Preserving Life: How Retinoic Acid (RA) Enhances Cell Viability and Reduces Apoptosis in Cryopreserved Blastocyst Cells of Pudong Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fertilized Eggs and Animal Care
2.2. Isolation and Culture of BCs
2.3. Cryopreservation and Thawing of BCs
2.4. Post-Thaw Evaluation of BCs
2.4.1. Cell Viability Measurement
2.4.2. Adherent Cell Analysis
2.4.3. Flow Cytometric Analysis of Mitochondrial Membrane Potential (MMP)
2.4.4. Apoptosis Analysis
2.4.5. Expression Analysis of Apoptosis-Related Genes
2.4.6. Flow Cytometric Analysis of Cell Cycle Distribution
2.5. Statistical Analysis
3. Results
3.1. Effects of RA Treatment on the Viability and Adhesion of Thawed Chicken BCs
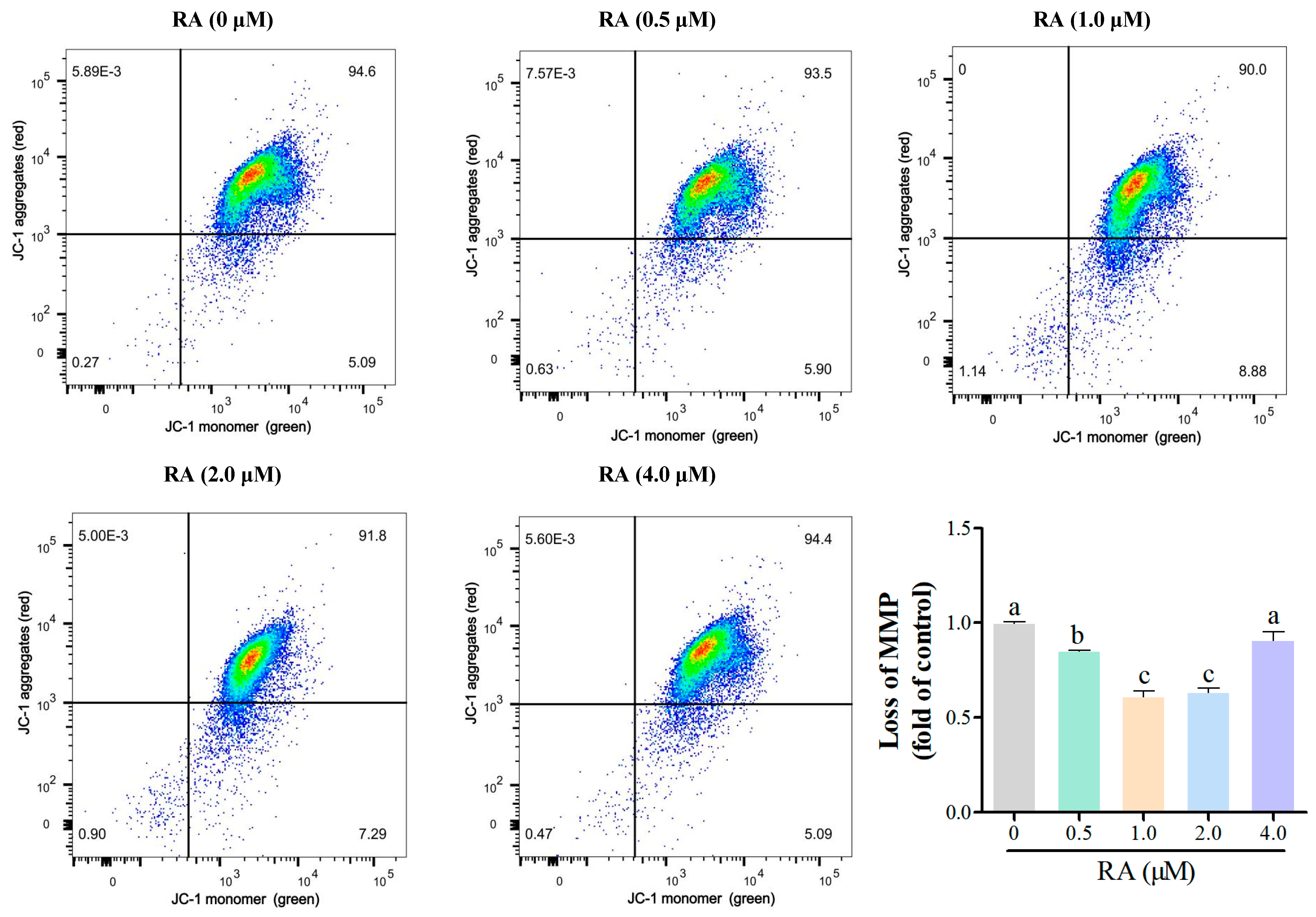
3.2. Effects of RA Treatment on the Mitochondrial Function of Thawed Chicken BCs
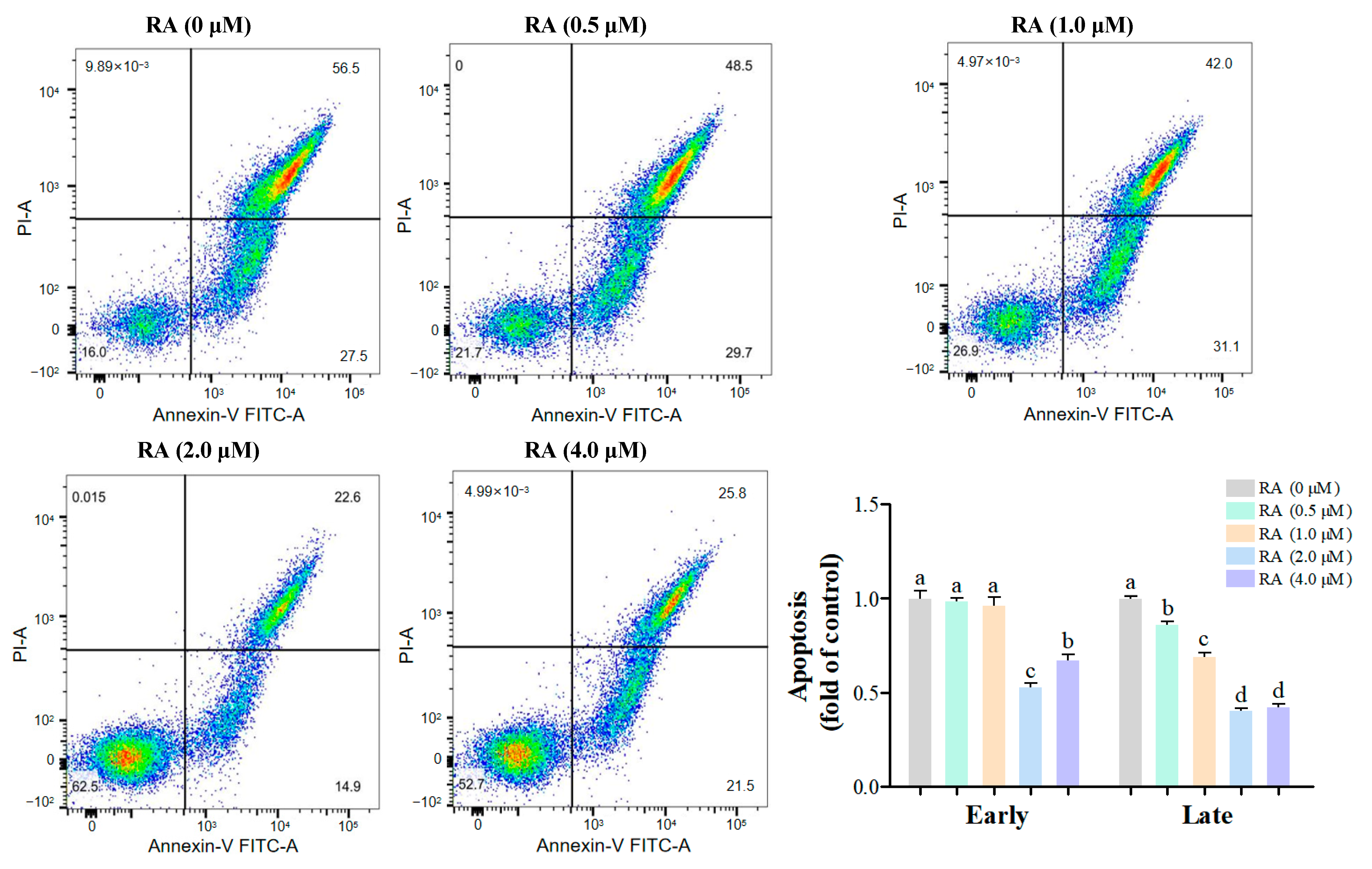
3.3. Effects of RA Treatment on the Apoptosis of Thawed Chicken BCs
3.4. Effects of RA Treatment on the Expression of Apoptosis-Related Genes in Thawed Chicken BCs
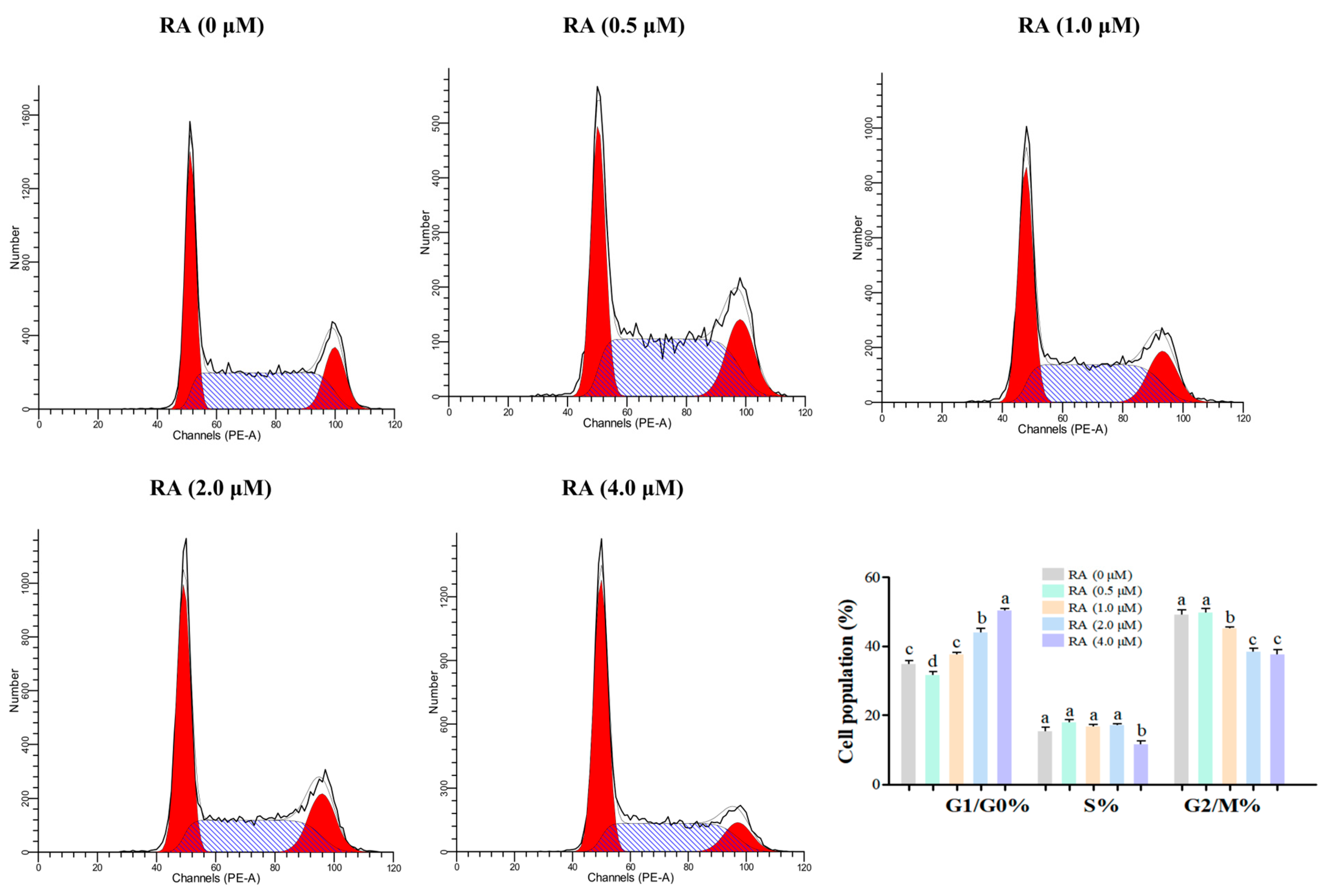
3.5. Effects of RA Treatment on the Cell Cycle Distribution of Thawed Chicken BCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, R.J.; Napolitano, A.; Garcia-Pereira, F.L.; Baldo, C.F.; Suhr, S.T.; King, L.E.; Cibelli, J.B.; Karcher, D.M.; McNiel, E.A.; Perez, G.I. Conservation of avian germplasm by xenogeneic transplantation of spermatogonia from sexually mature donors. Stem Cells Dev. 2013, 22, 735–749. [Google Scholar] [CrossRef]
- Altgilbers, S.; Klein, S.; Dierks, C.; Weigend, S.; Kues, W.A. Cultivation and characterization of primordial germ cells from blue layer hybrids (Araucana crossbreeds) and generation of germline chimeric chickens. Sci. Rep. 2021, 11, 12923. [Google Scholar] [CrossRef]
- Blesbois, E.; Seigneurin, F.; Grasseau, I.; Limouzin, C.; Besnard, J.; Gourichon, D.; Coquerelle, G.; Rault, P.; Tixier-Boichard, M. Semen cryopreservation for ex situ management of genetic diversity in chicken: Creation of the French avian cryobank. Poulty Sci. 2007, 86, 555–564. [Google Scholar]
- Thananurak, P.; Chuaychu-Noo, N.; Thélie, A.; Aurore, T.; Rattanawong, K.; Blesbois, E. Different concentrations of cysteamine, ergothioneine, and serine modulate quality and fertilizing ability of cryopreserved chicken sperm. Poulty Sci. 2020, 99, 1185–1198. [Google Scholar] [CrossRef]
- Etches, R.J.; Carsience, R.S.; Clark, M.E.; Fraser, R.A.; Toner, A.; Verrinder Gibbins, A.M. Chimeric chickens and their use in manipulation of the chicken genome. Poultrt Sci. 1993, 72, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Thananurak, P.; Chuaychu-Noo, N.; Thélie, A.; Phasuk, Y.; Vongpralub, T.; Blesbois, E. Sucrose increases the quality and fertilizing ability of cryopreserved chicken sperm in contrast to raffinose. Poultry Sci. 2019, 98, 4161–4171. [Google Scholar]
- Li, J.; Li, Q.; Geng, S. All-trans retinoic acid alters the expression of the tight junction proteins Claudin-1 and -4 and epidermal barrier function-associated genes in the epidermis. Int. J. Mol. Med. 2019, 43, 1789–1805. [Google Scholar] [CrossRef]
- Malivindi, R.; Rago, V.; De Rose, D.; Gervasi, M.C.; Cione, E.; Russo, G.; Santoro, M.; Aquila, S. Influence of all-trans retinoic acid on sperm metabolism and oxidative stress: Itsinvolvement in the physiopathology of varicocele-associated male infertility. J. Cell. Physiol. 2018, 233, 9526–9537. [Google Scholar]
- Tang, X.; Shi, J.; Qin, X.; Xiao, N.; Li, R.; Hu, H.; Yang, F.; Shi, D.; Wang, X. Retinoic acid (RA) and bone morphogenetic protein 4 (BMP4) restore the germline competence of in vitro cultured chicken blastodermal cells. Vitr. Cell. Deve. Biol. Anim. 2019, 55, 169–176. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, L.B.; Mao, H. Induction of ciliated cells from avian embryonic stem cells using three-dimensional matrix. Tissue Eng. Part C. Methods 2010, 16, 929–936. [Google Scholar] [CrossRef]
- Li, P.; Li, K.; Zou, C.; Tong, C.; Sun, L.; Cao, Z.; Yang, S.; Lyu, Q. Selenium yeast Alleviates ochratoxin a-induced hepatotoxicity via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in chickens. Toxins 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Grötter, L.G.; Cattaneo, L.; Marini, P.E.; Kjelland, M.E.; Ferré, L.B. Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post-thaw quality optimization. Reprod. Domest. Anim. 2019, 54, 655–665. [Google Scholar] [PubMed]
- Zhu, S.; Lin, G.; Song, C.; Wu, Y.; Feng, N.; Chen, W.; He, Z.; Chen, Y.Q. RA and ω-3 PUFA co-treatment activates autophagy in cancer cells. Oncotarget 2017, 8, 109135–109150. [Google Scholar]
- Jørgensen, A.; Nielsen, J.E.; Perlman, S.; Lundvall, L.; Mitchell, R.T.; Juul, A.; Rajpert-De Meyts, E. Ex vivo culture of human fetal gonads: Manipulation of meiosis signalling by retinoic acid treatment disrupts testis development. Hum. Reprod. 2015, 30, 2351–2363. [Google Scholar]
- López-Carballo, G.; Moreno, L.; Masiá, S.; Pérez, P.; Barettino, D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J. Biol. Chem. 2002, 277, 25297–25304. [Google Scholar] [PubMed]
- Gao, R.W.; Kong, X.Y.; Zhu, X.X.; Zhu, G.Q.; Ma, J.S.; Liu, X.X. Retinoic acid promotes primary fetal alveolar epithelial type II cell proliferation and differentiation to alveolar epithelial type I cells. In Vitro Cell. Dev. Biol. 2015, 51, 479–487. [Google Scholar] [CrossRef]
- Lowndes, M.; Rakshit, S.; Shafraz, O.; Borghi, N.; Harmon, R.M.; Green, K.J.; Sivasankar, S.; Nelson, W.J. Different roles of cadherins in the assembly and structural integrity of the desmosome complex. J. Cell Sci. 2014, 127, 2339–2350. [Google Scholar]
- Kim, M.H.; Taparowsky, E.J.; Kim, C.H. Retinoic acid differentially regulates the migration of innate lymphoid cell subsets to the gut. Immunity 2015, 43, 107–119. [Google Scholar] [CrossRef]
- Brown, G. Targeting the retinoic acid pathway to eradicate cancer stem cells. Int. J. Mol. Sci. 2023, 24, 2373. [Google Scholar] [CrossRef]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic acid and its derivatives in skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef] [PubMed]
- Pena-Rodríguez, E.; Moreno, M.C.; Blanco-Fernandez, B.; González, J.; Fernández-Campos, F. Epidermal delivery of retinyl palmitate loaded transfersomes: Penetration and biodistribution studies. Pharmaceutics 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Wang, S.; Sang, Y.; Sun, P.; Lal, B.; Goodwin, C.R.; Goodwin, C.R.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Laterra, J.; et al. Regulation of glioblastoma stem cells by retinoic acid: Role for Notch pathway inhibition. Oncogene 2011, 30, 3454–3467. [Google Scholar] [CrossRef] [PubMed]
- Aquino, J.B.; Lallemend, F.; Marmigère, F.; Adameyko, I.; Golemis, E.A.; Ernfors, P. The retinoic acid inducible Cas-family signaling protein Nedd9 regulates neural crest cell migration by modulating adhesion and actin dynamics. Neuroscience 2009, 162, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.J.; Else, K.J. The retinoic acid-producing capacity of gut dendritic cells and macrophages is reduced during persistent T. muris infection. Parasite Immunol. 2013, 35, 229–233. [Google Scholar] [CrossRef]
- Li, Y.; Gong, H.; Ding, J.; Zhao, F.; Du, J.; Wan, J.; Zhang, J.; Liu, S.; Li, J.; Wang, L.; et al. Inhibition of GSK3 represses the expression of retinoic acid synthetic enzyme ALDH1A2 via Wnt/β-catenin signaling in WiT49 cells. Front. Cell Dev. Biol. 2020, 8, 94. [Google Scholar] [CrossRef]
- Everts, H.B.; Claassen, D.O.; Hermoyian, C.L.; Berdanier, C.D. Nutrient-gene interactions: Dietary vitamin A and mitochondrial gene expression. IUBMB Life 2002, 53, 295–301. [Google Scholar] [CrossRef]
- El-Metwally, T.H.; Hussein, M.R.; Pour, P.M.; Kuszynski, C.A.; Adrian, T.E. High concentrations of retinoids induce differentiation and late apoptosis in pancreatic cancer cells in vitro. Cancer Biol. Ther. 2005, 4, 602–611. [Google Scholar] [CrossRef]
- Chidipi, B.; Shah, S.I.; Reiser, M.; Kanithi, M.; Garces, A.; Cha, B.J.; Ullah, G.; Noujaim, S.F. All-trans retinoic acid increases DRP1 levels and promotes mitochondrial fission. Cells 2021, 10, 1202. [Google Scholar] [CrossRef]
- Tourniaire, F.; Musinovic, H.; Gouranton, E.; Astier, J.; Marcotorchino, J.; Arreguin, A.; Bernot, D.; Palou, A.; Bonet, M.L.; Ribot, J.; et al. All-trans retinoic acid induces oxidative phosphorylation and mitochondria biogenesis in adipocytes. J. Lipid Res. 2015, 56, 1100–1109. [Google Scholar] [CrossRef]
- Karmakar, E.; Das, N.; Mukherjee, B.; Das, P.; Mukhopadhyay, S.; Roy, S.S. Lipid-induced alteration in retinoic acid signaling leads to mitochondrial dysfunction in HepG2 and Huh7 cells. Biochem. Cell Biol. 2023, 101, 220–234. [Google Scholar] [CrossRef]
- van der Pouw Kraan, T.C.; Schirmer, S.H.; Fledderus, J.O.; Moerland, P.D.; Baggen, J.M.; Leyen, T.A.; van der Laan, A.M.; Piek, J.J.; van Royen, N.; Horrevoets, A.J. Expression of a retinoic acid signature in circulating CD34 cells from coronary artery disease patients. BMC Genom. 2010, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar]
- Noy, N. Between death and survival: Retinoic acid in regulation of apoptosis. Annu. Rev. Nutr. 2010, 30, 201–217. [Google Scholar]
- Pan, J.; Guleria, R.S.; Zhu, S.; Baker, K.M. Molecular mechanisms of retinoid receptors in diabetes-induced cardiac remodeling. J. Clin. Med. 2014, 3, 566–594. [Google Scholar] [CrossRef]
- Songthaveesin, C.; Sa-Nongdej, W.; Limboonreung, T.; Chongthammakun, S. Combination of metformin and 9-cis retinoic acid increases apoptosis in C6 glioma stem-like cells. Heliyon 2018, 4, e00638. [Google Scholar]
- Hadjidaniel, M.D.; Reynolds, C.P. Antagonism of cytotoxicchemotherapy in neuroblastoma cell lines by 13-cis-retinoicacid is mediated by the antiapoptotic Bcl-2 family proteins. Mol. Cancer Ther. 2010, 9, 3164–3174. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M. Analysis of the mechanisms underlying the anticancer and biological activity of retinoic acid and chitosan nanoparticles containing retinoic acid. Med. Oncol. 2024, 41, 251. [Google Scholar] [PubMed]
- Sun, Y.; Cao, Y. Effects of Xinfeng capsule on the Fas/FasL-mediated apoptotic pathway in patients with rheumatoid arthritis. J. Tradit. Chin. Med. 2018, 38, 601–609. [Google Scholar]
- Dimberg, A.; Bahram, F.; Karlberg, I.; Larsson, L.G.; Nilsson, K.; Oberg, F. Retinoic acid-induced cell cycle arrest of human myeloid cell lines is associated with sequential down-regulation of c-Myc and cyclin E and posttranscriptional up-regulation of p27Kip1. Blood 2002, 99, 2199–2206. [Google Scholar]
- Miranda-Sayago, J.M.; Fernandez-Arcas, N.; Benito, C.; Reyes-Engel, A.; Herrero, J.R.; Alonso, A. Evaluation of a low cost cryopreservation system on the biology of human amniotic fluid-derived mesenchymal stromal cells. Cryobiology 2012, 64, 160–166. [Google Scholar]
- Tamanoue, Y.; Yamagishi, M.; Hongo, I.; Okamoto, H. Polypyrimidine tract-binding protein is required for the repression of gene expression by all-trans retinoic acid. Dev. Growth Differ. 2010, 52, 469–479. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Liu, F.; He, M.; Xu, J.; Wu, C.; Zhagn, S.; Gao, J.; Dai, J. Preserving Life: How Retinoic Acid (RA) Enhances Cell Viability and Reduces Apoptosis in Cryopreserved Blastocyst Cells of Pudong Chickens. Cells 2025, 14, 504. https://doi.org/10.3390/cells14070504
Sun L, Liu F, He M, Xu J, Wu C, Zhagn S, Gao J, Dai J. Preserving Life: How Retinoic Acid (RA) Enhances Cell Viability and Reduces Apoptosis in Cryopreserved Blastocyst Cells of Pudong Chickens. Cells. 2025; 14(7):504. https://doi.org/10.3390/cells14070504
Chicago/Turabian StyleSun, Lingwei, Fuqin Liu, Mengqian He, Jiehuan Xu, Caifeng Wu, Shushan Zhagn, Jun Gao, and Jianjun Dai. 2025. "Preserving Life: How Retinoic Acid (RA) Enhances Cell Viability and Reduces Apoptosis in Cryopreserved Blastocyst Cells of Pudong Chickens" Cells 14, no. 7: 504. https://doi.org/10.3390/cells14070504
APA StyleSun, L., Liu, F., He, M., Xu, J., Wu, C., Zhagn, S., Gao, J., & Dai, J. (2025). Preserving Life: How Retinoic Acid (RA) Enhances Cell Viability and Reduces Apoptosis in Cryopreserved Blastocyst Cells of Pudong Chickens. Cells, 14(7), 504. https://doi.org/10.3390/cells14070504


.png)


