Differential Effects of Hearing Loss Mutations in Homomeric P2X2 and Heteromeric P2X2/3 Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Biology and Cell Culture
2.2. Viability Assay
2.3. Electrophysiology
2.4. Confocal Microscopy
2.5. Analysis
3. Results
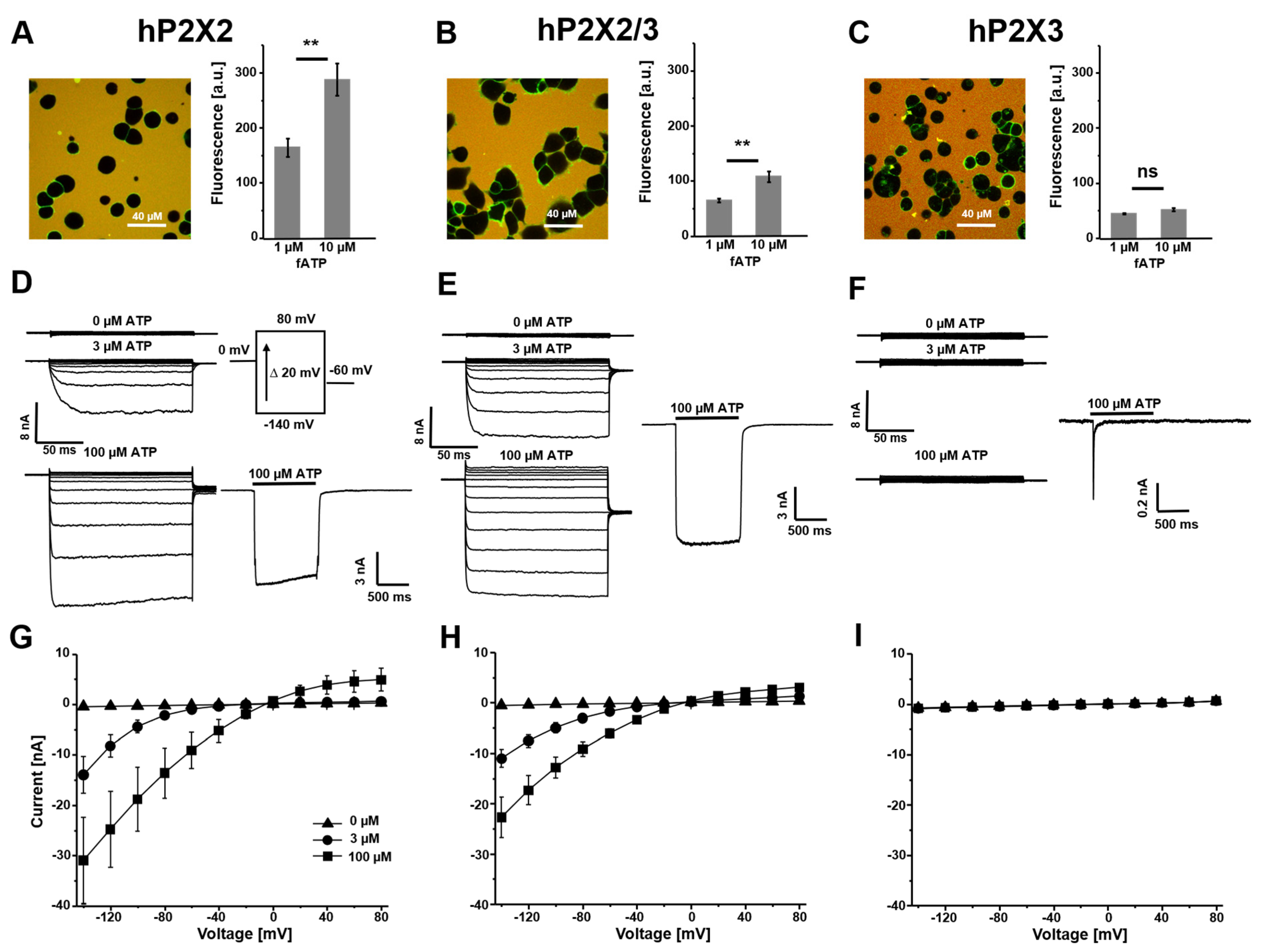
3.1. Binding of fATP to Wildtype P2X Receptors and Their Activation with Potassium as the Permeating Ion
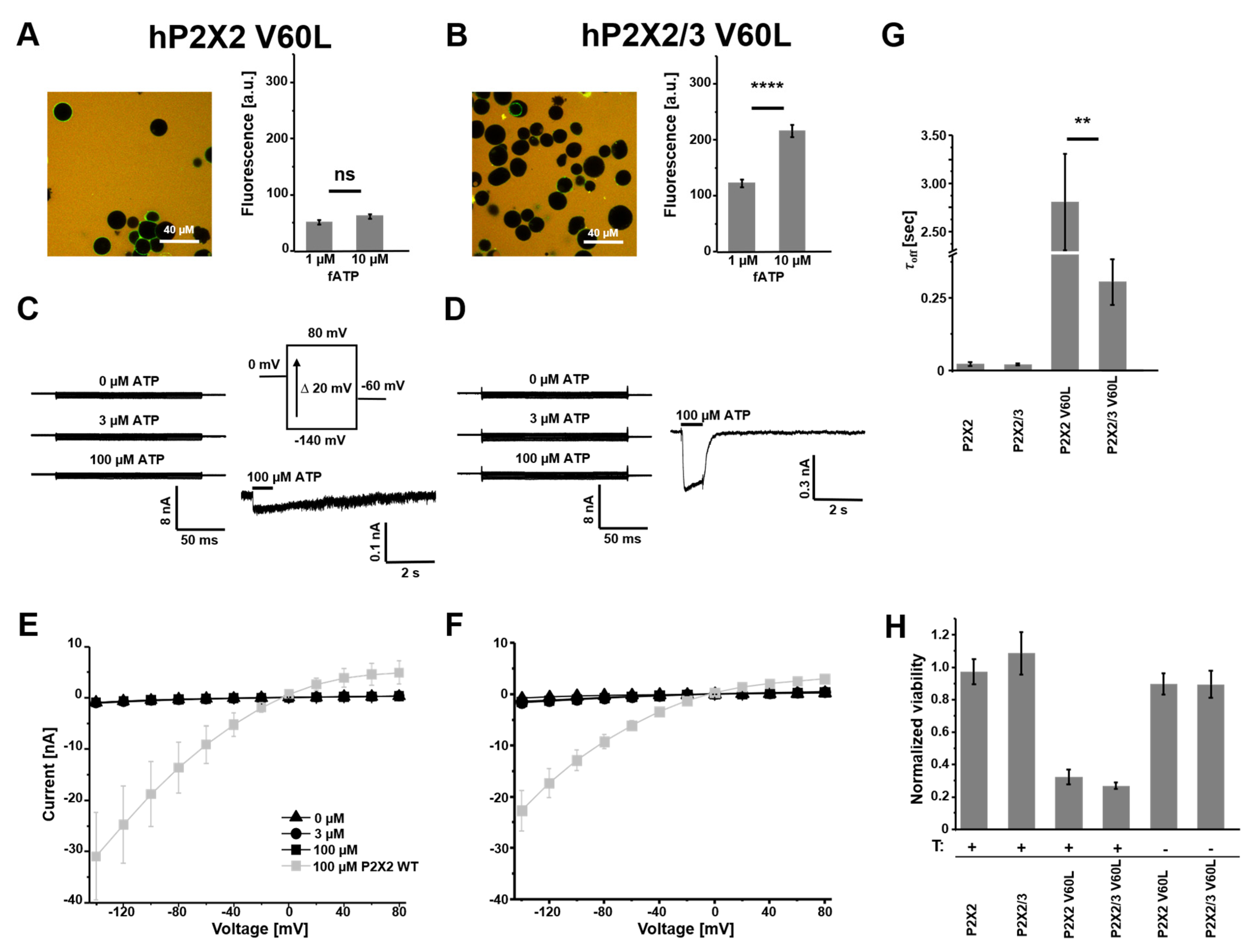
3.2. Homomeric hP2X2 V60L Receptors Show an Increased Affinity to fATP and Cause a Decrease in Cell Viability
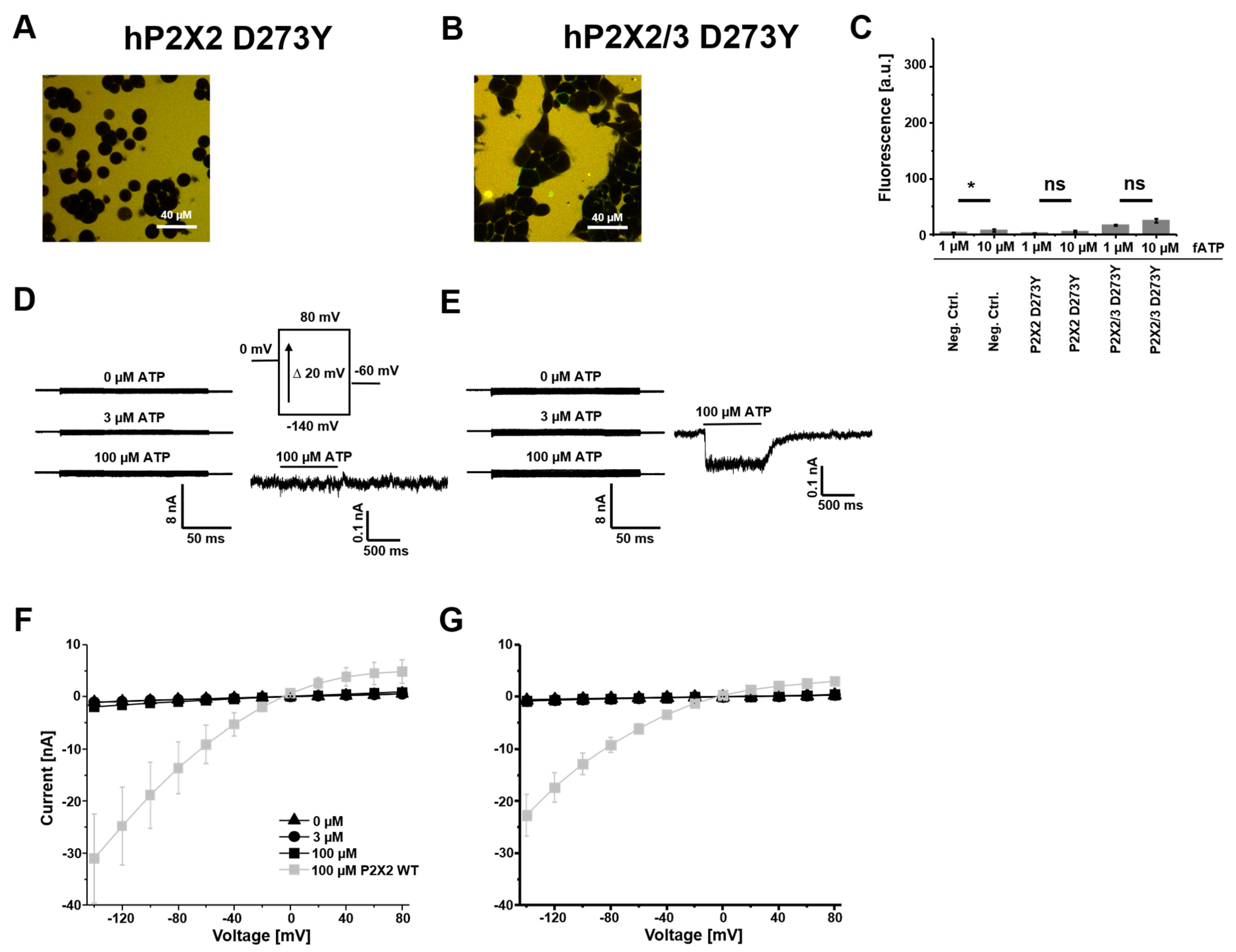
3.3. hP2X2 D273Y Subunits Do Not Form Functional Homomers but Can Assemble into P2X2/3 D273Y Heteromers
3.4. Homomeric hP2X2 G353R Receptors Show Reduced Function with Potassium as the Permeating Ion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| fATP | Fluorescent adenosine triphosphate (2-[DY-547P1]-AHT-ATP) |
| hP2X2 | Human P2X2 receptor |
| hP2X2/3 | Human P2X2/3 receptor |
| KI | Knock-in |
| PBS | Phosphate-buffered saline |
| SEM | Standard error of the mean |
| SGNs | Spiral ganglion neurons |
| TM1 | Transmembrane domain 1 |
| TM2 | Transmembrane domain 2 |
| wt | Wildtype |
References
- Berekméri, E.; Szepesy, J.; Köles, L.; Zelles, T. Purinergic Signaling in the Organ of Corti: Potential Therapeutic Targets of Sensorineural Hearing Losses. Brain Res. Bull. 2019, 151, 109–118. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Evans, R.J. ATP-Gated P2X Receptor Channels: Molecular Insights into Functional Roles. Annu. Rev. Physiol. 2019, 81, 43–62. [Google Scholar] [CrossRef]
- Coddou, C.; Yan, Z.; Obsil, T.; Huidobro-Toro, J.P.; Stojilkovic, S.S. Activation and Regulation of Purinergic P2X Receptor Channels. Pharmacol. Rev. 2011, 63, 641–683. [Google Scholar] [CrossRef]
- Sattler, C.; Benndorf, K. Enlightening Activation Gating in P2X Receptors. Purinergic Signal. 2022, 18, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal Structure of the ATP-Gated P2X4 Ion Channel in the Closed State. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Hausmann, R.; Bodnar, M.; Woltersdorf, R.; Wang, H.; Fuchs, M.; Messemer, N.; Qin, Y.; Günther, J.; Riedel, T.; Grohmann, M.; et al. ATP Binding Site Mutagenesis Reveals Different Subunit Stoichiometry of Functional P2X2/3 and P2X2/6 Receptors. J. Biol. Chem. 2012, 287, 13930–13943. [Google Scholar] [CrossRef]
- Keceli, B.; Kubo, Y. Signal Transmission within the P2X2 Trimeric Receptor. J. Gen. Physiol. 2014, 143, 761–782. [Google Scholar] [CrossRef]
- Stelmashenko, O.; Lalo, U.; Yang, Y.; Bragg, L.; North, R.A.; Compan, V. Activation of Trimeric P2X2 Receptors by Fewer than Three ATP Molecules. Mol. Pharmacol. 2012, 82, 760–766. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; Kanjhan, R.; Raybould, N.P.; Greenwood, D.; Salih, S.G.; Järlebark, L.; Burton, L.D.; Setz, V.C.M.; Cannell, M.B.; Soeller, C.; et al. Expression of the P2X2 Receptor Subunit of the ATP-Gated Ion Channel in the Cochlea: Implications for Sound Transduction and Auditory Neurotransmission. J. Neurosci. 1999, 19, 8377–8388. [Google Scholar] [CrossRef]
- Järlebark, L.E.; Housley, G.D.; Thorne, P.R. Immunohistochemical Localization of Adenosine 5′-Triphosphate-Gated Ion Channel P2X(2) Receptor Subunits in Adult and Developing Rat Cochlea. J. Comp. Neurol. 2000, 421, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, H.B. ATP-Mediated Potassium Recycling in the Cochlear Supporting Cells. Purinergic Signal. 2010, 6, 221–229. [Google Scholar] [CrossRef]
- Zhao, H.B.; Yu, N.; Fleming, C.R. Gap Junctional Hemichannel-Mediated ATP Release and Hearing Controls in the Inner Ear. Proc. Natl. Acad. Sci. USA 2005, 102, 18724–18729. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, H.-B. ATP Activates P2X Receptors to Mediate Gap Junctional Coupling in the Cochlea. Biochem. Biophys. Res. Commun. 2012, 426, 528–532. [Google Scholar] [CrossRef]
- Muñoz, D.J.B.; Thorne, P.R.; Housley, G.D.; Billett, T.E. Adenosine 5′-Triphosphate (ATP) Concentrations in the Endolymph and Perilymph of the Guinea-Pig Cochlea. Hear. Res. 1995, 90, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.J.; Kendrick, I.S.; Rassam, M.; Thorne, P.R. Vesicular Storage of Adenosine Triphosphate in the Guinea-Pig Cochlear Lateral Wall and Concentrations of ATP in the Endolymph during Sound Exposure and Hypoxia. Acta Otolaryngol. 2001, 121, 10–15. [Google Scholar] [CrossRef]
- Evans, R.J.; Lewis, C.; Virginio, C.; Lundstrom, K.; Buell, G.; Surprenant, A.; North, R.A. Ionic Permeability of, and Divalent Cation Effects on, Two ATP-Gated Cation Channels (P2X Receptors) Expressed in Mammalian Cells. J. Physiol. 1996, 497, 413–422. [Google Scholar] [CrossRef]
- Housley, G.D.; Jagger, D.J.; Greenwood, D.; Raybould, N.P.; Salih, S.G.; Järlebark, L.E.; Vlajkovic, S.M.; Kanjhan, R.; Nikolic, P.; Muñoz, D.J.M.; et al. Purinergic Regulation of Sound Transduction and Auditory Neurotransmission. Audiol. Neuro Otol. 2002, 7, 55–61. [Google Scholar] [CrossRef]
- Thorne, P.R.; Muñoz, D.J.B.; Housley, G.D. Purinergic Modulation of Cochlear Partition Resistance and Its Effect on the Endocochlear Potential in the Guinea Pig. J. Assoc. Res. Otolaryngol. 2004, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; Morton-Jones, R.; Vlajkovic, S.M.; Telang, R.S.; Paramananthasivam, V.; Tadros, S.F.; Wong, A.C.Y.; Froud, K.E.; Cederholm, J.M.E.; Sivakumaran, Y.; et al. ATP-Gated Ion Channels Mediate Adaptation to Elevated Sound Levels. Proc. Natl. Acad. Sci. USA 2013, 110, 7494–7499. [Google Scholar] [CrossRef]
- Yan, D.; Zhu, Y.; Walsh, T.; Xie, D.; Yuan, H.; Sirmaci, A.; Fujikawa, T.; Wong, A.C.Y.; Loh, T.L.; Du, L.; et al. Mutation of the ATP-Gated P2X2 Receptor Leads to Progressive Hearing Loss and Increased Susceptibility to Noise. Proc. Natl. Acad. Sci. USA 2013, 110, 2228–2233. [Google Scholar] [CrossRef]
- Blanton, S.H.; Liang, C.Y.; Cai, M.W.; Pandya, A.; Du, L.L.; Landa, B.; Mummalanni, S.; Li, K.S.; Chen, Z.Y.; Qin, X.N.; et al. A Novel Locus for Autosomal Dominant Non-Syndromic Deafness (DFNA41) Maps to Chromosome 12q24-Qter. J. Med. Genet. 2002, 39, 567–570. [Google Scholar] [CrossRef]
- Moteki, H.; Azaiez, H.; Booth, K.T.; Hattori, M.; Sato, A.; Sato, Y.; Motobayashi, M.; Sloan, C.M.; Kolbe, D.L.; Shearer, A.E.; et al. Hearing Loss Caused by a P2RX2 Mutation Identified in a MELAS Family with a Coexisting Mitochondrial 3243AG Mutation. Ann. Otol. Rhinol. Laryngol. 2015, 124, 177S–183S. [Google Scholar] [CrossRef]
- Faletra, F.; Girotto, G.; D’Adamo, A.P.; Vozzi, D.; Morgan, A.; Gasparini, P. A Novel P2RX2 Mutation in an Italian Family Affected by Autosomal Dominant Nonsyndromic Hearing Loss. Gene 2014, 534, 236–239. [Google Scholar] [CrossRef]
- Zhu, Y.; Beudez, J.; Yu, N.; Grutter, T.; Zhao, H.B. P2x2 Dominant Deafness Mutations Have No Negative Effect on Wild-Type Isoform: Implications for Functional Rescue and in Deafness Mechanism. Front. Mol. Neurosci. 2017, 10, 371. [Google Scholar] [CrossRef]
- George, B.; Swartz, K.J.; Li, M. Hearing Loss Mutations Alter the Functional Properties of Human P2X2 Receptor Channels through Distinct Mechanisms. Proc. Natl. Acad. Sci. USA 2019, 116, 22862–22871. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Keceli, B.; Nakajo, K.; Kubo, Y. Voltage- and [ATP]-Dependent Gating of the P2X2 ATP Receptor Channel. J. Gen. Physiol. 2009, 133, 93–109. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular Mechanism of ATP Binding and Ion Channel Activation in P2X Receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef]
- Habermacher, C.; Martz, A.; Calimet, N.; Lemoine, D.; Peverini, L.; Specht, A.; Cecchini, M.; Grutter, T. Photo-Switchable Tweezers Illuminate Pore-Opening Motions of an ATP-Gated P2X Ion Channel. eLife 2016, 5, e11050. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-C.; Greenwood, D.; Thorne, P.R.; Housley, G.D. Developmental Regulation of Neuron-Specific P2X3 Receptor Expression in the Rat Cochlea. J. Comp. Neurol. 2005, 484, 133–143. [Google Scholar] [CrossRef]
- Huang, L.-C.; Ryan, A.F.; Cockayne, D.A.; Housley, G.D. Developmentally Regulated Expression of the P2X3 Receptor in the Mouse Cochlea. Histochem. Cell Biol. 2006, 125, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.; Radulovic, T.; Coddou, C.; Dietz, B.; Nerlich, J.; Stojilkovic, S.S.; Rübsamen, R.; Milenkovic, I. Tonotopic Action Potential Tuning of Maturing Auditory Neurons through Endogenous ATP. J. Physiol. 2017, 595, 1315–1337. [Google Scholar] [CrossRef]
- Kowalski, M.; Hausmann, R.; Schmid, J.; Dopychai, A.; Stephan, G.; Tang, Y.; Schmalzing, G.; Illes, P.; Rubini, P. Flexible Subunit Stoichiometry of Functional Human P2X2/3 Heteromeric Receptors. Neuropharmacology 2015, 99, 115–130. [Google Scholar] [CrossRef]
- Lewis, C.; Neidhart, S.; Holy, C.; North, R.A.; Buell, G.; Surprenant, A. Coexpression of P2X2 and P2X3 Receptor Subunits Can Account for ATP-Gated Currents in Sensory Neurons. Nature 1995, 377, 432–435. [Google Scholar] [CrossRef]
- Liu, M.; King, B.F.; Dunn, P.M.; Rong, W.; Townsend-Nicholson, A.; Burnstock, G. Coexpression of P2X(3) and P2X(2) Receptor Subunits in Varying Amounts Generates Heterogeneous Populations of P2X Receptors That Evoke a Spectrum of Agonist Responses Comparable to That Seen in Sensory Neurons. J. Pharmacol. Exp. Ther. 2001, 296, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.; Jagger, D.J.; Huang, L.-C.; Hoya, N.; Thorne, P.R.; Wildman, S.S.; King, B.F.; Pak, K.; Ryan, A.F.; Housley, G.D. P2X Receptor Signaling Inhibits BDNF-Mediated Spiral Ganglion Neuron Development in the Neonatal Rat Cochlea. Development 2007, 134, 1407–1417. [Google Scholar] [CrossRef]
- Brünings, X.; Schmauder, R.; Mrowka, R.; Benndorf, K.; Sattler, C. Subtype-Specific Ligand Binding and Activation Gating in Homomeric and Heteromeric P2X Receptors. Biomolecules 2024, 14, 942. [Google Scholar] [CrossRef]
- Hassan, M.I.A.; Keller, M.; Hillger, M.; Binder, U.; Reuter, S.; Herold, K.; Telagathoti, A.; Dahse, H.-M.; Wicht, S.; Trinks, N.; et al. The Impact of Episporic Modification of Lichtheimia Corymbifera on Virulence and Interaction with Phagocytes. Comput. Struct. Biotechnol. J. 2021, 19, 880–896. [Google Scholar] [CrossRef]
- Karimi, E.; Nikkhah, M.; Hosseinkhani, S. Label-Free and Bioluminescence-Based Nano-Biosensor for ATP Detection. Biosensors 2022, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved Patch-Clamp Techniques for High-Resolution Current Recording from Cells and Cell-Free Membrane Patches. Pflug. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Sattler, C.; Schmauder, R.; Schwabe, T.; Schweinitz, A.; Unzeitig, C.; Schwede, F.; Otte, M.; Benndorf, K. Relating Ligand Binding to Activation Gating in P2X2 Receptors Using a Novel Fluorescent ATP Derivative. J. Neurochem. 2020, 154, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.E.; Lü, W.; Oosterheert, W.; Shekhar, M.; Tajkhorshid, E.; Gouaux, E. X-Ray Structures Define Human P2X 3 Receptor Gating Cycle and Antagonist Action. Nature 2016, 538, 66–71. [Google Scholar] [CrossRef]
- King, M.; Housley, G.D.; Raybould, N.P.; Greenwood, D.; Salih, S.G. Expression of ATP-Gated Ion Channels by Reissner’s Membrane Epithelial Cells. Neuroreport 1998, 9, 2467–2474. [Google Scholar] [CrossRef]
- Li, Z.; Migita, K.; Samways, D.S.K.; Voigt, M.M.; Egan, T.M. Gain and Loss of Channel Function by Alanine Substitutions in the Transmembrane Segments of the Rat ATP-Gated P2X2 Receptor. J. Neurosci. 2004, 24, 7378–7386. [Google Scholar] [CrossRef]
- Jiang, L.H.; Rassendren, F.; Spelta, V.; Surprenant, A.; North, R.A. Amino Acid Residues Involved in Gating Identified in the First Membrane-Spanning Domain of the Rat P2X(2) Receptor. J. Biol. Chem. 2001, 276, 14902–14908. [Google Scholar] [CrossRef]
- Khakh, B.S.; Egan, T.M. Contribution of Transmembrane Regions to ATP-Gated P2X2 Channel Permeability Dynamics. J. Biol. Chem. 2005, 280, 6118–6129. [Google Scholar] [CrossRef]
- Chen, X.; Abad, C.; Chen, Z.-Y.; Young, J.I.; Gurumurthy, C.B.; Walz, K.; Liu, X.Z. Generation and Characterization of a P2rx2 V60L Mouse Model for DFNA41. Hum. Mol. Genet. 2021, 30, 985–995. [Google Scholar] [CrossRef]
- Lee, J.H.; Chiba, T.; Marcus, D.C. P2X2 Receptor Mediates Stimulation of Parasensory Cation Absorption by Cochlear Outer Sulcus Cells and Vestibular Transitional Cells. J. Neurosci. 2001, 21, 9168–9174. [Google Scholar] [CrossRef]
- Wangemann, P. K+ Cycling and the Endocochlear Potential. Hear. Res. 2002, 165, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.M.; Bobinski, F.; Parada, C.A.; Sluka, K.A.; Tambeli, C.H. P2X3 and P2X2/3 Receptors Play a Crucial Role in Articular Hyperalgesia Development Through Inflammatory Mechanisms in the Knee Joint Experimental Synovitis. Mol. Neurobiol. 2017, 54, 6174–6186. [Google Scholar] [CrossRef]
- Kushnir, R.; Cherkas, P.S.; Hanani, M. Peripheral Inflammation Upregulates P2X Receptor Expression in Satellite Glial Cells of Mouse Trigeminal Ganglia: A Calcium Imaging Study. Neuropharmacology 2011, 61, 739–746. [Google Scholar] [CrossRef]
- Zheng, X.-B.; Zhang, Y.-L.; Li, Q.; Liu, Y.-G.; Wang, X.-D.; Yang, B.-L.; Zhu, G.-C.; Zhou, C.-F.; Gao, Y.; Liu, Z.-X. Effects of 1,8-Cineole on Neuropathic Pain Mediated by P2X2 Receptor in the Spinal Cord Dorsal Horn. Sci. Rep. 2019, 9, 7909. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.-J.; Zhang, C.-Y.; Yan, B.-Y.; Cai, W.-Z.; Lu, C.-L.; Xie, L.-J.; Li, S.-J.; Kong, P.-L.; Fan, J.; Pan, S.-M.; et al. P2X2 Receptors in Pyramidal Neurons Are Critical for Regulating Vulnerability to Chronic Stress. Theranostics 2022, 12, 3703–3718. [Google Scholar] [CrossRef] [PubMed]
- Huerta, M.Á.; Marcos-Frutos, D.; de la Nava, J.; García-Ramos, A.; Tejada, M.Á.; Roza, C. P2X3 and P2X2/3 Receptors Inhibition Produces a Consistent Analgesic Efficacy: A Systematic Review and Meta-Analysis of Preclinical Studies. Eur. J. Pharmacol. 2024, 984, 177052. [Google Scholar] [CrossRef]
- Finger, T.E.; Danilova, V.; Barrows, J.; Bartel, D.L.; Vigers, A.J.; Stone, L.; Hellekant, G.; Kinnamon, S.C. ATP Signaling Is Crucial for Communication from Taste Buds to Gustatory Nerves. Science 2005, 310, 1495–1499. [Google Scholar] [CrossRef]
- Takimoto, Y.; Ishida, Y.; Kondo, M.; Imai, T.; Hanada, Y.; Ozono, Y.; Kamakura, T.; Inohara, H.; Shimada, S. P2X2 Receptor Deficiency in Mouse Vestibular End Organs Attenuates Vestibular Function. Neuroscience 2018, 386, 41–50. [Google Scholar] [CrossRef]
- Navarro, B.; Miki, K.; Clapham, D.E. ATP-Activated P2X2 Current in Mouse Spermatozoa. Proc. Natl. Acad. Sci. USA 2011, 108, 14342–14347. [Google Scholar] [CrossRef]
- Gusic, M.; Benndorf, K.; Sattler, C. Dissecting Activation Steps in P2X7 Receptors. Biochem. Biophys. Res. Commun. 2021, 569, 112–117. [Google Scholar] [CrossRef]
- Schirmeyer, J.; Hummert, S.; Eick, T.; Schulz, E.; Schwabe, T.; Ehrlich, G.; Kukaj, T.; Wiegand, M.; Sattler, C.; Schmauder, R.; et al. Thermodynamic Profile of Mutual Subunit Control in a Heteromeric Receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2100469118. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Sattler, C.; Benndorf, K. Functional Properties of a Disease Mutation for Migraine in Kv2.1/6.4 Channels. Biochem. Biophys. Res. Commun. 2024, 738, 150560. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Lindström, S.H.; Kaneko, M.; Wang, K.; Minguez-Viñas, T.; Angelini, M.; Steccanella, F.; Holder, D.; Ottolia, M.; Olcese, R.; et al. An Epilepsy-Associated KV1.2 Charge-Transfer-Center Mutation Impairs KV1.2 and KV1.4 Trafficking. Proc. Natl. Acad. Sci. USA 2022, 119, e2113675119. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wand, P.-L.; Brünings, X.; Tewari, D.; Reuter, S.; Mrowka, R.; Benndorf, K.; Zimmer, T.; Sattler, C. Differential Effects of Hearing Loss Mutations in Homomeric P2X2 and Heteromeric P2X2/3 Receptors. Cells 2025, 14, 510. https://doi.org/10.3390/cells14070510
Wand P-L, Brünings X, Tewari D, Reuter S, Mrowka R, Benndorf K, Zimmer T, Sattler C. Differential Effects of Hearing Loss Mutations in Homomeric P2X2 and Heteromeric P2X2/3 Receptors. Cells. 2025; 14(7):510. https://doi.org/10.3390/cells14070510
Chicago/Turabian StyleWand, Paula-Luise, Xenia Brünings, Debanjan Tewari, Stefanie Reuter, Ralf Mrowka, Klaus Benndorf, Thomas Zimmer, and Christian Sattler. 2025. "Differential Effects of Hearing Loss Mutations in Homomeric P2X2 and Heteromeric P2X2/3 Receptors" Cells 14, no. 7: 510. https://doi.org/10.3390/cells14070510
APA StyleWand, P.-L., Brünings, X., Tewari, D., Reuter, S., Mrowka, R., Benndorf, K., Zimmer, T., & Sattler, C. (2025). Differential Effects of Hearing Loss Mutations in Homomeric P2X2 and Heteromeric P2X2/3 Receptors. Cells, 14(7), 510. https://doi.org/10.3390/cells14070510





