Targeting of Extracellular Vesicle-Based Therapeutics to the Brain
Abstract
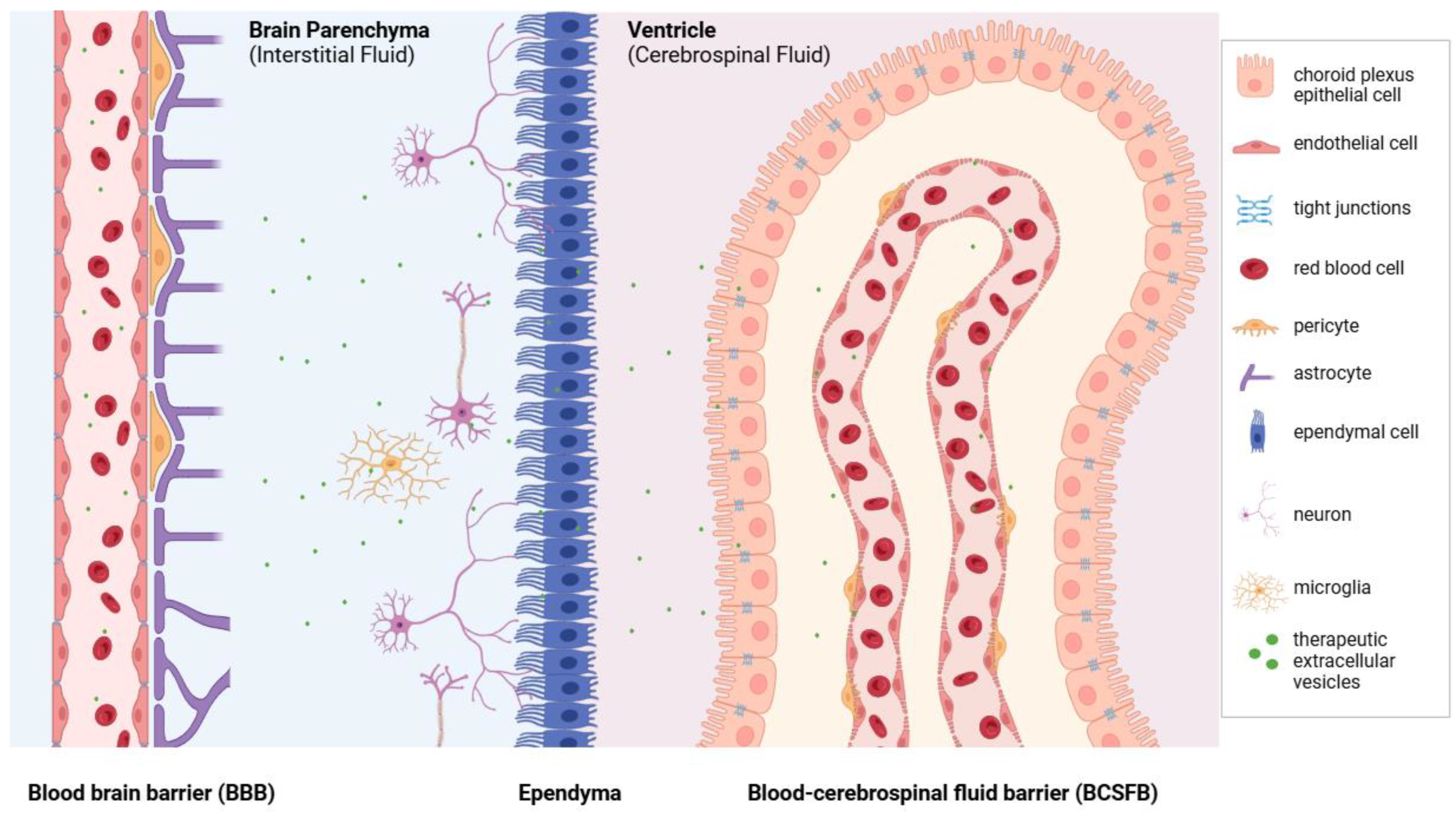
:1. Introduction
2. Importance of Administration Routes
2.1. Systemic Administration
2.2. Local Administration
2.3. Intranasal (IN) Administration
3. Capitalizing on the Biological Origin of EVs
3.1. Organotropism of EVs
3.2. Intrinsic Properties of Unmodified EVs
4. Targeting CNS with Brain-Specific Ligands
4.1. Conjugation of Vector Moieties on the Surface of EVs
4.2. Prolonged Circulation of EVs in the Blood Stream
5. Using External Stimuli for Brain Targeting
6. Challenges Surrounding EV-Based Therapeutics
6.1. Scalability and Standardization of EV Production
6.2. Cargo Loading Efficiency
7. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated Virus |
| AD | Alzheimer’s Disease |
| Ang-1 | Angiopoietin 1 |
| BBB | Blood–Brain Barrier |
| BCSFB | Blood–Cerebrospinal Fluid Barrier |
| BDNF | Brain-derived Neurotrophic Factor |
| BME | Brain Microvessel Endothelium |
| BMSCs | Bone Marrow Mesenchymal Stem Cells |
| cGMPs | current Good Manufacturing Practices |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| CuSNPs | Copper Sulfide Nanoparticles |
| DOX | Doxorubicin |
| FDA | Food and Drug Administration (FDA) |
| FGF2 | Fibroblast Growth Factor 2 |
| GBM | Glioblastoma Multiforme |
| GDNF | Glial-cell-derived Neurotrophic Factor |
| GI | Gastrointestinal Tract |
| HD | Huntington’s Disease |
| HGF | Hepatocyte Growth Factor |
| hNSCs | Human Neural Stem Cells |
| IC | Intracranial |
| IN | Intranasal |
| LINCL | Late-infantile Neuronal Ceroid Lipofuscinosis |
| LPS | Lipopolysaccharide |
| MCAO | Middle-Cerebral-Artery-Occlusion |
| MPS | Mononuclear Phagocyte System |
| MSCs | Mesenchymal Stromal Cells |
| NHP | Non-human Primates |
| NT3 | Neurotrophin-3 |
| OPC | Oligodendrocyte Precursor Cell |
| PBS | Phosphate-buffered Saline |
| PD | Parkinson’s Disease |
| PDGF | Platelet-derived Growth Factor |
| PEG | Polyethylene Glycol |
| qRT-PCR | Real-time Polymerase Chain Reaction |
| RES | Reticuloendothelial system |
| RIF | Rifampin |
| RVG | Rabies Viral Glycoprotein |
| SAH | Subarachnoid Hemorrhage |
| SC | Subcutaneous |
| SCI | Spinal Cord Injury |
| SPION | Superparamagnetic Iron Oxide Nanoparticle |
| TBI | Traumatic Brain Injury |
| TFF | Tangential Flow Filtration |
| TfR | Transferrin Receptor |
| TGF | Transforming Growth Factor |
| TME | Tumor-microenvironment |
| TMZ | Temozolomide |
| TPP1 | Tripeptidyl Peptidase-1 |
| Tregs | Regulatory T cells |
| VEGF | Vascular Endothelial Growth Factor |
References
- The Lancet Neurology: Neurological Conditions Now Leading Cause of Ill Health and Disability Globally, Affecting 3.4 Billion People Worldwide Institute for Health Metrics and Evaluation. Available online: https://www.healthdata.org/news-events/newsroom/news-releases/lancet-neurology-neurological-conditions-now-leading-cause-ill (accessed on 7 January 2025).
- Global, Regional, and National Burden of Disorders Affecting the Nervous System, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood–Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Dziegielewska, K.M.; Ek, J.; Habgood, M.D.; Saunders, N.R. Development of the Choroid Plexus. Microsc. Res. Tech. 2001, 52, 5–20. [Google Scholar] [CrossRef]
- Johansson, P.A.; Dziegielewska, K.M.; Liddelow, S.A.; Saunders, N.R. The Blood-CSF Barrier Explained: When Development Is Not Immaturity. BioEssays News Rev. Mol. Cell Dev. Biol. 2008, 30, 237–248. [Google Scholar] [CrossRef]
- Johanson, C.; Stopa, E.; McMillan, P.; Roth, D.; Funk, J.; Krinke, G. The Distributional Nexus of Choroid Plexus to Cerebrospinal Fluid, Ependyma and Brain: Toxicologic/Pathologic Phenomena, Periventricular Destabilization, and Lesion Spread. Toxicol. Pathol. 2011, 39, 186–212. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Cote, D. Epidemiology of Brain Tumors. Neurol. Clin. 2018, 36, 395–419. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Feigin, V.L.; A Stark, B.; Johnson, C.O.; A Roth, G.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Mol. Basel Switz. 2020, 25, 5789. [Google Scholar] [CrossRef]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. North Am. 2019, 103, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Putz, K.; Hayani, K.; Zar, F.A. Meningitis. Prim. Care 2013, 40, 707–726. [Google Scholar] [CrossRef]
- Mammoser, A.G.; Groves, M.D. Biology and Therapy of Neoplastic Meningitis. Curr. Oncol. Rep. 2010, 12, 41–49. [Google Scholar] [CrossRef]
- Abboud, H.; Probasco, J.C.; Irani, S.; Ances, B.; Benavides, D.R.; Bradshaw, M.; Christo, P.P.; Dale, R.C.; Fernandez-Fournier, M.; Flanagan, E.P.; et al. Autoimmune Encephalitis: Proposed Best Practice Recommendations for Diagnosis and Acute Management. J. Neurol. Neurosurg. Psychiatry 2021, 92, 757–768. [Google Scholar] [CrossRef]
- Uy, C.E.; Binks, S.; Irani, S.R. Autoimmune Encephalitis: Clinical Spectrum and Management. Pract. Neurol. 2021, 21, 412–423. [Google Scholar] [CrossRef]
- Piñar-Morales, R.; Barrero-Hernández, F.; Aliaga-Martínez, L. Human Prion Diseases: An Overview. Med. Clin. 2023, 160, 554–560. [Google Scholar] [CrossRef]
- Rackstraw, S. HIV-Related Neurocognitive Impairment—A Review. Psychol. Health Med. 2011, 16, 548–563. [Google Scholar] [CrossRef]
- Rayi, A.; Alnahhas, I.; Ong, S.; Giglio, P.; Puduvalli, V.K. Targeted Therapy for BRAF Mutant Brain Tumors. Curr. Treat. Options Oncol. 2021, 22, 105. [Google Scholar] [CrossRef]
- Hasan, I.; Roy, S.; Guo, B.; Du, S.; Tao, W.; Chang, C. Recent Progress in Nanomedicines for Imaging and Therapy of Brain Tumors. Biomater. Sci. 2023, 11, 1270–1310. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.M.; Harkness, K.L. Major Depression and Its Recurrences: Life Course Matters. Annu. Rev. Clin. Psychol. 2022, 18, 329–357. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.-W.; Yang, C.-C.; Lai, T.-H.; Wu, Y.-H.; Yang, C.-P. Light Therapy in Chronic Migraine. Curr. Pain Headache Rep. 2024, 28, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Góralska, K.; Blaszkowska, J.; Dzikowiec, M. Neuroinfections Caused by Fungi. Infection 2018, 46, 443–459. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary Medical, Device, and Surgical Therapies for Obesity in Adults. Lancet Lond. Engl. 2023, 401, 1116–1130. [Google Scholar] [CrossRef]
- Platt, F.M.; d’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal Storage Diseases. Nat. Rev. Dis. Primer 2018, 4, 27. [Google Scholar] [CrossRef]
- Naseer, S.; Khalid, S.; Parveen, S.; Abbass, K.; Song, H.; Achim, M.V. COVID-19 Outbreak: Impact on Global Economy. Front. Public Health 2023, 10, 1009393. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Z.; Wang, L.; Xing, D.; Lin, J. Global Research Trends in Extracellular Vesicles Based on Stem Cells from 1991 to 2021: A Bibliometric and Visualized Study. Front. Bioeng. Biotechnol. 2022, 10, 956058. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Wood, M.J.A.; O’Loughlin, A.J.; Samira, L. Exosomes and the Blood-Brain Barrier: Implications for Neurological Diseases. Ther. Deliv. 2011, 2, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.-E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of α-Synuclein-Containing Erythrocyte-Derived Extracellular Vesicles across the Blood-Brain Barrier via Adsorptive Mediated Transcytosis: Another Mechanism for Initiation and Progression of Parkinson’s Disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef]
- Vandendriessche, C.; Balusu, S.; Van Cauwenberghe, C.; Brkic, M.; Pauwels, M.; Plehiers, N.; Bruggeman, A.; Dujardin, P.; Van Imschoot, G.; Van Wonterghem, E.; et al. Importance of Extracellular Vesicle Secretion at the Blood-Cerebrospinal Fluid Interface in the Pathogenesis of Alzheimer’s Disease. Acta Neuropathol. Commun. 2021, 9, 143. [Google Scholar] [CrossRef]
- Erb, U.; Hikel, J.; Meyer, S.; Ishikawa, H.; Worst, T.S.; Nitschke, K.; Nuhn, P.; Porubsky, S.; Weiss, C.; Schroten, H.; et al. The Impact of Small Extracellular Vesicles on Lymphoblast Trafficking across the Blood-Cerebrospinal Fluid Barrier In Vitro. Int. J. Mol. Sci. 2020, 21, 5491. [Google Scholar] [CrossRef]
- Ortenlöf, N.; Vallius, S.; Karlsson, H.; Ekström, C.; Kristiansson, A.; Holmqvist, B.; Pankratova, S.; Barton, N.; Ley, D.; Gram, M. Choroid Plexus Extracellular Vesicle Transport of Blood-Borne Insulin-like Growth Factor 1 to the Hippocampus of the Immature Brain. PNAS Nexus 2024, 3, 496. [Google Scholar] [CrossRef]
- Grapp, M.; Wrede, A.; Schweizer, M.; Hüwel, S.; Galla, H.-J.; Snaidero, N.; Simons, M.; Bückers, J.; Low, P.S.; Urlaub, H.; et al. Choroid Plexus Transcytosis and Exosome Shuttling Deliver Folate into Brain Parenchyma. Nat. Commun. 2013, 4, 2123. [Google Scholar] [CrossRef]
- Balusu, S.; Van Wonterghem, E.; De Rycke, R.; Raemdonck, K.; Stremersch, S.; Gevaert, K.; Brkic, M.; Demeestere, D.; Vanhooren, V.; Hendrix, A.; et al. Identification of a Novel Mechanism of Blood-Brain Communication during Peripheral Inflammation via Choroid Plexus-Derived Extracellular Vesicles. EMBO Mol. Med. 2016, 8, 1162–1183. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular Vesicles for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.Y.; Kang, M.; Hisey, C.L.; Chamley, L.W. Studying Exogenous Extracellular Vesicle Biodistribution by in Vivo Fluorescence Microscopy. Dis. Model. Mech. 2023, 16, dmm050074. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Yuan, H.; Shipley, S.T.; Wu, Z.; Zhao, Y.; Pate, K.; Frank, J.E.; Massoud, N.; Stewart, P.W.; Perlmutter, J.S.; et al. Biodistribution of Biomimetic Drug Carriers, Mononuclear Cells and Extracellular Vesicles, in Nonhuman Primates. Adv. Biol. 2022, 6, e2101293. [Google Scholar] [CrossRef]
- Haney, M.J.; Zhao, Y.; Jin, Y.S.; Batrakova, E.V. Extracellular Vesicles as Drug Carriers for Enzyme Replacement Therapy to Treat CLN2 Batten Disease: Optimization of Drug Administration Routes. Cells 2020, 9, 1273. [Google Scholar] [CrossRef]
- Lakhal, S.; Wood, M.J.A. Exosome Nanotechnology: An Emerging Paradigm Shift in Drug Delivery: Exploitation of Exosome Nanovesicles for Systemic in Vivo Delivery of RNAi Heralds New Horizons for Drug Delivery across Biological Barriers. BioEssays News Rev. Mol. Cell. Dev. Biol. 2011, 33, 737–741. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage Exosomes as Natural Nanocarriers for Protein Delivery to Inflamed Brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Harrison, E.B.; Zhao, Y.; Kabanov, A.V.; Batrakova, E.V. TPP1 Delivery to Lysosomes with Extracellular Vesicles and Their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv. Healthc. Mater. 2019, 8, e1801271. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of Exosomes Derived from Multipluripotent Mesenchymal Stromal Cells on Functional Recovery and Neurovascular Plasticity in Rats after Traumatic Brain Injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yin, X.-M.; Xu, Y.; Xu, C.-C.; Lin, X.; Ye, F.-B.; Cao, Y.; Lin, F.-Y. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma 2017, 34, 3388–3396. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b Promotes Neural Plasticity and Functional Recovery after Treatment of Stroke with Multipotent Mesenchymal Stromal Cells in Rats via Transfer of Exosome-Enriched Extracellular Particles. Stem Cells Dayt. Ohio 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.M.; Hossain, A.; Gumin, J.; Momin, E.N.; Shimizu, Y.; Ledbetter, D.; Shahar, T.; Yamashita, S.; Parker Kerrigan, B.; Fueyo, J.; et al. Mesenchymal Stem Cells as Natural Biofactories for Exosomes Carrying miR-124a in the Treatment of Gliomas. Neuro-Oncol. 2018, 20, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Zaazaa, A.M.; Abd El-Motelp, B.A.; Ali, N.A.; Youssef, A.M.; Sayed, M.A.; Mohamed, S.H. Stem Cell-Derived Exosomes and Copper Sulfide Nanoparticles Attenuate the Progression of Neurodegenerative Disorders Induced by Cadmium in Rats. Heliyon 2022, 8, e08622. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity after Stroke in Rats. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef]
- Chang, C.-L.; Chen, H.-H.; Chen, K.-H.; Chiang, J.Y.; Li, Y.-C.; Lin, H.-S.; Sung, P.-H.; Yip, H.-K. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomes Markedly Protected the Brain against Sepsis Syndrome Induced Injury in Rat. Am. J. Transl. Res. 2019, 11, 3955–3971. [Google Scholar]
- Qi, Y.; Guo, L.; Jiang, Y.; Shi, Y.; Sui, H.; Zhao, L. Brain Delivery of Quercetin-Loaded Exosomes Improved Cognitive Function in AD Mice by Inhibiting Phosphorylated Tau-Mediated Neurofibrillary Tangles. Drug Deliv. 2020, 27, 745–755. [Google Scholar] [CrossRef]
- Kang, S.; Min, H. Ginseng, the “Immunity Boost”: The Effects of Panax Ginseng on Immune System. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef]
- Kim, J.; Zhu, Y.; Chen, S.; Wang, D.; Zhang, S.; Xia, J.; Li, S.; Qiu, Q.; Lee, H.; Wang, J. Anti-Glioma Effect of Ginseng-Derived Exosomes-like Nanoparticles by Active Blood-Brain-Barrier Penetration and Tumor Microenvironment Modulation. J. Nanobiotechnol. 2023, 21, 253. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Pharmacokinetics of Exosomes-An Important Factor for Elucidating the Biological Roles of Exosomes and for the Development of Exosome-Based Therapeutics. J. Pharm. Sci. 2017, 106, 2265–2269. [Google Scholar] [CrossRef]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-Dependent Clearance of Systemically Administered B16BL6-Derived Exosomes from the Blood Circulation in Mice. J. Extracell. Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and Delivery Efficiency of Unmodified Tumor-Derived Exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Zhao, Y.; Fallon, J.K.; Yue, W.; Li, S.M.; Lentz, E.E.; Erie, D.; Smith, P.C.; Batrakova, E.V. Extracellular Vesicles as Drug Delivery System for Treatment of Neurodegenerative Disorders: Optimization of the Cell Source. Adv. Nanobiomed Res. 2021, 1, 2100064. [Google Scholar] [CrossRef]
- El-Hage, N.; Haney, M.J.; Zhao, Y.; Rodriguez, M.; Wu, Z.; Liu, M.; Swain, C.J.; Yuan, H.; Batrakova, E.V. Extracellular Vesicles Released by Genetically Modified Macrophages Activate Autophagy and Produce Potent Neuroprotection in Mouse Model of Lysosomal Storage Disorder, Batten Disease. Cells 2023, 12, 1497. [Google Scholar] [CrossRef]
- Lee, S.-T.; Im, W.; Ban, J.-J.; Lee, M.; Jung, K.-H.; Lee, S.K.; Chu, K.; Kim, M. Exosome-Based Delivery of miR-124 in a Huntington’s Disease Model. J. Mov. Disord. 2017, 10, 45–52. [Google Scholar] [CrossRef]
- Didiot, M.-C.; Hall, L.M.; Coles, A.H.; Haraszti, R.A.; Godinho, B.M.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.F.; Hassler, M.R.; et al. Exosome-Mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1836–1847. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Hernández-Sapiéns, M.A.; Gutiérrez-Mercado, Y.K.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.L.; Vázquez-Méndez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal Stem Cell-Derived Exosomes Promote Neurogenesis and Cognitive Function Recovery in a Mouse Model of Alzheimer’s Disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.-I.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. Decreased Amyloid-β Pathologies by Intracerebral Loading of Glycosphingolipid-Enriched Exosomes in Alzheimer Model Mice. J. Biol. Chem. 2014, 289, 24488–24498. [Google Scholar] [CrossRef]
- Orefice, N.S.; Souchet, B.; Braudeau, J.; Alves, S.; Piguet, F.; Collaud, F.; Ronzitti, G.; Tada, S.; Hantraye, P.; Mingozzi, F.; et al. Real-Time Monitoring of Exosome Enveloped-AAV Spreading by Endomicroscopy Approach: A New Tool for Gene Delivery in the Brain. Mol. Ther. Methods Clin. Dev. 2019, 14, 237–251. [Google Scholar] [CrossRef]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal Stem Cell-Derived Exosomes as a Nanotherapeutic Agent for Amelioration of Inflammation-Induced Astrocyte Alterations in Mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.-Y.; Ren, J.-L.; Xu, F.; Chen, F.-M.; Li, A. Exosomes Secreted by Stem Cells from Human Exfoliated Deciduous Teeth Contribute to Functional Recovery after Traumatic Brain Injury by Shifting Microglia M1/M2 Polarization in Rats. Stem Cell Res. Ther. 2017, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from Marrow Stromal Cells Expressing miR-146b Inhibit Glioma Growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Hayes, S.H.; Liu, Q.; Selvakumaran, S.; Haney, M.J.; Batrakova, E.V.; Allman, B.L.; Walton, P.A.; Kiser, P.; Whitehead, S.N. Brain Targeting and Toxicological Assessment of the Extracellular Vesicle-Packaged Antioxidant Catalase-SKL Following Intranasal Administration in Mice. Neurotox. Res. 2021, 39, 1418–1429. [Google Scholar] [CrossRef]
- Pusic, A.D.; Kraig, R.P. Youth and Environmental Enrichment Generate Serum Exosomes Containing miR-219 That Promote CNS Myelination. Glia 2014, 62, 284–299. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-Inflammatory Drugs from the Nasal Region to the Brain. Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Nie, H.; Xie, X.; Zhang, D.; Zhou, Y.; Li, B.; Li, F.; Li, F.; Cheng, Y.; Mei, H.; Meng, H.; et al. Use of Lung-Specific Exosomes for miRNA-126 Delivery in Non-Small Cell Lung Cancer. Nanoscale 2020, 12, 877–887. [Google Scholar] [CrossRef]
- Emam, S.E.; Abu Lila, A.S.; Elsadek, N.E.; Ando, H.; Shimizu, T.; Okuhira, K.; Ishima, Y.; Mahdy, M.A.; Ghazy, F.-E.S.; Ishida, T. Cancer Cell-Type Tropism Is One of Crucial Determinants for the Efficient Systemic Delivery of Cancer Cell-Derived Exosomes to Tumor Tissues. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2019, 145, 27–34. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.L.; Kaiser, E.E.; Jurgielewicz, B.J.; Spellicy, S.; Scoville, S.L.; Thompson, T.A.; Swetenburg, R.L.; Hess, D.C.; West, F.D.; Stice, S.L. Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke. Stroke 2018, 49, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S.; et al. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl. Stroke Res. 2018, 9, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhu, Z.; Wang, H.; Yu, Y.; Chen, W.; Waqas, A.; Wang, Y.; Chen, L. Exosomes Derived from Human Neural Stem Cells Stimulated by Interferon Gamma Improve Therapeutic Ability in Ischemic Stroke Model. J. Adv. Res. 2020, 24, 435–445. [Google Scholar] [CrossRef]
- Ayyubova, G.; Kodali, M.; Upadhya, R.; Madhu, L.N.; Attaluri, S.; Somayaji, Y.; Shuai, B.; Rao, S.; Shankar, G.; Shetty, A.K. Extracellular Vesicles from hiPSC-NSCs Can Prevent Peripheral Inflammation-Induced Cognitive Dysfunction with Inflammasome Inhibition and Improved Neurogenesis in the Hippocampus. J. Neuroinflamm. 2023, 20, 297. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Usuki, S.; Sakai, S.; Hanamatsu, H.; Mioka, T.; Kimura, N.; Okada, M.; Tahara, H.; Furukawa, J.; et al. A Potential Function for Neuronal Exosomes: Sequestering Intracerebral Amyloid-β Peptide. FEBS Lett. 2015, 589, 84–88. [Google Scholar] [CrossRef]
- Millimaggi, D.; Mari, M.; D’Ascenzo, S.; Carosa, E.; Jannini, E.A.; Zucker, S.; Carta, G.; Pavan, A.; Dolo, V. Tumor Vesicle-Associated CD147 Modulates the Angiogenic Capability of Endothelial Cells. Neoplasia 2007, 9, 349–357. [Google Scholar] [CrossRef]
- Iero, M.; Valenti, R.; Huber, V.; Filipazzi, P.; Parmiani, G.; Fais, S.; Rivoltini, L. Tumour-Released Exosomes and Their Implications in Cancer Immunity. Cell Death Differ. 2008, 15, 80–88. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I.-I. The Role of Exosomes in Cancer Metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Youn, Y.S.; Oh, K.T.; Lee, E.S. Tumor-Homing pH-Sensitive Extracellular Vesicles for Targeting Heterogeneous Tumors. Pharmaceutics 2020, 12, 372. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, N.L.; Haney, M.J.; Zhao, Y.; Manickam, D.S.; Mahajan, V.; Suresh, P.; Hingtgen, S.D.; Mosley, R.L.; Gendelman, H.E.; Kabanov, A.V.; et al. Macrophages Offer a Paradigm Switch for CNS Delivery of Therapeutic Proteins. Nanomedicine 2014, 9, 1403–1422. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Q.; Huyan, T.; Wang, Y.; Huang, Q.; Shi, J. Bifacial Effects of Engineering Tumour Cell-Derived Exosomes on Human Natural Killer Cells. Exp. Cell Res. 2018, 363, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Stevanato, L.; Thanabalasundaram, L.; Vysokov, N.; Sinden, J.D. Investigation of Content, Stoichiometry and Transfer of miRNA from Human Neural Stem Cell Line Derived Exosomes. PLoS ONE 2016, 11, e0146353. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, A.I.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Jin, R.; Yang, G.; Li, G. Inflammatory Mechanisms in Ischemic Stroke: Role of Inflammatory Cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef]
- Galvão, R.P.; Zong, H. Inflammation and Gliomagenesis: Bi-Directional Communication at Early and Late Stages of Tumor Progression. Curr. Pathobiol. Rep. 2013, 1, 19–28. [Google Scholar] [CrossRef]
- Chan, P.; Spudich, S. HIV Compartmentalization in the CNS and Its Impact in Treatment Outcomes and Cure Strategies. Curr. HIV/AIDS Rep. 2022, 19, 207–216. [Google Scholar] [CrossRef]
- Postolache, T.T.; Wadhawan, A.; Can, A.; Lowry, C.A.; Woodbury, M.; Makkar, H.; Hoisington, A.J.; Scott, A.J.; Potocki, E.; Benros, M.E.; et al. Inflammation in Traumatic Brain Injury. J. Alzheimers Dis. JAD 2020, 74, 1–28. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The Role of Macrophages in the Resolution of Inflammation. J. Clin. Invest. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A Novel Nanoparticle Drug Delivery System: The Anti-Inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering Macrophage-Derived Exosomes for Targeted Paclitaxel Delivery to Pulmonary Metastases: In Vitro and in Vivo Evaluations. Nanomedicine Nanotechnol. Biol. Med. 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Carman, C.V. Mechanisms for Transcellular Diapedesis: Probing and Pathfinding by “Invadosome-like Protrusions”. J. Cell Sci. 2009, 122, 3025–3035. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Wang, J.; Yang, B.; Weng, Q.; He, Q. Targeting Microglia and Macrophages: A Potential Treatment Strategy for Multiple Sclerosis. Front. Pharmacol. 2019, 10, 286. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-Derived Extracellular Vesicles: Diverse Mediators of Pathology and Therapeutics in Multiple Diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S.P.; Elkahloun, A.G.; Wu, W.; Abu-Asab, M.S.; Roberts, D.D. CD47-Dependent Immunomodulatory and Angiogenic Activities of Extracellular Vesicles Produced by T Cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 37, 49–59. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-Loaded Blood Exosomes Targeted to Brain for Better Treatment of Parkinson’s Disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef]
- Nicholls, C.; Mulley, J.C. Distribution of Six TF C (Transferrin) Subtypes in Cord Bloods and Blood Donors. Aust. J. Exp. Biol. Med. Sci. 1982, 60, 433–436. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Zhang, S.; Deng, Z.-B.; Grizzle, W.; Miller, D.; Zhang, H.-G. Exosomes Are Endogenous Nanoparticles That Can Deliver Biological Information between Cells. Adv. Drug Deliv. Rev. 2013, 65, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Dong, T.; Zhou, C.; Deng, L.; Liu, H.B.; Wang, W.; Liu, G.; Ying, M.; Li, P.P. Genetically Engineered Human Induced Pluripotent Stem Cells for the Production of Brain-Targeting Extracellular Vesicles. Stem Cell Res. Ther. 2024, 15, 345. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic Exosomal siRNA Delivery Reduced Alpha-Synuclein Aggregates in Brains of Transgenic Mice. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Liu, Z.; Zhou, Y.; Chu, D.; Li, X.; Jiang, X.; Hou, D.; Chen, X.; Chen, Y.; et al. Targeted Exosome-Mediated Delivery of Opioid Receptor Mu siRNA for the Treatment of Morphine Relapse. Sci. Rep. 2015, 5, 17543. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol. Ther. Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef]
- Cui, G.-H.; Guo, H.-D.; Li, H.; Zhai, Y.; Gong, Z.-B.; Wu, J.; Liu, J.-S.; Dong, Y.-R.; Hou, S.-X.; Liu, J.-R. RVG-Modified Exosomes Derived from Mesenchymal Stem Cells Rescue Memory Deficits by Regulating Inflammatory Responses in a Mouse Model of Alzheimer’s Disease. Immun. Ageing A 2019, 16, 10. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, Y.; Xue, F.; Zheng, Y.; Huang, H.; Wang, W.; Chang, Y.; Yang, H.; Zhang, J. Exosomal DNA Aptamer Targeting α-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Mol. Ther. Nucleic Acids 2019, 17, 726–740. [Google Scholar] [CrossRef]
- Izco, M.; Blesa, J.; Schleef, M.; Schmeer, M.; Porcari, R.; Al-Shawi, R.; Ellmerich, S.; de Toro, M.; Gardiner, C.; Seow, Y.; et al. Systemic Exosomal Delivery of shRNA Minicircles Prevents Parkinsonian Pathology. Mol. Ther. 2019, 27, 2111–2122. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Peng, H.; Liu, R.; Ji, W.; Shi, Z.; Shen, J.; Ma, G.; Zhang, X. Targeted Exosome Coating Gene-Chem Nanocomplex as “Nanoscavenger” for Clearing α-Synuclein and Immune Activation of Parkinson’s Disease. Sci. Adv. 2020, 6, eaba3967. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.; Wu, D.; Liang, T.; Pan, P.; Yuan, G.; Li, X.; Li, H.; Shen, H.; Wang, Z.; Chen, G. Systemic Exosomal miR-193b-3p Delivery Attenuates Neuroinflammation in Early Brain Injury after Subarachnoid Hemorrhage in Mice. J. Neuroinflamm. 2020, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface Modification of Gold Nanoparticles with Neuron-Targeted Exosome for Enhanced Blood–Brain Barrier Penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface Functionalized Exosomes as Targeted Drug Delivery Vehicles for Cerebral Ischemia Therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, J.; Wu, W.-C.; Gao, Y.-H.; Guo, Y.-X.; Song, S.-J.; Gao, R.; Wang, L.-B.; Wu, X.-Y.; Zhang, Y.; et al. Targeting Central Nervous System Extracellular Vesicles Enhanced Triiodothyronine Remyelination Effect on Experimental Autoimmune Encephalomyelitis. Bioact. Mater. 2022, 9, 373–384. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Li, Y.-J.; Wang, J.; Hu, X.-B.; Huang, S.; Luo, S.; Xiang, D.-X. Multifunctional Exosome-Mimetics for Targeted Anti-Glioblastoma Therapy by Manipulating Protein Corona. J. Nanobiotechnol. 2021, 19, 405. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 Targeted and Cargo-Loaded Exosomes Facilitate Simultaneous Imaging and Therapy of Glioma in Vitro and in Vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Liang, S.; Xu, H.; Ye, B.-C. Membrane-Decorated Exosomes for Combination Drug Delivery and Improved Glioma Therapy. Langmuir ACS J. Surf. Colloids 2022, 38, 299–308. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Chen, L.; Wu, W.; Yang, Z.; Wang, Y.; Wang, A.; Jiang, S.; Qin, X.; Ye, Z.; et al. Glioblastoma Cell-Derived Exosomes Functionalized with Peptides as Efficient Nanocarriers for Synergistic Chemotherapy of Glioblastoma with Improved Biosafety. Nano Res. 2023, 16, 13283–13293. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Huang, J.; Zhao, Y.; Chen, W.; Tang, Q.; An, Y.; Chen, R.; Hu, C. Angiopep-2 Modified Exosomes Load Rifampicin with Potential for Treating Central Nervous System Tuberculosis. Int. J. Nanomed. 2023, 18, 489–503. [Google Scholar] [CrossRef]
- Shi, S.; Li, T.; Wen, X.; Wu, S.Y.; Xiong, C.; Zhao, J.; Lincha, V.R.; Chow, D.S.; Liu, Y.; Sood, A.K.; et al. Copper-64 Labeled PEGylated Exosomes for In Vivo Positron Emission Tomography and Enhanced Tumor Retention. Bioconjug. Chem. 2019, 30, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, T.J.; Kang, L.; Kim, Y.-J.; Kang, M.K.; Kim, J.; Ryu, J.H.; Hyeon, T.; Yoon, B.-W.; Ko, S.-B.; et al. Mesenchymal Stem Cell-Derived Magnetic Extracellular Nanovesicles for Targeting and Treatment of Ischemic Stroke. Biomaterials 2020, 243, 119942. [Google Scholar] [CrossRef]
- Zhuang, M.; Chen, X.; Du, D.; Shi, J.; Deng, M.; Long, Q.; Yin, X.; Wang, Y.; Rao, L. SPION Decorated Exosome Delivery of TNF-α to Cancer Cell Membranes through Magnetism. Nanoscale 2020, 12, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Secchi, V.; Macchi, M.; Tripodi, L.; Trombetta, E.; Zambroni, D.; Padelli, F.; Mauri, M.; Molinaro, M.; Oddone, R.; et al. Magnetic-Field-Driven Targeting of Exosomes Modulates Immune and Metabolic Changes in Dystrophic Muscle. Nat. Nanotechnol. 2024, 19, 1532–1543. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Bao, L.; Liu, T.; Yuan, P.; Yang, X.; Qiu, X.; Gooding, J.J.; Bai, Y.; Xiao, J.; et al. Treatment of Infarcted Heart Tissue via the Capture and Local Delivery of Circulating Exosomes through Antibody-Conjugated Magnetic Nanoparticles. Nat. Biomed. Eng. 2020, 4, 1063–1075. [Google Scholar] [CrossRef]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef]
- Srivatsa Palakurthi, S.; Shah, B.; Kapre, S.; Charbe, N.; Immanuel, S.; Pasham, S.; Thalla, M.; Jain, A.; Palakurthi, S. A Comprehensive Review of Challenges and Advances in Exosome-Based Drug Delivery Systems. Nanoscale Adv. 2024, 6, 5803–5826. [Google Scholar] [CrossRef]
- Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. [Google Scholar] [CrossRef]
- Casajuana Ester, M.; Day, R.M. Production and Utility of Extracellular Vesicles with 3D Culture Methods. Pharmaceutics 2023, 15, 663. [Google Scholar] [CrossRef]
- Sun, L.; Ji, Y.; Chi, B.; Xiao, T.; Li, C.; Yan, X.; Xiong, X.; Mao, L.; Cai, D.; Zou, A.; et al. A 3D Culture System Improves the Yield of MSCs-Derived Extracellular Vesicles and Enhances Their Therapeutic Efficacy for Heart Repair. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 161, 114557. [Google Scholar] [CrossRef]
- Zhou, Y.; Huo, C. Three-Dimensional and Serum-Free Culture in Fixed-Bed Bioreactor Enhance Exosome Production by Affecting the Cytoskeleton. 2024. Available online: https://ssrn.com/abstract=4914000 (accessed on 1 March 2025).
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-Dimensional Culture of MSCs Produces Exosomes with Improved Yield and Enhanced Therapeutic Efficacy for Cisplatin-Induced Acute Kidney Injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wu, X. Exosomes Produced from 3D Cultures of Umbilical Cord Mesenchymal Stem Cells in a Hollow-Fiber Bioreactor Show Improved Osteochondral Regeneration Activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Almeria, C.; Weiss, R.; Keck, M.; Weber, V.; Kasper, C.; Egger, D. Dynamic Cultivation of Human Mesenchymal Stem/Stromal Cells for the Production of Extracellular Vesicles in a 3D Bioreactor System. Biotechnol. Lett. 2024, 46, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.C.; Bayik, D.; Srivatsan, A.; Bergamaschi, C.; Valentin, A.; Niu, G.; Bear, J.; Monninger, M.; Sun, M.; Morales-Kastresana, A.; et al. Efficient Production and Enhanced Tumor Delivery of Engineered Extracellular Vesicles. Biomaterials 2016, 105, 195–205. [Google Scholar] [CrossRef]
- Guo, S.; Debbi, L.; Zohar, B.; Samuel, R.; Arzi, R.S.; Fried, A.I.; Carmon, T.; Shevach, D.; Redenski, I.; Schlachet, I.; et al. Stimulating Extracellular Vesicles Production from Engineered Tissues by Mechanical Forces. Nano Lett. 2021, 21, 2497–2504. [Google Scholar] [CrossRef]
- Kink, J.A.; Bellio, M.A.; Forsberg, M.H.; Lobo, A.; Thickens, A.S.; Lewis, B.M.; Ong, I.M.; Khan, A.; Capitini, C.M.; Hematti, P. Large-Scale Bioreactor Production of Extracellular Vesicles from Mesenchymal Stromal Cells for Treatment of Acute Radiation Syndrome. Stem Cell Res. Ther. 2024, 15, 72. [Google Scholar] [CrossRef]
- Hindle, J.; Williams, A.; Kim, Y.; Kim, D.; Patil, K.; Khatkar, P.; Osgood, Q.; Nelson, C.; Routenberg, D.A.; Howard, M.; et al. hTERT-Immortalized Mesenchymal Stem Cell-Derived Extracellular Vesicles: Large-Scale Manufacturing, Cargo Profiling, and Functional Effects in Retinal Epithelial Cells. Cells 2024, 13, 861. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.-C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- You, D.G.; Lim, G.T.; Kwon, S.; Um, W.; Oh, B.H.; Song, S.H.; Lee, J.; Jo, D.-G.; Cho, Y.W.; Park, J.H. Metabolically Engineered Stem Cell–Derived Exosomes to Regulate Macrophage Heterogeneity in Rheumatoid Arthritis. Sci. Adv. 2021, 7, eabe0083. [Google Scholar] [CrossRef]
- Son, J.P.; Kim, E.H.; Shin, E.K.; Kim, D.H.; Sung, J.H.; Oh, M.J.; Cha, J.M.; Chopp, M.; Bang, O.Y. Mesenchymal Stem Cell-Extracellular Vesicle Therapy for Stroke: Scalable Production and Imaging Biomarker Studies. Stem Cells Transl. Med. 2023, 12, 459–473. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, D.-S.; Kim, H.-M.; Lee, J.-K.; Hwang, D.-Y.; Kim, T.-H.; You, S.; Han, D.K. Human Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Neural Differentiation of Neural Progenitor Cells. Int. J. Mol. Sci. 2022, 23, 7047. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, N.L.; Arzt, C.J.; Li, S.M.; Gololobova, O.A.; Batrakova, E.V. Extracellular Vesicle-Based Therapeutics: Preclinical and Clinical Investigations. Pharmaceutics 2020, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular Vesicles: A Rising Star for Therapeutics and Drug Delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, B.; Lian, J.; Wang, X. Extracellular Vesicles as Drug Delivery System for Cancer Therapy. Pharmaceutics 2024, 16, 1029. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, H.; Liu, J.; Chen, J.; Cui, Y.; Wang, S.; Zhang, X.; Yang, Z. Extracellular Vesicles: A New Star for Gene Drug Delivery. Int. J. Nanomed. 2024, 19, 2241–2264. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, Y.; Jiang, X.; Wang, Y.; Chen, Z.; Wu, D. Nature’s Carriers: Leveraging Extracellular Vesicles for Targeted Drug Delivery. Drug Deliv. 2024, 31, 2361165. [Google Scholar] [CrossRef]

| Donor Cell | Disease Model | Major Findings | Study |
|---|---|---|---|
| Human Neural Stem Cells (hNSCs) | Stroke (pig, murine, and rat models) | Enhanced neurological recovery. Of note, IFN-γ-hNSC-EVs showed better outcomes. | [83,84,85] |
| Chronic neural inflammation (LPS-induced in mice) | hNSC-EVs were shown reduce microglial activation and inflammation with marked reduction in proinflammatory NLRP3 pathway. | [86] | |
| Mesenchymal Stem Cells | Stroke (murine model) | MSC-EVs improved neurological recovery. However, MSC-EVs were less effective than NSC-EVs. | [84] |
| Neuronal Cells vs. Glial cells | AD (Aβ aggregation in AAPP mice) | EVs from neuronal cells (not glial cells) were shown to capture and clear Aβ as well as reduce amyloid deposits. | [87] |
| Cancer Cells | Brain tumor (GL26 mouse model) | Delayed brain tumor growth; cell-type specificity observed in EV biodistribution. | [79] |
| EV biodistribution in various tumor murine models | EVs from different cancer cell lines were found to have distinct tropism and may have detrimental effects (e.g., promoting angiogenesis, inducing chemoresistance). | [63,81,82,88,89,90] |
| Disorder | Donor Cell | Ligand | Loading Mechanism | Other EV Modifications | Major Findings | Study |
|---|---|---|---|---|---|---|
| Targeted Delivery | HEK293T | RVG-Lamp2b | Transfection | Gold nanoparticles were mechanically loaded | RVG-EVs effectively carried AuNPs to the brain. | [124] |
| human iPSCs | RVG-Lamp2b-HA | CRISPR/Cas9-assisted homologous recombination | Labeled with NIR dye | RVG-EVs have improved targeting to the brain compared to EV controls. | [115] | |
| AD | Murine dendritic cells | Lamp2b-RVG | Transfection | GAPDH siRNA loaded via electroporation | Significant knockdown of GAPDH mRNA throughout the brain. Knockdown of protein BACE1. | [114] |
| MSC | DOPE-RVG | DOPE-NHS linker | Labeled with lipophilic dye DiI | Significant decrease in proinflammatory cytokines and increase in anti-inflammatory cytokines. Improved learning and memory capabilities. Decreased plaque depositions, Aβ levels, and astrocyte activation. | [119] | |
| PD | Murine dendritic cells | RVG-Lamp2b | Transfection | α-Syn siRNA loaded via electroporation | Delivery of EV-associated siRNA to the brain. Mice had decrease in α-Syn mRNA and protein. | [116] |
| Murine dendritic cells | RVG-Lamp2b | Transfection | shRNA-mini circles (MC) loaded via electroporation | Decrease in α-Syn aggregation, reduction in loss of dopaminergic neurons, improved clinical symptoms. | [121] | |
| HEK293T | RVG-Lamp2b | Transfection | α-Syn aptamer loaded via transfection | Aptamer-loaded EVs were delivered into neurons, reduced α-Syn PFF, and reduced the loss of dopaminergic neurons. | [120] | |
| Murine dendritic cells | RVG | Ultrasonic assembly | C/ANP/S core loaded via ultrasonic assembly | RVG-EVs with C/ANP/S cores were shown to decrease α-Syn and improved motor behavior in mice. | [122] | |
| SAH | Murine BM-MSCs | RVG-Lamp2b | Transfection | FAM-labeled miR-193b-3p or scrambled miRNA were loaded via electroporation | RVG-EVs with miR-193b-3p were more effective at delivery of miR-19b-3p to the sight of injury compared to miR-19b-3p alone, and reduced behavioral impairment, brain edema, BBB injury, and neurodegeneration. | [123] |
| Cortical ischemia | Murine BM-MSC | RVG-Lamp2b | Transfection | Fluorescently labeled; miR-124 or scrambled miRNA were loaded via electroporation | RVG-EVs loaded with miR-124 promoted neuron differentiation and protected ischemic injury. | [118] |
| Morphine addiction | 293T | RVG-Lamp2b | Transfection | MOR siRNA transfection fluorescence-labeled siRNA | RVG-EVs efficiently transfer siRNA to CNS and downregulate MOR expression inhibiting morphine relapse. | [117] |
| Cerebral Ischemia | Murine BM-MSC | c(RGDyK) peptide | Bio-orthogonal chemistry | Curcumin and triiodothyronine (T3) loaded | Reduction in inflammatory response and apoptosis near the lesion. | [125] |
| Autoimmune Encephalomyelitis | Murine BM-NSC | PDGF-A | Transfection | Via sonication | Slowed down disease development by reducing myelin damage and promoting oligodendrocyte survival and myelin regeneration. | [126] |
| Glioma | U87-MG | Angiopep-2 | Conjugation with DSPE-PEG2000 as a linker | Plasma membrane of EVs were isolated and used to synthesize liposomes via extrusion and loaded with docetaxel | Docetaxel loaded “exo-mimics” showed increased ability to deliver DTX to the tumor area and reduced GBM growth. | [127] |
| Raw264.7 cells | Neuropilin-1-targeted peptide (RGE) | Click chemistry | SPION/Cur were loaded via electroporation | Synergistic anti-tumor effect with SPIONs and Cur, with increased delivery and decreased therapeutic side effects when delivered via RGE-labeled EVs. | [128] | |
| THP-1 | Angiopep-2 and CD133 RNA | Amphiphilic molecule bridge | Temozolomide (TMZ) and O6-benzylguanine (BG) were loaded via sonication | Extended life in mice with less side effects than therapeutics alone. | [129] | |
| U251 GBM | Angiopep-2 and CD133 RNA | Click chemistry | Temozolomide (TMZ) and O6-benzylguanine (BG) were loaded via sonication | Were able to penetrate tumor environment and suppress tumor growth, increasing survival time in mice. | [130] | |
| CNS-TB | BMSCs | Angiopep-2 | Click chemistry | Rifampin (RIF) loaded via electroporation | Higher targeting capacity compared to unmodified EVs; furthermore, modified EVs did not change the MIC or MBC (although this was determined in vitro). | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.; Branscome, H.; Kashanchi, F.; Batrakova, E.V. Targeting of Extracellular Vesicle-Based Therapeutics to the Brain. Cells 2025, 14, 548. https://doi.org/10.3390/cells14070548
Williams A, Branscome H, Kashanchi F, Batrakova EV. Targeting of Extracellular Vesicle-Based Therapeutics to the Brain. Cells. 2025; 14(7):548. https://doi.org/10.3390/cells14070548
Chicago/Turabian StyleWilliams, Anastasia, Heather Branscome, Fatah Kashanchi, and Elena V. Batrakova. 2025. "Targeting of Extracellular Vesicle-Based Therapeutics to the Brain" Cells 14, no. 7: 548. https://doi.org/10.3390/cells14070548
APA StyleWilliams, A., Branscome, H., Kashanchi, F., & Batrakova, E. V. (2025). Targeting of Extracellular Vesicle-Based Therapeutics to the Brain. Cells, 14(7), 548. https://doi.org/10.3390/cells14070548









