.; Knobler, H.; Maor, Y.; Stein, A.; et al. Fecal microbiome signatures of pancreatic cancer patients. Sci. Rep. 2019, 1, 16801. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, D.; Li, Z.; Jiang, H.; Li, J.; Ren, R.; Gao, X.; Li, J.; Wang, X.; Wang, W.; et al. The fecal microbiota of patients with pancreatic ductal adenocarcinoma and autoimmune pancreatitis characterized by metagenomic sequencing. J. Transl. Med. 2021, 19, 215. [Google Scholar] [CrossRef]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 1, 33–37.e6. [Google Scholar] [CrossRef]
- Pfisterer, N.; Ammer-Herrmenau, C.; Antweiler, K.; Küffer, S.; Ellenrieder, V.; Neesse, A. Dynamics of intestinal and intratumoral microbiome signatures in genetically engineered mice and human pancreatic ductal adenocarcinoma. Pancreatology 2023, 6, 663–673. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 6356, 1156–1160. [Google Scholar] [CrossRef]
- Tintelnot, J.; Xu, Y.; Lesker, T.R.; Schönlein, M.; Konczalla, L.; Giannou, A.D.; Pelczar, P.; Kylies, D.; Puelles, V.G.; Bielecka, A.A.; et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 2023, 7950, 168–174. [Google Scholar] [CrossRef]
- Chen, S.M.; Hsu, L.J.; Lee, H.L.; Lin, C.P.; Huang, S.W.; Lai, C.J.; Lin, C.W.; Chen, W.T.; Chen, Y.J.; Lin, Y.C.; et al. Lactobacillus Attenuate the Progression of Pancreatic Cancer Promoted by Porphyromonas Gingivalis in K-ras(G12D) Transgenic Mice. Cancers 2020, 12, 3522. [Google Scholar] [CrossRef]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 4, 795–806.e12. [Google Scholar] [CrossRef]
- Kaune, T.; Griesmann, H.; Theuerkorn, K.; Hämmerle, M.; Laumen, H.; Krug, S.; Plumeier, I.; Kahl, S.; Junca, H.; Gustavo Dos Anjos Borges, L.; et al. Gender-specific changes of the gut microbiome correlate with tumor development in murine models of pancreatic cancer. iScience 2023, 6, 106841. [Google Scholar] [CrossRef]
- Daniluk, J.; Liu, Y.; Deng, D.; Chu, J.; Huang, H.; Gaiser, S.; Cruz-Monserrate, Z.; Wang, H.; Ji, B.; Logsdon, C.D. An NF-κB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J. Clin. Investig. 2012, 4, 1519–1528. [Google Scholar] [CrossRef]
- Philip, B.; Roland, C.L.; Daniluk, J.; Liu, Y.; Chatterjee, D.; Gomez, S.B.; Ji, B.; Huang, H.; Wang, H.; Fleming, J.B.; et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology 2013, 6, 1449–1458. [Google Scholar] [CrossRef]
- Ji, B.; Song, J.; Tsou, L.; Bi, Y.; Gaiser, S.; Mortensen, R.; Logsdon, C. Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis 2008, 8, 390–395. [Google Scholar] [CrossRef]
- Stebegg, M.; Silva-Cayetano, A.; Innocentin, S.; Jenkins, T.P.; Cantacessi, C.; Gilbert, C.; Linterman, M.A. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun. 2019, 1, 2443. [Google Scholar] [CrossRef]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018, 8, 1068–1078. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Cruz-Monserrate, Z.; Davis, B.M.; Edderkaoui, M.; Eibl, G.; Epouhe, A.Y.; Gedeon, J.Y.; Gorelick, F.S.; Grippo, P.J.; et al. Animal Models: Challenges and Opportunities to Determine Optimal Experimental Models of Pancreatitis and Pancreatic Cancer. Pancreas 2019, 48, 759–779. [Google Scholar] [CrossRef]
- Gomez-Chou, S.B.; Swidnicka-Siergiejko, A.K.; Badi, N.; Chavez-Tomar, M.; Lesinski, G.B.; Bekaii-Saab, T.; Farren, M.R.; Mace, T.A.; Schmidt, C.; Liu, Y.; et al. Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment. Cancer Res. 2017, 77, 2647–2660. [Google Scholar] [CrossRef]
- Baydogan, S.; Mohindroo, C.; Montiel, M.F.; Petrosino, J.; Maitra, A.; Kim, M.P.; Bhutani, M.S.; White, J.R.; McAllister, F. Prospective characterization of oral and gut microbiome in a high-risk pancreatic cancer cohort. Abstract. J. Clin. Oncol. 2023, 41, 691. [Google Scholar] [CrossRef]
- Wu, C.; Li, M.; Chen, W. Characteristics of Gut Microbiota in Cerulein-Induced Chronic Pancreatitis. Diabetes Metab. Syndr. Obes. 2021, 14, 285–294. [Google Scholar] [CrossRef]
- Genton, L.; Lazarevic, V.; Stojanovic, O.; Spiljar, M.; Djaafar, S.; Koessler, T.; Dutoit, V.; Gaïa, N.; Mareschal, J.; Macpherson, A.J.; et al. Metataxonomic and Metabolic Impact of Fecal Microbiota Transplantation From Patients With Pancreatic Cancer Into Germ-Free Mice: A Pilot Study. Front. Cell Infect. Microbiol. 2021, 11, 752889. [Google Scholar] [CrossRef]
- Chai, Y.; Huang, Z.; Shen, X.; Lin, T.; Zhang, Y.; Feng, X.; Mao, Q.; Liang, Y. Microbiota regulates pancreatic cancer carcinogenesis through altered immune response. Microorganisms 2023, 11, 1240. [Google Scholar] [CrossRef]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell-Derived IL1beta Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Łuksza, M.; Zhao, J.N.; Makarov, V.; Moral, J.A.; Remark, R.; Herbst, B.; Askan, G.; Bhanot, U.; Senbabaoglu, Y.; et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017, 551, 512–516. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Khani Ali Akbari, S.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef]
- Temel, H.Y.; Kaymak, Ö.; Kaplan, S.; Bahcivanci, B.; Gkoutos, G.V.; Acharjee, A. Role of microbiota and microbiota-derived short-chain fatty acids in PDAC. Cancer Med. 2023, 5, 5661–5675. [Google Scholar] [CrossRef]
- Frost, F.; Weiss, F.U.; Sendler, M.; Kacprowski, T.; Rühlemann, M.; Bang, C.; Franke, A.; Völker, U.; Völzke, H.; Lamprecht, G.; et al. The Gut Microbiome in Patients With Chronic Pancreatitis Is Characterized by Significant Dysbiosis and Overgrowth by Opportunistic Pathogens. Clin. Transl. Gastroenterol. 2020, 9, e00232. [Google Scholar] [CrossRef]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity 2015, 2, 304–317. [Google Scholar] [CrossRef]
- Panebianco, C.; Villani, A.; Pisati, F.; Orsenigo, F.; Ulaszewska, M.; Latiano, T.P.; Potenza, A.; Andolfo, A.; Terracciano, F.; Tripodo, C.; et al. Butyrate, a postbiotic of intestinal bacteria, affects pancreatic cancer and gemcitabine response in in vitro and in vivo models. Biomed. Pharmacother. 2022, 151, 113163. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 6529, 595–602. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Lee, P.C.; Wu, C.J.; Hung, Y.W.; Lee, C.J.; Chi, C.T.; Lee, I.C.; Lun, K.Y.; Chou, S.H.; Lou, J.C.; Hou, M.C.; et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2022, 6, e004779. [Google Scholar] [CrossRef]
- Zhao, R.; Li, J.; Chen, B.; Zhao, J.; Hu, L.; Huang, K.; Chen, Q.; Yao, J.; Lin, G.; Bao, L.; et al. The enrichment of the gut microbiota Lachnoclostridium is associated with the presence of intratumoral tertiary lymphoid structures in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1289753. [Google Scholar] [CrossRef]
- Diehl, G.E.; Longman, R.S.; Zhang, J.X.; Breart, B.; Galan, C.; Cuesta, A.; Schwab, S.R.; Littman, D.R. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 2013, 7435, 116–120. [Google Scholar] [CrossRef]
- Kohi, S.; Macgregor-Das, A.; Dbouk, M.; Yoshida, T.; Chuidian, M.; Abe, T.; Borges, M.; Lennon, A.M.; Shin, E.J.; Canto, M.I.; et al. Alterations in the Duodenal Fluid Microbiome of Patients with Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 2022, 2, 196–227. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Todor, S.B.; Anderco, P.; Popa, M.L. The importance of microbiota and fecal microbiota transplantation in pancreatic disorders. Diagnostics 2023, 14, 861. [Google Scholar] [CrossRef]
- Zhang, Z.; Tanaka, I.; Pan, Z.; Ernst, P.B.; Kiyono, H.; Kurashima, Y. Intestinal homeostasis and inflammation: Gut microbiota at the crossroads of pancreas-intestinal barrier axis. Eur. J. Immunol. 2022, 7, 1035–1046. [Google Scholar] [CrossRef]
- Partelli, S.; Frulloni, L.; Minniti, C.; Bassi, C.; Barugola, G.; D’Onofrio, M.; Crippa, S.; Falconi, M. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig. Liver Dis. 2012, 11, 945–951. [Google Scholar] [CrossRef]
- Rogers, M.B.; Aveson, V.; Firek, B.; Yeh, A.; Brooks, B.; Brower-Sinning, R.; Steve, J.; Banfield, J.F.; Zureikat, A.; Hogg, M.; et al. Disturbances of the Perioperative Microbiome Across Multiple Body Sites in Patients Undergoing Pancreaticoduodenectomy. Pancreas 2017, 2, 260–267. [Google Scholar] [CrossRef]
- Ritz, S.; Hahn, D.; Wami, H.T.; Tegelkamp, K.; Dobrindt, U.; Schnekenbuger, J. Gut microbiome as a response marker for pancreatic enzyme replacement therapy in a porcine model of exocrine pancreas insufficiency. Microb. Cell Fact. 2020, 19, 221. [Google Scholar] [CrossRef]
- Isaiah, A.; Parambeth, J.C.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe 2017, 45, 50–58. [Google Scholar] [CrossRef]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Bülow, R.; Kühn, J.P.; Franke, A.; Heinsen, F.A.; Pietzner, M.; Nauck, M.; Völker, U.; et al. Impaired Exocrine Pancreatic Function Associates with Changes in Intestinal Microbiota Composition and Diversity. Gastroenterology 2019, 4, 1010–1015. [Google Scholar] [CrossRef]
- Ahuja, M.; Schwartz, D.M.; Tandon, M.; Son, A.; Zeng, M.; Swaim, W.; Eckhaus, M.; Hoffman, V.; Cui, Y.; Xiao, B.; et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017, 3, 635–646. [Google Scholar] [CrossRef]
- Avril, M.; DePaolo, R.W. “Driver-passenger” bacteria and their metabolites in the pathogenesis of colorectal cancer. Gut Microbes 2021, 1, 1941710. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świdnicka-Siergiejko, A.; Daniluk, J.; Miniewska, K.; Daniluk, U.; Guzińska-Ustymowicz, K.; Pryczynicz, A.; Dąbrowska, M.; Rusak, M.; Ciborowski, M.; Dąbrowski, A. Inflammatory Stimuli and Fecal Microbiota Transplantation Accelerate Pancreatic Carcinogenesis in Transgenic Mice, Accompanied by Changes in the Microbiota Composition. Cells 2025, 14, 361. https://doi.org/10.3390/cells14050361
Świdnicka-Siergiejko A, Daniluk J, Miniewska K, Daniluk U, Guzińska-Ustymowicz K, Pryczynicz A, Dąbrowska M, Rusak M, Ciborowski M, Dąbrowski A. Inflammatory Stimuli and Fecal Microbiota Transplantation Accelerate Pancreatic Carcinogenesis in Transgenic Mice, Accompanied by Changes in the Microbiota Composition. Cells. 2025; 14(5):361. https://doi.org/10.3390/cells14050361
Chicago/Turabian StyleŚwidnicka-Siergiejko, Agnieszka, Jarosław Daniluk, Katarzyna Miniewska, Urszula Daniluk, Katarzyna Guzińska-Ustymowicz, Anna Pryczynicz, Milena Dąbrowska, Małgorzata Rusak, Michał Ciborowski, and Andrzej Dąbrowski. 2025. "Inflammatory Stimuli and Fecal Microbiota Transplantation Accelerate Pancreatic Carcinogenesis in Transgenic Mice, Accompanied by Changes in the Microbiota Composition" Cells 14, no. 5: 361. https://doi.org/10.3390/cells14050361
APA StyleŚwidnicka-Siergiejko, A., Daniluk, J., Miniewska, K., Daniluk, U., Guzińska-Ustymowicz, K., Pryczynicz, A., Dąbrowska, M., Rusak, M., Ciborowski, M., & Dąbrowski, A. (2025). Inflammatory Stimuli and Fecal Microbiota Transplantation Accelerate Pancreatic Carcinogenesis in Transgenic Mice, Accompanied by Changes in the Microbiota Composition. Cells, 14(5), 361. https://doi.org/10.3390/cells14050361






