Latest Update on lncRNA in Epithelial Ovarian Cancer—A Scoping Review
Abstract
1. Introduction
1.1. Long Noncoding RNA (lncRNA)—Definition, Description
1.2. LncRNA—From the Discovery to the Role in Carcinogenesis
1.3. Epithelial Ovarian Cancer—Pathological and Molecular Background
- (a)
- Endometriosis-associated ovarian cancer (EAOC)
- EAOC consists of ovarian endometrioid carcinoma (OEC) and ovarian clear cell carcinoma (OCCC) [25]. When examining tissue remodeling, one should remember that endometriosis is a benign condition that can become cancerous, with atypical endometriosis being an intermediate stage.
- Despite numerous reports on EAOC, the precise mechanism of malignant transformation from endometriosis to ovarian cancer remains unclear. Kok et al. stated that endometriosis increased the risk of EOC fourfold [26]. A critical factor in the pathogenesis of EAOC is the ARID1A (AT-rich interactive domain 1A) suppressor gene, which encodes the BAF250a protein. The mutation rate of ARID1A in OCCC ranges from 40% to 95%, while in OEC, it is around 30%. It is confirmed as a first step in EAOC carcinogenesis [25]. Kristen rat sarcoma virus (KRAS) is another crucial gene involved in EAOC pathogenesis, with mutations identified in 29% of EAOC cases [27].
- (b)
- High-grade serous ovarian cancer (HGSOC)
- HGSOC accounts for 70% of all ovarian cancers and is characterized by high-grade histology, rapid progression, poor prognosis, and high recurrence rate. Genetically, HGSOC is distinguished from low-grade EOC by frequent mutations in the TP53 gene, which are found in nearly all cases, along with other molecular alterations, such as BRCA1 and BRCA2 mutations. HGSOC is often diagnosed at an advanced stage due to its asymptomatic progression. Despite advances in surgery and chemotherapy, overall survival is still unsatisfactory. Platinum-based chemotherapy combined with paclitaxel remains the cornerstone of treatment but, unfortunately, is usually followed by chemo-resistant recurrence. However, recent advancements in targeted therapies, including PARP inhibitors and anti-angiogenic agents, like bevacizumab, have improved outcomes for some patients.
2. Material and Methods
3. Results
4. Discussion
4.1. XIST
4.2. H19
4.3. NEAT1
4.4. MALAT1
4.5. UCA1
4.6. HOTAIR
Major Concerns
5. Future Perspectives and Clinical Utility
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.Y.; Lu, A.Q.; Chen, L.J. LncRNAs in ovarian cancer. Clin. Chim. Acta 2019, 490, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.; Pirlog, R.; Chiroi, P.; Nutu, A.; Puia, V.R.; Fetti, A.C.; Rusu, D.R.; Berindan-Neagoe, I.; Al Hajjar, N. The Implications of Noncoding RNAs in the Evolution and Progression of Nonalcoholic Fatty Liver Disease (NAFLD)-Related, HCC. Int. J. Mol. Sci. 2022, 23, 12370. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Qin, C.; Jiang, B.; Fang, S.; Pan, X.; Peng, L.; Liu, Z.; Li, W.; Li, Y.; Li, G. Down-regulation of MALAT1 inhibits cervical cancer cell invasion and metastasis by inhibition of epithelial-mesenchymal transition. Mol. Biosyst. 2016, 12, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Geng, G.; Zhao, C.; Gao, T.; Wei, B. LncRNA MEG3 promotes cisplatin sensitivity of cervical cancer cells by regulating the miR-21/PTEN axis. BMC Cancer 2022, 22, 1145. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, T.; Wang, Y.; Yu, J.; Liu, Y.; Lin, Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol. Ther. 2016, 17, 104–113. [Google Scholar] [CrossRef]
- Huang, J.; Ke, P.; Guo, L.; Wang, W.; Tan, H.; Liang, Y.; Yao, S. Lentivirus-mediated RNA interference targeting the long noncoding RNA HOTAIR inhibits proliferation and invasion of endometrial carcinoma cells in vitro and in vivo. Int. J. Gynecol. Cancer 2014, 24, 635–642. [Google Scholar] [CrossRef]
- Guo, C.; Song, W.Q.; Sun, P.; Jin, L.; Dai, H.Y. LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J. Biomed. Sci. 2015, 22, 100. [Google Scholar] [CrossRef]
- Jiang, J.; Duan, M.; Wang, Z.; Lai, Y.; Zhang, C.; Duan, C. RNA epigenetics in pulmonary diseases: Insights into methylation modification of lncRNAs in lung cancer. Biomed. Pharmacother. 2024, 175, 116704. [Google Scholar] [CrossRef]
- Dai, S.; Wang, Q.; Lyu, Y.; Chen, Z.; Liu, X.; Zhao, G.; Zhang, H. LncRNA AC100826.1 regulated PLCB1 to promote progression in non-small cell lung cancer. Thorac. Cancer 2024, 15, 1477–1489. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, H.; Hu, Z.; Gu, Y.; Zhang, R.; Shu, H.; Liu, H.; Sun, X. Comprehensive Transcriptome Analysis Expands lncRNA Functional Profiles in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 8456. [Google Scholar] [CrossRef]
- Thangavelu, L.; Moglad, E.; Gupta, G.; Menon, S.V.; Gaur, A.; Sharma, S.; Kaur, M.; Chahar, M.; Sivaprasad, G.V.; Deorari, M. GAS5 lncRNA: A biomarker and therapeutic target in breast cancer. Pathol. Res. Pract. 2024, 260, 155424. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Y.; Chen, J.; Yang, J.; Yuan, Y.; Wu, W. Exosomal lncRNA SNHG12 promotes angiogenesis and breast cancer progression. Breast Cancer 2024, 31, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, Y.; Gu, B. A novel immune-related lncRNA as a prognostic biomarker in HER2+ breast cancer. Oncol. Lett. 2024, 27, 269. [Google Scholar] [CrossRef]
- Yu, J.M.; Sun, C.Q.; Xu, H.H.; Jiang, Y.L.; Jiang, X.Y.; Ni, S.Q.; Zhao, T.Y.; Liu, L.X. Navigating the labyrinth of long non-coding RNAs in colorectal cancer: From chemoresistance to autophagy. World J. Gastrointest. Oncol. 2024, 16, 3376–3381. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Jiang, Q.; Wang, Z.; Zhang, H.; Fu, Z. The roles of lncRNA AP001469.3 in clinical implications, immune landscape and carcinogenesis of colorectal cancer. Transl. Cancer Res. 2024, 13, 3465–3481. [Google Scholar] [CrossRef]
- Luo, X.J.; Lu, Y.X.; Wang, Y.; Huang, R.; Liu, J.; Jin, Y.; Liu, Z.K.; Liu, Z.X.; Huang, Q.T.; Pu, H.Y.; et al. M6A-modified lncRNA FAM83H-AS1 promotes colorectal cancer progression through PTBP1. Cancer Lett. 2024, 598, 217085. [Google Scholar] [CrossRef]
- Mao, R.; Xu, C.; Zhang, Q.; Wang, Z.; Liu, Y.; Peng, Y.; Li, M. Predictive significance of glycolysis-associated lncRNA profiles in colorectal cancer progression. BMC Med. Genom. 2024, 17, 112. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Norquist, B.; Lacchetti, C.; Armstrong, D.; Grisham, R.N.; Goodfellow, P.J.; Kohn, E.C.; Levine, D.A.; Liu, J.F.; Lu, K.H.; et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1222–1245. [Google Scholar] [CrossRef]
- Kaku, T.; Ogawa, S.; Kawano, Y.; Ohishi, Y.; Kobayashi, H.; Hirakawa, T.; Nakano, H. Histological classification of ovarian cancer. Med. Electron. Microsc. 2003, 36, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.C.; Kwong, J.; Welch, W.R.; Samimi, G.; Ozbun, L.; Bonome, T.; Birrer, M.J.; Berkowitz, R.S.; Wong, K.K. Etiology and pathogenesis of epithelial ovarian cancer. Dis. Markers 2007, 23, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef]
- Chien, J.R.; Aletti, G.; Bell, D.A.; Keeney, G.L.; Shridhar, V.; Hartmann, L.C. Molecular pathogenesis and therapeutic targets in epithelial ovarian cancer. J. Cell Biochem. 2007, 102, 1117–1129. [Google Scholar] [CrossRef]
- Pejovic, T.; Thisted, S.; White, M.; Nezhat, F.R. Endometriosis and Endometriosis-Associated Ovarian Cancer (EAOC). Adv. Exp. Med. Biol. 2020, 1242, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C.; Tsai, H.J.; Su, C.F.; Lee, C.K. The risks for ovarian, endometrial, breast, colorectal, and other cancers in women with newly diagnosed endometriosis or adenomyosis: A population-based study. Int. J. Gynecol. Cancer. 2015, 25, 968–976. [Google Scholar]
- Stewart, C.J.; Leung, Y.; Walsh, M.D.; Walters, R.J.; Young, J.P.; Buchanan, D.D. KRAS mutations in ovarian low-grade endometrioid adenocarcinoma: Association with concurrent endometriosis. Hum. Pathol. 2012, 43, 1177–1183. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.M.; Huang, Y.; Wang, K.N.; Xie, Y.; Yang, N. LncRNA GAS5 inhibits the proliferation and invasion of ovarian clear cell carcinoma via the miR-31-5p/ARID1A axis. Kaohsiung J. Med. Sci. 2021, 37, 940–950. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, Y.; Guo, Q.; Zhu, J.; Cao, L.; Guo, X.; Xu, F.; Weng, W.; Ju, X.; Wu, X. Long non-coding RNA SNHG6 promotes cell proliferation and migration through sponging miR-4465 in ovarian clear cell carcinoma. J. Cell Mol. Med. 2019, 23, 5025–5036. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Buttarelli, M.; De Donato, M.; Raspaglio, G.; Babini, G.; Ciucci, A.; Martinelli, E.; Baccaro, P.; Pasciuto, T.; Fagotti, A.; Scambia, G.; et al. Clinical Value of lncRNA MEG3 in High-Grade Serous Ovarian Cancer. Cancers 2020, 12, 966. [Google Scholar] [CrossRef]
- Filippov-Levy, N.; Cohen-Schussheim, H.; Tropé, C.G.; Hetland Falkenthal, T.E.; Smith, Y.; Davidson, B.; Reich, R. Expression and clinical role of long non-coding RNA in high-grade serous carcinoma. Gynecol. Oncol. 2018, 148, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tang, X.; Zheng, T.; Qiu, J.; Hua, K. Long non-coding RNA AOC4P suppresses epithelial ovarian cancer metastasis by regulating epithelial-mesenchymal transition. J. Ovarian Res. 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tang, Y.; Liu, L.; Yan, J.; Qin, L. Downregulation of lncRNA ASMTL-AS1 in Epithelial Ovarian Cancer Correlates with Worse Prognosis and Cancer Progression. Horm. Metab. Res. 2022, 54, 481–488. [Google Scholar] [CrossRef]
- Cui, K.; Zhu, G. LncRNA CTBP1-AS2 regulates miR-216a/PTEN to suppress ovarian cancer cell proliferation. J. Ovarian Res. 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yang, P.; Gao, Y. Long non-coding RNA EPB41L4A-AS2 suppresses progression of ovarian cancer by sequestering microRNA-103a to upregulate transcription factor RUNX1T1. Exp. Physiol. 2020, 105, 75–87. [Google Scholar] [CrossRef]
- Yao, C.; Zeng, L.; Liu, Q.; Qiu, X.; Chen, C. LncRNA FAM225B Regulates PDIA4-Mediated Ovarian Cancer Cell Invasion and Migration via Modulating Transcription Factor DDX17. Breast J. 2023, 2023, 3970444. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, J.; Liu, G. Abnormal expression of long non-coding RNA FGD5-AS1 affects the development of ovarian cancer through regulating miR-107/RBBP6 axis. Chin. J. Physiol. 2023, 66, 171–180. [Google Scholar] [CrossRef]
- Dong, Q.; Long, X.; Cheng, J.; Wang, W.; Tian, Q.; Di, W. LncRNA GAS5 suppresses ovarian cancer progression by targeting the miR-96-5p/PTEN axis. Ann. Transl. Med. 2021, 9, 1770. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, H.; Lin, L.; Li, Y.; Li, J. lncRNA GAS5 suppression of the malignant phenotype of ovarian cancer via the miR-23a-WT1 axis. Ann. Transl. Med. 2023, 11, 119. [Google Scholar] [CrossRef]
- Zhang, T.; Leng, Y.; Duan, M.; Li, Z.; Ma, Y.; Huang, C.; Shi, Q.; Wang, Y.; Wang, C.; Liu, D.; et al. LncRNA GAS5-hnRNPK axis inhibited ovarian cancer progression via inhibition of AKT signaling in ovarian cancer cells. Discov. Oncol. 2023, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Jiang, P.; Zhai, H.; Dong, J.S. LncRNA GAS8-AS1 Inhibits Ovarian Cancer Progression Through Activating Beclin1-Mediated Autophagy. Onco Targets Ther. 2020, 13, 10431–10440. [Google Scholar] [CrossRef]
- Gokulnath, P.; de Cristofaro, T.; Manipur, I.; Di Palma, T.; Soriano, A.A.; Guarracino, M.R.; Zannini, M. Long Non-Coding RNA HAND2-AS1 Acts as a Tumor Suppressor in High-Grade Serous Ovarian Carcinoma. Int. J. Mol. Sci. 2020, 21, 4059. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, L.; Hu, L.; Li, T.; Xie, D.; Liu, X. Long non-coding RNA HAND2-AS1/miR-106a/PTEN axis re-sensitizes cisplatin-resistant ovarian cells to cisplatin treatment. Mol. Med. Rep. 2021, 24, 762. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Sun, J.; Hu, C.; Ge, X.; Li, R. LncRNA HCG11 represses ovarian cancer cell growth via AKT signaling pathway. J. Obstet. Gynaecol. Res. 2022, 48, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Zhang, M.; Hou, H.; Zhang, Z.; Li, N. HOTAIRM1 suppresses cell proliferation and invasion in ovarian cancer through facilitating ARHGAP24 expression by sponging miR-106a-5p. Life Sci. 2020, 243, 117296. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, S.; Li, C.; Huang, L.; Chen, C.; Tang, X.; Su, X.; Zhu, H. Downregulation of LEMD1-AS1 and Its Influences on the Diagnosis, Prognosis, and Immune Infiltrates of Epithelial Ovarian Cancer. Dis. Markers 2022, 2022, 6408879. [Google Scholar] [CrossRef]
- Guo, R.; Qin, Y. LEMD1-AS1 Suppresses Ovarian Cancer Progression Through Regulating miR-183-5p/TP53 Axis. Onco Targets Ther. 2020, 13, 7387–7398. [Google Scholar] [CrossRef]
- Liu, F.; Cao, L.; Zhang, Y.; Xia, X.; Ji, Y. LncRNA LIFR-AS1 overexpression suppressed the progression of serous ovarian carcinoma. J. Clin. Lab. Anal. 2022, 36, e25470. [Google Scholar] [CrossRef]
- Hao, T.; Huang, S.; Han, F. LINC-PINT suppresses tumour cell proliferation, migration and invasion through targeting miR-374a-5p in ovarian cancer. Cell Biochem. Funct. 2020, 38, 1089–1099. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Wang, H.; Han, C.; Wang, Y.; Tang, R. LINC00629, a HOXB4-downregulated long noncoding RNA, inhibits glycolysis and ovarian cancer progression by destabilizing c-Myc. Cancer Sci. 2024, 115, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Wang, W.; Wu, L.; Qi, C.; Yan, W.; Lu, W.; Tian, J.; Shang, A. LINC00936/microRNA-221-3p Regulates Tumor Progression in Ovarian Cancer by Interacting with LAMA3. Recent Patents Anti-Cancer Drug Discov. 2023, 18, 66–79. [Google Scholar] [CrossRef]
- Xu, H.; Mao, H.L.; Zhao, X.R.; Li, Y.; Liu, P.S. MiR-29c-3p, a target miRNA of LINC01296, accelerates tumor malignancy: Therapeutic potential of a LINC01296/miR-29c-3p axis in ovarian cancer. J. Ovarian Res. 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Shi, X.Y.; Li, Z.L.; Li, M.; Zhang, M.M.; Yan, S.J.; Wei, Z.L. Downregulation of LINC01508 contributes to cisplatin resistance in ovarian cancer via the regulation of the Hippo-YAP pathway. J. Gynecol. Oncol. 2021, 32, e77. [Google Scholar] [CrossRef]
- Luo, T.; Jiang, Y.; Yang, J. Long Noncoding RNA LINC01554 as a Novel Biomarker for Diagnosis and Prognosis Prediction of Epithelial Ovarian Cancer. Dis. Markers 2021, 2021, 1244612. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zhang, X.; Li, B.; Meng, X. MAGI2-AS3 suppresses MYC signaling to inhibit cell proliferation and migration in ovarian cancer through targeting miR-525-5p/MXD1 axis. Cancer Med. 2020, 9, 6377–6386. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Q.; Xie, F. LncRNA-MALAT1 regulates proliferation and apoptosis of ovarian cancer cells by targeting miR-503-5p. Onco Targets Ther. 2019, 12, 6297–6307. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Ding, L.; Yu, L.; Zhang, B.; Wei, D. LncRNA MEG3 suppressed the progression of ovarian cancer via sponging miR-30e-3p and regulating LAMA4 expression. Cancer Cell Int. 2020, 20, 181. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Zhao, Y.; Peng, H.; Zhang, N.; Bai, W. MEG3 sponges miRNA-376a and YBX1 to regulate angiogenesis in ovarian cancer endothelial cells. Heliyon 2023, 9, e13204. [Google Scholar] [CrossRef]
- Tian, J.; Yang, L.; Wang, Z.; Yan, H. MIR503HG impeded ovarian cancer progression by interacting with SPI1 and preventing TMEFF1 transcription. Aging 2022, 14, 5390–5405. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, D.; Zhu, D.; Xu, T.; Huang, A.; Jiang, L.; Liu, C.; Qian, H.; Bu, X. LncRNA-MSC-AS1 inhibits the ovarian cancer progression by targeting miR-425-5p. J. Ovarian Res. 2021, 14, 109. [Google Scholar] [CrossRef]
- Liu, S.; Du, Q.; Rao, Y.; Liu, C.; Qu, P. Long non-coding RNA NPBWR1-2 affects the development of ovarian cancer via multiple microRNAs. Oncol. Lett. 2020, 20, 685–692. [Google Scholar] [CrossRef]
- Chen, B.; Lu, X.; Zhou, Q.; Chen, Q.; Zhu, S.; Li, G.; Liu, H. PAXIP1-AS1 is associated with immune infiltration and predicts poor prognosis in ovarian cancer. PLoS ONE 2023, 18, e0290031. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, M.; Chen, Z.; Yu, B.; He, X.; Luo, Y.; Ai, F.; Hu, W. PITPNA-AS1 Inhibits Cell Proliferation and Migration in Ovarian Cancer by Regulating the MIR-223-3p/RHOB Axis. Rev. Investig. Clin. 2024, 76, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, S.; Yao, X.; Liu, Y.; Qian, L.; Wang, Y.; Zhang, T.; Shan, G.; Chen, L.; Zhou, Y. Ascites exosomal lncRNA PLADE enhances platinum sensitivity by inducing R-loops in ovarian cancer. Oncogene 2024, 43, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; Huang, K.; Xiong, X.; Shi, Y.; Wang, X.; Pan, X.; Cong, Y.; Sun, Y.; Ge, L.; et al. Long noncoding RNA RFPL1S-202 inhibits ovarian cancer progression by downregulating the IFN-β/STAT1 signaling. Exp. Cell Res. 2023, 422, 113438. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Peng, S.; Zhang, K. LncRNA RP11-499E18.1 Inhibits Proliferation, Migration, and Epithelial-Mesenchymal Transition Process of Ovarian Cancer Cells by Dissociating PAK2-SOX2 Interaction. Front. Cell Dev. Biol. 2021, 9, 697831. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Zhang, A.; Wang, Q.; Ge, J.; Li, Q.; Xiao, J. Long non-coding RNA SDCBP2-AS1 delays the progression of ovarian cancer via microRNA-100-5p-targeted EPDR1. World J. Surg. Oncol. 2021, 19, 199. [Google Scholar] [CrossRef]
- Huang, K.; Chen, X.; Geng, Z.; Xiong, X.; Cong, Y.; Pan, X.; Liu, S.; Ge, L.; Xu, J.; Jia, X. LncRNA SLC25A21-AS1 increases the chemosensitivity and inhibits the progression of ovarian cancer by upregulating the expression of KCNK4. Funct. Integr. Genom. 2023, 23, 110. [Google Scholar] [CrossRef]
- Li, S.; Shen, S.; Ge, W.; Cen, Y.; Zhang, S.; Cheng, X.; Wang, X.; Xie, X.; Lu, W. Long non-coding RNA SLC25A21-AS1 inhibits the development of epithelial ovarian cancer by specifically inducing PTBP3 degradation. Biomark. Res. 2023, 11, 12. [Google Scholar] [CrossRef]
- Lv, W.; Jia, Y.; Wang, J.; Duan, Y.; Wang, X.; Liu, T.; Hao, S.; Liu, L. Long non-coding RNA SNHG10 upregulates BIN1 to suppress the tumorigenesis and epithelial-mesenchymal transition of epithelial ovarian cancer via sponging miR-200a-3p. Cell Death Discov. 2022, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shen, L.; Lin, Q.; Dong, C.; Maswela, B.; Illahi, G.S.; Wu, X. SNHG5 enhances Paclitaxel sensitivity of ovarian cancer cells through sponging miR-23a. Biomed. Pharmacother. 2020, 123, 109711. [Google Scholar] [CrossRef]
- Chen, G.Y.; Zhang, Z.S.; Chen, Y.; Li, Y. Long non-coding RNA SNHG9 inhibits ovarian cancer progression by sponging microRNA-214-5p. Oncol. Lett. 2021, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Wang, J.; Xuan, L.; Liu, X. LncRNA TTN-AS1 acts as sponge for miR-15b-5p to regulate FBXW7 expression in ovarian cancer. Biofactors 2020, 46, 600–607. [Google Scholar] [CrossRef]
- Zhu, L.M.; Li, N. Downregulation of long noncoding RNA TUSC7 promoted cell growth, invasion and migration through sponging with miR-616-5p/GSK3β pathway in ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7253–7265. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Wang, Y.; Zhang, Y.; Yuan, J.; Zhu, C.; Jiang, W. lncRNA AC005224.4/miR-140-3p/SNAI2 regulating axis facilitates the invasion and metastasis of ovarian cancer through epithelial-mesenchymal transition. Chin. Med. J. 2023, 136, 1098–1110. [Google Scholar] [CrossRef]
- Lin, C.; Zheng, M.; Yang, Y.; Chen, Y.; Zhang, X.; Zhu, L.; Zhang, H. Knockdown of lncRNA ACTA2-AS1 reverses cisplatin resistance of ovarian cancer cells via inhibition of miR-378a-3p-regulated Wnt5a. Bioengineered 2022, 13, 9829–9838. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Chen, J. Mechanism underlying the regulation of lncRNA ACTA2-AS1 on CXCL2 by absorbing miRNA-532-5p as ceRNA in the development of ovarian cancer. Int. J. Clin. Exp. Pathol. 2021, 14, 596–607. [Google Scholar]
- Cai, L.; Hu, X.; Ye, L.; Bai, P.; Jie, Y.; Shu, K. Long non-coding RNA ADAMTS9-AS1 attenuates ferroptosis by Targeting microRNA-587/solute carrier family 7 member 11 axis in epithelial ovarian cancer. Bioengineered 2022, 13, 8226–8239. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, X.; Wei, W.; Chen, X.; Sun, L. Regulations of Exosomal-Transmitted AFAP1-AS1 LncRNA on Ovarian Cancer Cell Migration and Invasion. Discov. Med. 2023, 35, 877–886. [Google Scholar] [CrossRef]
- Liu, B.; Yan, L.; Chi, Y.; Sun, Y.; Yang, X. Long non-coding RNA AFAP1-AS1 facilitates ovarian cancer progression by regulating the miR-107/PDK4 axis. J. Ovarian Res. 2021, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dong, B.; Xu, X.; Li, Y.; Wang, Y.; Li, X. Correction to: LncRNA ARAP1-AS1 aggravates the malignant phenotypes of ovarian cancer cells through sponging miR-4735-3p to enhance PLAGL2 expression. Cytotechnology 2022, 74, 201, Erratum in: Cytotechnology 2021, 73, 363–372. https://doi.org/10.1007/s10616-021-00463-6. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Y.B.; Zhao, Y.; Ye, C. Long non-coding RNA ASAP1-IT1 suppresses ovarian cancer progression by regulating Hippo/YAP signaling. Int. J. Mol. Med. 2021, 47, 44. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, L.; Han, X.; Ma, H.; Zhang, N.; She, L. LncRNA ASB16-AS1 accelerates cellular process and chemoresistance of ovarian cancer cells by regulating GOLM1 expression via targeting miR-3918. Biochem. Biophys. Res. Commun. 2023, 675, 1–9. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, X.; Zhao, Y.; Qian, H.; Wang, H.; He, C.; Liu, X.; Guo, T.; Lin, M.; Yu, H.; et al. Role of lncRNA-ATB in ovarian cancer and its mechanisms of action. Exp. Ther. Med. 2020, 19, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; An, N. Long noncoding RNA ATB promotes ovarian cancer tumorigenesis by mediating histone H3 lysine 27 trimethylation through binding to EZH2. J. Cell Mol. Med. 2021, 25, 37–46. [Google Scholar] [CrossRef]
- Yuan, D.; Guo, T.; Zhu, D.; Ge, H.; Zhao, Y.; Huang, A.; Wang, X.; Cao, X.; He, C.; Qian, H.; et al. Exosomal lncRNA ATB Derived from Ovarian Cancer Cells Promotes Angiogenesis via Regulating miR-204-3p/TGFβR2 Axis. Cancer Manag. Res. 2022, 14, 327–337. [Google Scholar] [CrossRef]
- Yao, H.; Chen, R.; Yang, Y.; Jiang, J. LncRNA BBOX1-AS1 Aggravates the Development of Ovarian Cancer by Sequestering miR-361-3p to Augment PODXL Expression. Reprod. Sci. 2021, 28, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.M.; Guo, Y.R.; Zhou, M.Y.; Wang, Y. Expression and clinical significance of lncRNA BC041954 in ovarian cancer. Exp. Ther. Med. 2022, 23, 408. [Google Scholar] [CrossRef]
- Yang, H.; Qi, Y.; Wang, X.L.; Gu, J.J.; Shi, T.M. Down-regulation of lncRNA BLACAT1 inhibits ovarian cancer progression by suppressing the Wnt/β-catenin signaling pathway via regulating miR-519d-3p. Mol. Cell Biochem. 2020, 467, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qiu, J.; Lu, X.; Ma, Y.; Li, G. LncRNA CACNA1G-AS1 up-regulates FTH1 to inhibit ferroptosis and promote malignant phenotypes in ovarian cancer cells. Oncol. Res. 2023, 31, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Rivera, R.; Rivera-Serrano, M.; Rabelo-Fernandez, R.J.; Pérez-Santiago, J.; Valiyeva, F.; Vivas-Mejía, P.E. Upregulation of the Long Noncoding RNA CASC10 Promotes Cisplatin Resistance in High-Grade Serous Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 7737. [Google Scholar] [CrossRef]
- Lin, H.; Xu, X.; Chen, K.; Fu, Z.; Wang, S.; Chen, Y.; Zhang, H.; Niu, Y.; Chen, H.; Yu, H.; et al. LncRNA CASC15, MiR-23b Cluster and SMAD3 form a Novel Positive Feedback Loop to promote Epithelial-Mesenchymal Transition and Metastasis in Ovarian Cancer. Int. J. Biol. Sci. 2022, 18, 1989–2002. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Li, N.; Cui, Y.L. Long Non-coding RNA CCAT1 Sponges miR-454 to Promote Chemoresistance of Ovarian Cancer Cells to Cisplatin by Regulation of Surviving. Cancer Res. Treat. 2020, 52, 798–814. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Y.; Liu, X.; Cui, Y. LncRNACASC9 promotes proliferation, metastasis, and cell cycle inovarian carcinoma cells through cyclinG1/TP53/MMP7 signaling. Bioengineered 2021, 12, 8006–8019. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, D.; Chu, H.; Man, L.; Huang, X.; Yin, L.; Zhao, D.; Mu, L.; Gao, C.; Che, J.; et al. lncRNA CDKN2A-AS1 facilitates tumorigenesis and progression of epithelial ovarian cancer via modulating the SOSTDC1-mediated BMP-SMAD signaling pathway. Cell Cycle 2021, 20, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhai, J.; Fu, Y. LncRNA CDKN2B-AS1 promotes the progression of ovarian cancer by miR-143-3p/SMAD3 axis and predicts a poor prognosis. Neoplasma 2020, 67, 782–793. [Google Scholar] [CrossRef]
- Wang, H.M.; Shen, S.L.; Li, N.M.; Su, H.F.; Li, W.Y. LncRNA CDKN2BAS aggravates the progression of ovarian cancer by positively interacting with GAS6. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5946–5952. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.X.; Zhang, C.Y.; Bai, N.; Feng, C.; Zhang, Z.M.; Wang, L.; Gao, Z.Z. LncRNA CRNDE promotes cell proliferation, migration and invasion of ovarian cancer via miR-423-5p/FSCN1 axis. Mol. Cell Biochem. 2022, 477, 1477–1488. [Google Scholar] [CrossRef]
- Wu, J.; Ni, X.; Yu, Z.; Wu, S.; Liu, Z. CRNDE inducing cisplatin resistance through SRSF1/TIA1 signaling pathway in ovarian cancer. Pathol. Res. Pract. 2022, 235, 153957. [Google Scholar] [CrossRef]
- Liu, P.; Fu, R.; Chen, K.; Zhang, L.; Wang, S.; Liang, W.; Zou, H.; Tao, L.; Jia, W. ETV5-mediated upregulation of lncRNA CTBP1-DT as a ceRNA facilitates HGSOC progression by regulating miR-188-5p/MAP3K3 axis. Cell Death Dis. 2021, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Qing, X.; Wei, J.; Mo, H.; Liu, Y.; Zhi, Y.; Lu, W.; Zheng, M.; Zhang, W.; Chen, Y.; et al. The DDUP protein encoded by the DNA damage-induced CTBP1-DT lncRNA confers cisplatin resistance in ovarian cancer. Cell Death Dis. 2023, 14, 568. [Google Scholar] [CrossRef]
- Liu, T.; Shen, J.; He, Q.; Xu, S. Identification of a Novel Immune-Related lncRNA CTD-2288O8.1 Regulating Cisplatin Resistance in Ovarian Cancer Based on Integrated Analysis. Front. Genet. 2022, 13, 814291. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wang, X.; Lin, F.; Cheng, X.; Wang, Z.; Wang, X. Long non-coding RNA CTSLP8 mediates ovarian cancer progression and chemotherapy resistance by modulating cellular glycolysis and regulating c-Myc expression through PKM2. Cell Biol. Toxicol. 2022, 38, 1027–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Lin, F.; Sun, H.; Lin, Y.; Wang, Z.; Wang, X. The lnc-CTSLP8 upregulates CTSL1 as a competitive endogenous RNA and promotes ovarian cancer metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 151. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Qi, B.; Yao, H.; Zhang, L.; Li, Y.; Li, Q. Knockdown of DANCR Suppressed the Biological Behaviors of Ovarian Cancer Cells Treated with Transforming Growth Factor-β (TGF-β) by Sponging MiR-214. Med. Sci. Monit. 2020, 26, e922760. [Google Scholar] [CrossRef]
- Zhou, M.; Cheng, H.; Fu, Y.; Zhang, J. Long noncoding RNA DARS-AS1 regulates TP53 ubiquitination and affects ovarian cancer progression by modulation miR-194-5p/RBX1 axis. J. Biochem. Mol. Toxicol. 2021, 35, e22865. [Google Scholar] [CrossRef]
- Qin, W.; Miao, Y.; Sun, G.; Chen, S.; Zang, Y.S.; Dong, C. Long noncoding RNA DATOC-1 that associate with DICER promotes development in epithelial ovarian cancer by upregulating miR-7 expression. Transl. Cancer Res. 2021, 10, 2379–2388. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Jiang, X. Silencing of lncRNA DLEU1 inhibits tumorigenesis of ovarian cancer via regulating miR-429/TFAP2A axis. Mol. Cell Biochem. 2021, 476, 1051–1061. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, C. LncRNA DLX6-AS1 aggravates the development of ovarian cancer via modulating FHL2 by sponging miR-195-5p. Cancer Cell Int. 2020, 20, 370. [Google Scholar] [CrossRef]
- He, L.; He, G. DNM3OS Facilitates Ovarian Cancer Progression by Regulating miR-193a-3p/MAP3K3 Axis. Yonsei Med. J. 2021, 62, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Cao, X.; Luo, Y.; Zhang, G.; Zhang, D. A Positive Feedback Loop of lncRNA DSCR8/miR-98-5p/STAT3/HIF-1α Plays a Role in the Progression of Ovarian Cancer. Front. Oncol. 2020, 10, 1713. [Google Scholar] [CrossRef]
- Li, L.M.; Hao, S.J.; Ni, M.; Jin, S.; Tian, Y.Q. DUXAP8 promotes the proliferation and migration of ovarian cancer cells via down-regulating microRNA-29a-3p expression. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1837–1844. [Google Scholar] [CrossRef]
- Meng, Q.; Li, Z.; Pan, J.; Sun, X. Long noncoding RNA DUXAP8 regulates proliferation and apoptosis of ovarian cancer cells via targeting miR-590-5p. Hum. Cell 2020, 33, 1240–1251. [Google Scholar] [CrossRef]
- Jie, Y.; Ye, L.; Chen, H.; Yu, X.; Cai, L.; He, W.; Fu, Y. ELFN1-AS1 accelerates cell proliferation, invasion and migration via regulating miR-497-3p/CLDN4 axis in ovarian cancer. Bioengineered 2020, 11, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Huang, Y.; Guo, M.; Hu, X.; Li, P. Long non-coding RNA FAM83H-AS1 acts as a potential oncogenic driver in human ovarian cancer. J. Ovarian Res. 2021, 14, 6. [Google Scholar] [CrossRef]
- Sun, Z.; Gao, S.; Xuan, L.; Liu, X. Long non-coding RNA FEZF1-AS1 induced progression of ovarian cancer via regulating miR-130a-5p/SOX4 axis. J. Cell Mol. Med. 2020, 24, 4275–4285. [Google Scholar] [CrossRef]
- Aichen, Z.; Kun, W.; Xiaochun, S.; Lingling, T. LncRNA FGD5-AS1 promotes the malignant phenotypes of ovarian cancer cells via targeting miR-142-5p. Apoptosis 2021, 26, 348–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Chen, T.; Yue, Y.; Zhao, L.; Zhang, D. Lncrna FGFR3-AS1 Is a Prognostic Indicator for Ovarian Cancer and Induces Cell Proliferation and Hinders Apoptosis. Iran. J. Public Health 2023, 52, 2412–2416. [Google Scholar] [CrossRef]
- Yan, H.; Li, H.; Silva, M.A.; Guan, Y.; Yang, L.; Zhu, L.; Zhang, Z.; Li, G.; Ren, C. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J. Exp. Clin. Cancer Res. 2019, 38, 356. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Zhao, X.; Zhang, P. Long non-coding RNA FOXD2-AS1 promotes proliferation, migration and invasion of ovarian cancer cells via regulating the expression of miR-4492. Exp. Ther. Med. 2021, 21, 307. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, X.; Zhang, C.; Yin, Y.; Chen, L.; Liu, Y.; He, A.; Xia, F. Long noncoding RNA FTX promotes epithelial-mesenchymal transition of epithelial ovarian cancer through modulating miR-7515/TPD52 and activating Met/Akt/mTOR. Histol. Histopathol. 2023, 38, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, Z.; Yang, X.; Chen, Y.; He, L.; Wan, T. LncRNA GClnc1 may contribute to the progression of ovarian cancer by regulating p53 signaling pathway. Eur. J. Histochem. 2020, 64, 3166. [Google Scholar] [CrossRef]
- Wu, D.; Ke, Y.; Xiao, R.; Liu, J.; Li, Q.; Wang, Y. Long non-coding RNA GClnc1 knockdown suppresses progression of epithelial ovarian cancer by recruiting FOXC2 to disrupt the NOTCH1/NF-κB/Snail pathway. Exp. Cell Res. 2021, 399, 112422. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Gao, L.; Yu, H.; Song, X. Long non-coding RNA H19 correlates with unfavorable prognosis and promotes cell migration and invasion in ovarian cancer. Ginekol. Pol. 2022, 93, 1. [Google Scholar] [CrossRef]
- Tian, X.; Zuo, X.; Hou, M.; Li, C.; Teng, Y. LncRNA-H19 regulates chemoresistance to carboplatin in epithelial ovarian cancer through microRNA-29b-3p and STAT3. J. Cancer 2021, 12, 5712–5722. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.J. Long non-coding RNA-H19 promotes ovarian cancer cell proliferation and migration via the microRNA-140/Wnt1 axis. Kaohsiung J. Med. Sci. 2021, 37, 768–775. [Google Scholar] [CrossRef]
- Xu, H.; Ding, Y.; Yang, X. Overexpression of Long Noncoding RNA H19 Downregulates miR-140-5p and Activates PI3K/AKT Signaling Pathway to Promote Invasion, Migration and Epithelial-Mesenchymal Transition of Ovarian Cancer Cells. Biomed. Res. Int. 2021, 2021, 6619730. [Google Scholar] [CrossRef]
- Zhu, L.; Mei, M. Interference of long non-coding RNA HAGLROS inhibits the proliferation and promotes the apoptosis of ovarian cancer cells by targeting miR-26b-5p. Exp. Ther. Med. 2021, 22, 879. [Google Scholar] [CrossRef]
- Li, X.F.; Hu, D.M.; Zhao, Y.X.; Zhang, L.; Jin, Y. Knockdown of lncRNA HCG11 suppresses cell progression in ovarian cancer by modulating miR-144-3p/PBX3. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11032–11040. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, B.H.; Wu, Q.H.; Li, Q.L.; Yang, K.D. LncRNA HCG18 upregulates TRAF4/TRAF5 to facilitate proliferation, migration and EMT of epithelial ovarian cancer by targeting miR-29a/b. Mol. Med. 2022, 28, 2. [Google Scholar] [CrossRef]
- Wang, L.; He, M.; Fu, L.; Jin, Y. Role of lncRNAHCP5/microRNA-525-5p/PRC1 crosstalk in the malignant behaviors of ovarian cancer cells. Exp. Cell Res. 2020, 394, 112129. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Zhang, C.; Zheng, X.; Li, Y.; Wu, P.; Chen, L.; Wei, X. LncRNA HCP5 Facilitates the Progression of Ovarian Cancer by Interacting with the PTBP1 Protein. Biochem. Genet. 2023, 62, 3136–3154. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, J.; Yang, S.; Liu, Z.; Chen, Y.; Peng, M.; Jia, Y. lncRNA HEIH accelerates cell proliferation and inhibits cell senescence by targeting miR-3619-5p/CTTNBP2 axis in ovarian cancer. Menopause 2020, 27, 1302–1314, Erratum in: Menopause 2021, 28, 601. https://doi.org/10.1097/GME.0000000000001764. [Google Scholar] [CrossRef]
- Xie, W.; Wang, W.; Meng, S.; Wu, X.; Liu, X.; Liu, Y.; Kang, X.; Su, Y.; Lv, X.; Guo, L.; et al. A novel hypoxia-stimulated lncRNA HIF1A-AS3 binds with YBX1 to promote ovarian cancer tumorigenesis by suppressing p21 and AJAP1 transcription. Mol. Carcinog. 2023, 62, 1860–1876. [Google Scholar] [CrossRef]
- Fang, W.; Xia, Y. LncRNA HLA-F-AS1 attenuates the ovarian cancer development by targeting miR-21-3p/PEG3 axis. Anticancer. Drugs 2022, 33, 671–681. [Google Scholar] [CrossRef]
- Fan, L.; Lei, H.; Lin, Y.; Zhou, Z.; Li, J.; Wu, A.; Shu, G.; Roger, S.; Yin, G. Hotair promotes the migration and proliferation in ovarian cancer by miR-222-3p/CDK19 axis. Cell Mol. Life Sci. 2022, 79, 254. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Shao, Y.; Yue, X.; Chu, Y.; Yang, C.; Chen, D. LncRNA HOTAIR down-expression inhibits the invasion and tumorigenicity of epithelial ovarian cancer cells by suppressing TGF-β1 and ZEB1. Discov. Oncol. 2023, 14, 228. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, S.; Wang, Z.; Cai, J.; Han, L.; Xie, L.; Han, Q.; Wang, W.; Zhang, Y.; He, X.; et al. HOTAIR promotes paclitaxel resistance by regulating CHEK1 in ovarian cancer. Cancer Chemother. Pharmacol. 2020, 86, 295–305. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Jin, S.M.; Luo, H.Q.; Leng, H.L.; Fang, J.D. LncRNA HOTAIR regulates anoikis-resistance capacity and spheroid formation of ovarian cancer cells by recruiting EZH2 and influencing H3K27 methylation. Neoplasma 2021, 68, 509–518. [Google Scholar] [CrossRef]
- Zhang, Y.; Ai, H.; Fan, X.; Chen, S.; Wang, Y.; Liu, L. Knockdown of long non-coding RNA HOTAIR reverses cisplatin resistance of ovarian cancer cells through inhibiting miR-138-5p-regulated EZH2 and SIRT1. Biol. Res. 2020, 53, 18. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Cai, E.; Cai, J.; Wen, Y.; Lu, S.; Li, X.; Han, Q.; Jiang, J.; Li, T.; et al. HOTAIR maintains the stemness of ovarian cancer stem cells via the miR-206/TBX3 axis. Exp. Cell Res. 2020, 395, 112218. [Google Scholar] [CrossRef]
- Ye, L.; Meng, X.; Xiang, R.; Li, W.; Wang, J. Investigating function of long noncoding RNA of HOTAIRM1 in progression of SKOV3 ovarian cancer cells. Drug Dev. Res. 2021, 82, 1162–1168. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Q.; Wu, X.; Chen, P. LncRNA HOTTIP Promotes Ovarian Cancer Cell Invasion And Metastasis By Stabilizing Hif-1α In The Anoxic Cellular Microenvironment. Acta Endocrinol. 2022, 18, 263–270. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.B.; Liu, Y.M.; Li, F.X.; Zhang, L.P.; Liao, Z.M. LncRNA HOTTIP promotes the proliferation and invasion of ovarian cancer cells by activating the MEK/ERK pathway. Mol. Med. Rep. 2020, 22, 3667–3676. [Google Scholar] [CrossRef]
- Tan, C.; Liu, W.; Zheng, Z.H.; Wan, X.G. LncRNA HOTTIP inhibits cell pyroptosis by targeting miR-148a-3p/AKT2 axis in ovarian cancer. Cell Biol. Int. 2021, 45, 1487–1497. [Google Scholar] [CrossRef]
- Dong, Y.J.; Feng, W.; Li, Y. HOTTIP-miR-205-ZEB2 Axis Confers Cisplatin Resistance to Ovarian Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 707424. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, W. LncRNA HOXA-AS2 promotes the progression of epithelial ovarian cancer via the regulation of miR-372. Oncol. Lett. 2024, 28, 394. [Google Scholar] [CrossRef]
- Eoh, K.J.; Lee, D.W.; Nam, E.J.; Kim, J.I.; Moon, H.; Kim, S.W.; Kim, Y.T. HOXA-AS3 induces tumor progression through the epithelial-mesenchymal transition pathway in epithelial ovarian cancer. Oncol. Rep. 2023, 49, 64. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Wu, Q.; Wang, H.; Xia, H.; Sun, Y. Long non-coding RNA HOXA11-AS knockout inhibits proliferation and overcomes drug resistance in ovarian cancer. Bioengineered 2022, 13, 13893–13905. [Google Scholar] [CrossRef]
- Xu, S.; Jia, G.; Zhang, H.; Wang, L.; Cong, Y.; Lv, M.; Xu, J.; Ruan, H.; Jia, X.; Xu, P.; et al. LncRNA HOXB-AS3 promotes growth, invasion and migration of epithelial ovarian cancer by altering glycolysis. Life Sci. 2021, 264, 118636. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Sun, L.; Liang, L. LncRNA HOXC-AS3 Suppresses the Formation of Mature miR-96 in Ovarian Cancer Cells to Promote Cell Proliferation. Reprod. Sci. 2021, 28, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wei, M.; Hong, L. Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis. Open Life Sci. 2021, 16, 667–681. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Y.; Wang, L.; Cong, Y.; Huang, K.; Pan, X.; Liu, G.; Li, W.; Dai, C.; Xu, P.; et al. LncRNA IDH1-AS1 sponges miR-518c-5p to suppress proliferation of epithelial ovarian cancer cell by targeting RMB47. J. Biomed. Res. 2023, 38, 51–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Yan, C.; Xu, S. LncRNA IL21-AS1 facilitates tumour progression by enhancing CD24-induced phagocytosis inhibition and tumorigenesis in ovarian cancer. Cell Death Dis. 2024, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, R.; Kang, F.; Lai, H.; Wang, Y. KCNQ1OT1 promotes ovarian cancer progression via modulating MIR-142-5p/CAPN10 axis. Mol. Genet. Genomic Med. 2020, 8, e1077. [Google Scholar] [CrossRef]
- Chen, P.; Sun, L.S.; Shen, H.M.; Qu, B. LncRNA KCNQ1OT1 accelerates ovarian cancer progression via miR-125b-5p/CD147 axis. Pathol. Res. Pract. 2022, 239, 154135. [Google Scholar] [CrossRef]
- He, S.L.; Chen, Y.L.; Chen, Q.H.; Tian, Q.; Yi, S.J. LncRNA KCNQ1OT1 promotes the metastasis of ovarian cancer by increasing the methylation of EIF2B5 promoter. Mol. Med. 2022, 28, 112. [Google Scholar] [CrossRef]
- Wu, X.; Qiu, L.; Feng, H.; Zhang, H.; Yu, H.; Du, Y.; Wu, H.; Zhu, S.; Ruan, Y.; Jiang, H. KHDRBS3 promotes paclitaxel resistance and induces glycolysis through modulated MIR17HG/CLDN6 signaling in epithelial ovarian cancer. Life Sci. 2022, 293, 120328. [Google Scholar] [CrossRef]
- Xie, X.; Wen, Q.; Yang, X.; Chen, W.; Liu, Y.; Liu, W.; Zhang, T.; Xu, C.; Shi, K. H3K27ac-activated lncRNA KTN1-AS1 aggravates tumor progression by miR-505-3p/ZNF326 axis in ovarian cancer. Ann. Transl. Med. 2022, 10, 599. [Google Scholar] [CrossRef]
- Gu, H.; Lin, R.; Zheng, F.; Zhang, Q. ELK1 activated-long noncoding RNA LBX2-AS1 aggravates the progression of ovarian cancer through targeting miR-4784/KDM5C axis. J. Mol. Histol. 2021, 52, 31–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, F. LncRNA LEF1-AS1 Promotes Ovarian Cancer Development Through Interacting with miR-1285-3p. Cancer Manag. Res. 2020, 12, 687–694. [Google Scholar] [CrossRef]
- Wang, S.; Weng, W.; Chen, T.; Xu, M.; Wei, P.; Li, J.; Lu, L.; Wang, Y. LINC00152 Promotes Tumor Progression and Predicts Poor Prognosis by Stabilizing BCL6 From Degradation in the Epithelial Ovarian Cancer. Front. Oncol. 2020, 10, 555132. [Google Scholar] [CrossRef]
- Zou, H.; Li, H. Knockdown of long non-coding RNA LINC00152 increases cisplatin sensitivity in ovarian cancer cells. Exp. Ther. Med. 2019, 18, 4510–4516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, L.; Niu, J.; Feng, Y. Knockdown of long non-coding RNA LINC00176 suppresses ovarian cancer progression by BCL3-mediated down-regulation of ceruloplasmin. J. Cell Mol. Med. 2020, 24, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Liu, L.; Xue, J.; Fei, J.; Chen, X.; Wang, J.; Yang, X.; Peng, Q.; Zhu, W. The downregulation of LINC00273 inhibits the proliferation, invasion, and migration of ovarian cancer cells in vivo and in vitro. Ann. Transl. Med. 2022, 10, 1139. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.G.; Zhang, K.Q. LINC00452 promotes ovarian carcinogenesis through increasing ROCK1 by sponging miR-501-3p and suppressing ubiquitin-mediated degradation. Aging 2020, 12, 21129–21146. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Zhang, H.; Li, J.; Shan, Y. LINC00494 Promotes Ovarian Cancer Development and Progression by Modulating NFκB1 and FBXO32. Front. Oncol. 2021, 10, 541410. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Chen, Y.; Cao, D. Long non-coding RNA LINC00504 regulates the Warburg effect in ovarian cancer through inhibition of miR-1244. Mol. Cell. Biochem. 2019, 464, 39–50. [Google Scholar] [CrossRef]
- Tao, L.M.; Gong, Y.F.; Yang, H.M.; Pei, J.H.; Zhao, X.J.; Liu, S.S. LINC00662 promotes glycolysis and cell survival by regulating miR- 375/HIF-1α axis in ovarian cancer. J. Biol. Regul. Homeost. Agents 2020, 34, 467–477. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Li, Y.; Qiao, J.; Chen, X.; Bao, J.; Li, R.; Xing, Y. LINC00665 affects the malignant biological behavior of ovarian cancer via the miR-148b-3p/KLF5. Syst. Biol. Reprod. Med. 2022, 68, 370–383. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Lu, J.; Wang, J. LncRNA LINC00665 Promotes Ovarian Cancer Cell Proliferation and Inhibits Apoptosis via Targeting miR-181a-5p/FHDC. Appl. Biochem. Biotechnol. 2022, 194, 3819–3832. [Google Scholar] [CrossRef]
- Zhao, M.W.; Lin, C.J.; Qiu, J.P. LINC00707 promotes multidrug resistance of ovarian cancer cells by targeting the miR-382-5p/LRRK2 axis. Acta Biochim. Pol. 2023, 70, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.W.; Jiang, Y.; Wang, L.; Wang, L.; Jiang, J.; Zhang, J.R.; Mu, P. LINC00852 promotes the proliferation and invasion of ovarian cancer cells by competitively binding with miR-140-3p to regulate AGTR1 expression. BMC Cancer 2021, 21, 1004. [Google Scholar] [CrossRef]
- Lin, X.; Feng, D.; Li, P.; Lv, Y. LncRNA LINC00857 regulates the progression and glycolysis in ovarian cancer by modulating the Hippo signaling pathway. Cancer Med. 2020, 9, 8122–8132. [Google Scholar] [CrossRef]
- Xue, H.; Wu, Z.; Rao, D.; Zhuo, B.; Chen, Q. Long non-coding RNA LINC00858 aggravates the oncogenic phenotypes of ovarian cancer cells through miR-134-5p/RAD18 signaling. Arch. Gynecol. Obstet. 2020, 302, 1243–1254. [Google Scholar] [CrossRef]
- Li, P.; Huang, G. Long noncoding RNA LINC00858 promotes the progression of ovarian cancer via regulating the miR-134-5p/TRIM44 axis. J. Recept. Signal Transduct. Res. 2022, 42, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, G.; Yang, F.; He, L.; Xie, X.; Li, L.; Yang, L.; Ma, Y.; Zhang, Q.; Chen, J.; et al. Elevated LINC00909 Promotes Tumor Progression of Ovarian Cancer via Regulating the miR-23b-3p/MRC2 Axis. Oxid. Med. Cell Longev. 2021, 2021, 5574130. [Google Scholar] [CrossRef]
- Wang, L.; Ren, C.; Xu, Y.; Yang, L.; Chen, Y.; Zhu, Y. The LINC00922 aggravates ovarian cancer progression via sponging miR-361-3p. J. Ovarian Res. 2021, 14, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, M.; Fu, Q.; Wang, P.P.; Cui, Y.L. STAT1-Induced Upregulation lncRNA LINC00958 Accelerates the Epithelial Ovarian Cancer Tumorigenesis by Regulating Wnt/β-Catenin Signaling. Dis. Markers 2021, 2021, 1405045. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, P.; Sun, H.; Liu, Y. LINC01094/miR-577 axis regulates the progression of ovarian cancer. J. Ovarian Res. 2020, 13, 122. [Google Scholar] [CrossRef]
- Dong, B.; Li, C.; Xu, X.; Wang, Y.; Li, Y.; Li, X. LncRNA LINC01123 promotes malignancy of ovarian cancer by targeting hsa-miR-516b-5p/VEGFA. Genes Genom. 2024, 46, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xiao, X.; Chen, J. Silencing of the long noncoding RNA LINC01132 alleviates the oncogenicity of epithelial ovarian cancer by regulating the microRNA-431-5p/SOX9 axis. Int. J. Mol. Med. 2021, 48, 151. [Google Scholar] [CrossRef]
- Liu, S.; Xi, X. LINC01133 contribute to epithelial ovarian cancer metastasis by regulating miR-495-3p/TPD52 axis. Biochem. Biophys. Res. Commun. 2020, 533, 1088–1094. [Google Scholar] [CrossRef]
- Liu, W.; Tan, S.; Bai, X.; Ma, S.; Chen, X. Long non-coding RNA LINC01215 promotes epithelial-mesenchymal transition and lymph node metastasis in epithelial ovarian cancer through RUNX3 promoter methylation. Transl. Oncol. 2021, 14, 101135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Zhang, Y.; Luo, C.; Zhu, T.; Zhang, R.; Yao, R. LINC01342 promotes the progression of ovarian cancer by absorbing microRNA-30c-2-3p to upregulate HIF3A. J. Cell Physiol. 2020, 235, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhai, Y.; Chen, Y. GATA1-induced upregulation of LINC01503 promotes carboplatin resistance in ovarian carcinoma by upregulating PD-L1 via sponging miR-766-5p. J. Ovarian Res. 2021, 14, 108. [Google Scholar] [CrossRef]
- Coan, M.; Toso, M.; Cesaratto, L.; Rigo, I.; Borgna, S.; Dalla Pietà, A.; Zandonà, L.; Iuri, L.; Zucchetto, A.; Piazza, C.; et al. LINC01605 Is a Novel Target of Mutant p53 in Breast and Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 13736. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Yang, L.; Zhang, J. Long Non-coding RNA LINC01969 Promotes Ovarian Cancer by Regulating the miR-144-5p/LARP1 Axis as a Competing Endogenous, R.N.A. Front. Cell Dev. Biol. 2021, 8, 625730. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Sun, D.; Li, Y. Novel long noncoding RNA LINC02323 promotes cell growth and migration of ovarian cancer via TGF-β receptor 1 by miR-1343-3p. J. Clin. Lab. Anal. 2021, 35, e23651. [Google Scholar] [CrossRef]
- Maleki, P.; Sheida, S.V.; Mowla, S.J.; Soleimani, V.; Taheri, M.; Raheb, J. LINK-A long non-coding RNA and VEGF RNA expression in epithelial ovarian cancer patients. Hum. Antibodies 2020, 28, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cheng, Y.; He, Y.; Cong, S.; Sun, L.; Wu, D.; Wu, H.; Zhang, G. LNC00115 Mediates Cisplatin Resistance by Regulating the miR-7/ERK Signalling Pathway in Ovarian Cancer. Cancer Manag. Res. 2021, 13, 3817–3826. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, M.; Deng, G.; Chen, L.; Sun, L.; Zhang, Y.; Luo, C.; Tang, J. lncRNA LOC102724169 plus cisplatin exhibit the synergistic anti-tumor effect in ovarian cancer with chronic stress. Mol. Ther. Nucleic Acids 2021, 24, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Yim, G.W.; Lee, D.W.; Kim, J.I.; Kim, Y.T. Long Non-coding RNA LOC285194 Promotes Epithelial Ovarian Cancer Progression via the Apoptosis Signaling Pathway. In Vivo 2022, 36, 121–131. [Google Scholar] [CrossRef]
- Filippov-Levy, N.; Reich, R.; Davidson, B. The Biological and Clinical Role of the Long Non-Coding RNA LOC642852 in Ovarian Carcinoma. Int. J. Mol. Sci. 2020, 21, 5237. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Y.; Yu, X.; Nan, Y.; Liu, S.; Li, B.; Cui, Z.; Liu, Z. Long noncoding RNA LOC646029 functions as a ceRNA to suppress ovarian cancer progression through the miR-627-3p/SPRED1 axis. Front. Med. 2023, 17, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Xu, Y.H.; Shen, C.C.; Qin, Z.L.; Zhou, H.B. Non-coding RNA LOXL1-AS1 exhibits oncogenic activity in ovarian cancer via regulation of miR-18b-5p/VMA21 axis. Biomed. Pharmacother. 2020, 125, 109568. [Google Scholar] [CrossRef]
- Liu, H.Z.; Liu, G.Y.; Pang, W.W.; Zhang, H.; Zeng, Z.J.; Wang, H.J. LncRNA LUCAT1 promotes proliferation of ovarian cancer cells by regulating miR-199a-5p expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1682–1687. [Google Scholar] [CrossRef]
- Bai, Y.; Ren, C.; Wang, B.; Xue, J.; Li, F.; Liu, J.; Yang, L. LncRNA MAFG-AS1 promotes the malignant phenotype of ovarian cancer by upregulating NFKB1-dependent IGF1. Cancer Gene Ther. 2021, 29, 277–291. [Google Scholar] [CrossRef]
- Mao, T.L.; Fan, M.H.; Dlamini, N.; Liu, C.L. LncRNA MALAT1 Facilitates Ovarian Cancer Progression through Promoting Chemoresistance and Invasiveness in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 10201. [Google Scholar] [CrossRef]
- Pei, C.; Gong, X.; Zhang, Y. LncRNA MALAT-1 promotes growth and metastasis of epithelial ovarian cancer via sponging microrna-22. Am. J. Transl. Res. 2020, 12, 6977–6987. [Google Scholar] [PubMed]
- Zhu, Y.; Yang, L.; Wang, J.; Li, Y.; Chen, Y. SP1-induced lncRNA MCF2L-AS1 promotes cisplatin resistance in ovarian cancer by regulating IGF2BP1/IGF2/MEK/ERK axis. J. Gynecol. Oncol. 2022, 33, e75. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, A.; Song, T.; Gao, F.; Sun, H.; Kong, X. lncRNA MIAT Regulates Cell Growth, Migration, and Invasion Through Sponging miR-150-5p in Ovarian Cancer. Cancer Biother. Radiopharm. 2020, 35, 650–660. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, L.; Han, X.C.; Ma, H.Y.; Zhang, N.; Zhe, L. LncRNA MIF-AS1 aggravates the progression of ovarian cancer by sponging miRNA-31-5p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Huang, H.; Qi, X.; Bian, C.; Cheng, M.; Liu, L.; Xue, L.; Zhao, X.; Yi, T.; Quan, Y. Hypoxia-Induced LncRNA-MIR210HG Promotes Cancer Progression By Inhibiting HIF-1α Degradation in Ovarian Cancer. Front. Oncol. 2021, 11, 701488. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, A.; Gao, M.; Duan, X.; Li, Z. LncRNA MIR4435-2HG triggers ovarian cancer progression by regulating miR-128-3p/CKD14 axis. Cancer Cell Int. 2020, 20, 145. [Google Scholar] [CrossRef]
- Wu, A.; Liu, J.; Zhang, X.; Niu, C.; Shu, G.; Yin, G. Comprehensive network analysis of dysregulated genes revealed MNX1-AS1/hsa-miR-4697-3p/HOXB13 axis in ovarian cancer chemotherapy response. Cancer Sci. 2022, 113, 2627–2641. [Google Scholar] [CrossRef]
- Shen, Y.; Lv, M.; Fang, Y.; Lu, J.; Wu, Y. LncRNA MNX1-AS1 promotes ovarian cancer process via targeting the miR-744-5p/SOX12 axis. J. Ovarian Res. 2021, 14, 161. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, D.; Chen, X.; Li, L.; Yu, J. miR-152-mediated MKK7 downregulation is attenuated by MYCNOS in ovarian adenocarcinoma. Oncol. Lett. 2021, 22, 841. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Q.; Wang, J.; Chen, S.; Chen, L. Long non-coding RNA MYU promotes ovarian cancer cell proliferation by sponging miR-6827-5p and upregulating HMGA1. Pathol. Oncol. Res. 2023, 29, 1610870. [Google Scholar] [CrossRef]
- Chang, H.; Li, B.; Zhang, X.; Meng, X. NCK1-AS1 promotes NCK1 expression to facilitate tumorigenesis and chemo-resistance in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 522, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wei, Q.; Jiang, X.; Chen, N.; Zuo, X.; Zhao, H.; Liu, Y.; Liu, X.; Xie, L.; Yang, Y.; et al. CSTF3 contributes to platinum resistance in ovarian cancer through alternative polyadenylation of lncRNA NEAT1 and generating the short isoform NEAT1_1. Cell Death Dis. 2024, 15, 432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Wu, Y.; Zhao, Y.; Hu, X.; Sun, C. The long non-coding RNA NEAT1 promotes the progression of human ovarian cancer by targeting miR-214-3p and regulating angiogenesis. J. Ovarian Res. 2023, 16, 219. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, L.; Wang, X. NEAT1 Knockdown Suppresses the Cisplatin Resistance in Ovarian Cancer by Regulating miR-770-5p/PARP1 Axis. Cancer Manag. Res. 2020, 12, 7277–7289. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, K.; Yang, L. LncRNA NEAT1 promotes proliferation of ovarian cancer cells and angiogenesis of co-incubated human umbilical vein endothelial cells by regulating FGF9 through sponging miR-365: An experimental study. Medicine 2021, 100, e23423. [Google Scholar] [CrossRef]
- Xu, H.; Sun, X.; Huang, Y.; Si, Q.; Li, M. Long non-coding RNA NEAT1 modifies cell proliferation, colony formation, apoptosis, migration and invasion via the miR-4500/BZW1 axis in ovarian cancer. Mol. Med. Rep. 2020, 22, 3347–3357. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Y. Long non-coding RNA NEAT1 facilitates the growth, migration, and invasion of ovarian cancer cells via the let-7 g/MEST/ATGL axis. Cancer Cell Int. 2021, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhang, L.; Yang, H.; Luo, K.; Qing, C. Long non-coding RNA NEAT1 promotes ovarian cancer cell invasion and migration by interacting with miR-1321 and regulating tight junction protein 3 expression. Mol. Med. Rep. 2020, 22, 3429–3439, Erratum in: Mol. Med. Rep. 2024, 29, 91. https://doi.org/10.3892/mmr.2024.13215. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, L.X.; Sun, D.M.; Yao, H.; Han, D.X. Regulatory mechanism of lncRNA NORAD on proliferation and invasion of ovarian cancer cells through miR-199a-3p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1672–1681. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, J.; Yang, Y. Long non-coding RNA NRSN2-AS1 facilitates tumorigenesis and progression of ovarian cancer via miR-744-5p/PRKX axis. Biol. Reprod. 2022, 106, 526–539. [Google Scholar] [CrossRef]
- Wu, Y.B.; Li, S.Y.; Liu, J.Y.; Xue, J.J.; Xu, J.F.; Chen, T.; Cao, T.Y.; Zhou, H.; Wu, T.T.; Dong, C.L.; et al. Long non-coding RNA NRSN2-AS1 promotes ovarian cancer progression through targeting PTK2/β-catenin pathway. Cell Death Dis. 2023, 14, 696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zhang, X.; Zhao, X. Long non-coding RNA OIP5-AS1 suppresses microRNA-92a to augment proliferation and metastasis of ovarian cancer cells through upregulating ITGA6. J. Ovarian Res. 2022, 15, 25. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Jiang, X.X.; Tian, H.N.; Guo, H.L.; Guo, H.; Guo, Y. Long non-coding RNA OIP5-AS1 plays an oncogenic role in ovarian cancer through targeting miR-324-3p/NFIB axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7266–7275. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Liu, D.; Liu, L. OIP5-AS1/miR-137/ZNF217 Axis Promotes Malignant Behaviors in Epithelial Ovarian Cancer. Cancer Manag. Res. 2020, 12, 6707–6717. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ye, Z.; Jiang, Y.; Yu, W.; Fang, Q. LncRNA OIP5-AS1 upregulates snail expression by sponging miR-34a to promote ovarian carcinoma cell invasion and migration. Biol. Res. 2020, 53, 49. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Wang, X.; Liu, Y.; Song, X. Long non-coding RNA OIP5-AS1 facilitates the progression of ovarian cancer via the miR-128-3p/CCNG1 axis. Mol. Med. Rep. 2021, 23, 388. [Google Scholar] [CrossRef]
- Guo, F.; Du, J.; Liu, L.; Gou, Y.; Zhang, M.; Sun, W.; Yu, H.; Fu, X. lncRNA OR3A4 Promotes the Proliferation and Metastasis of Ovarian Cancer Through KLF6 Pathway. Front. Pharmacol. 2021, 12, 727876. [Google Scholar] [CrossRef]
- Wang, J.; Han, Y.; Zhang, T.; Li, J.; Xu, B. LncRNA PART1Regulates Ovarian Carcinoma Development via the miR-150-5p/MYB Axis. Front. Biosci. 2023, 28, 270. [Google Scholar] [CrossRef]
- Li, H.; Lei, Y.; Li, S.; Li, F.; Lei, J. LncRNA PART1 Stimulates the Development of Ovarian Cancer by Up-regulating RACGAP1 and RRM2. Reprod. Sci. 2022, 29, 2224–2235. [Google Scholar] [CrossRef]
- Li, B.; Lou, G.; Zhang, J.; Cao, N.; Yu, X. Repression of lncRNA PART1 attenuates ovarian cancer cell viability, migration and invasion through the miR-503-5p/FOXK1 axis. BMC Cancer 2022, 22, 124. [Google Scholar] [CrossRef]
- Liu, Y.; Zong, Z.H.; Guan, X.; Wang, L.L.; Zhao, Y. The role of long non-coding RNA PCA3 in epithelial ovarian carcinoma tumorigenesis and progression. Gene 2017, 633, 42–47. [Google Scholar] [PubMed]
- He, Y.; Wei, L.; Zhang, S.; Liu, H.; Fang, F.; Li, Y. LncRNA PLAC2 Positively Regulates CDK2 to Promote Ovarian Carcinoma Cell Proliferation. Cancer Manag. Res. 2020, 12, 5713–5720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hong, L. lncRNA-PRLB Confers Paclitaxel Resistance of Ovarian Cancer Cells by Regulating RSF1/NF-κB Signaling Pathway. Cancer Biother. Radiopharm. 2021, 36, 202–210. [Google Scholar] [CrossRef]
- Qi, X.; Chen, D.; Yu, W.; Wang, L.; Liu, L.; Tao, X. Long non-coding RNA PRNCR1 promotes ovarian cancer cell proliferation, migration and invasion by targeting the miR-653-5p/ELF2 axis. Mol. Cell Biochem. 2022, 477, 1463–1475. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Zhang, S.; Qi, Y.A.; Wang, M. PRPF6 promotes metastasis and paclitaxel resistance of ovarian cancer via SNHG16/CEBPB/GATA3 axis. Oncol. Res. 2022, 29, 275–289. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, H.; Duan, X.; Liu, H.; Zhao, X.; Fan, S.; Wang, Y.; Yao, T. LncRNA PSMA3-AS1 promotes cell proliferation, migration, and invasion in ovarian cancer by activating the PI3K/Akt pathway via the miR-378a-3p/GALNT3 axis. Environ. Toxicol. 2021, 36, 2562–2577. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yu, M.; Yang, R.; Zhang, L.; Zhang, L.; Zhu, D.; Luo, H.; Hong, Y.; Yu, T.; Sun, J.; et al. A PTAL-miR-101-FN1 Axis Promotes EMT and Invasion-Metastasis in Serous Ovarian Cancer. Mol. Ther. Oncolytics 2019, 16, 53–62. [Google Scholar] [CrossRef]
- Zhang, R.; He, T.; Shi, H.; Yuan, C.; Wei, F.; Liu, Z.; Wang, W. Disregulations of PURPL and MiR-338-3p Could Serve As Prognosis Biomarkers for Epithelial Ovarian Cancer. J. Cancer 2021, 12, 5674–5680. [Google Scholar] [CrossRef]
- Dong, L.; Wang, H.; Gao, Y.; Wang, S.; Wang, W. Long non-coding RNA PVT1 promotes the proliferation, migration and EMT process of ovarian cancer cells by regulating CTGF. Oncol. Lett. 2022, 25, 71. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, W.; Han, X.; Jin, Z. LncRNA PVT1 promotes the progression of ovarian cancer by activating TGF-β pathway via miR-148a-3p/AGO1 axis. J. Cell Mol. Med. 2021, 25, 8229–8243. [Google Scholar] [CrossRef]
- Li, M.; Chi, C.; Zhou, L.; Chen, Y.; Tang, X. Circular PVT1 regulates cell proliferation and invasion via miR-149-5p/FOXM1 axis in ovarian cancer. J. Cancer 2021, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Hou, M.; Yuan, J.; Yang, L.; Zeng, X.; Xi, M.; Chen, J. LncRNA PVT1 epigenetically stabilizes and post-transcriptionally regulates FOXM1 by acting as a microRNA sponge and thus promotes malignant behaviors of ovarian cancer cells. Am. J. Transl. Res. 2020, 12, 2860–2874. [Google Scholar] [PubMed]
- Qu, C.; Dai, C.; Guo, Y.; Qin, R.; Liu, J. Long non-coding RNA PVT1-mediated miR-543/SERPINI1 axis plays a key role in the regulatory mechanism of ovarian cancer. Biosci. Rep. 2020, 40, BSR20200800. [Google Scholar] [CrossRef]
- Tabury, K.; Monavarian, M.; Listik, E.; Shelton, A.K.; Choi, A.S.; Quintens, R.; Arend, R.C.; Hempel, N.; Miller, C.R.; Györrfy, B.; et al. PVT1 is a stress-responsive lncRNA that drives ovarian cancer metastasis and chemoresistance. Life Sci. Alliance 2022, 5, e202201370. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, J.; Liu, W.; Su, X.; Zhao, B.; Wang, X.; He, X. Long non-coding RNA RAD51-AS1 promotes the tumorigenesis of ovarian cancer by elevating EIF5A2 expression. J. Cancer Res. Clin. Oncol. 2024, 150, 179. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, Y.; Zheng, T.; Jing, Y.; Cao, R.; Wu, M.; Fan, D.; Tao, Y.; Zhao, M. Application of long non-coding RNA RBAT1 in improving diagnosis and prognosis of ovarian carcinoma. Anticancer. Drugs 2023, 34, 9–14. [Google Scholar] [CrossRef]
- Cui, S. METTL3-mediated m6A modification of lnc RNA RHPN1-AS1 enhances cisplatin resistance in ovarian cancer by activating PI3K/AKT pathway. J. Clin. Lab. Anal. 2022, 36, e24761. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, T.; Zhang, X.; Zuo, D.; Liu, C. lncRNA RHPN1-AS1 Promotes Ovarian Cancer Growth and Invasiveness Through Inhibiting miR-1299. Onco Targets Ther. 2020, 13, 5337–5344. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, T.; Ji, J.; Zhao, F.; Li, C.; Han, X. RHPN1-AS1 promotes cell proliferation and migration via miR-665/Akt3 in ovarian cancer. Cancer Gene Ther. 2021, 28, 33–41. [Google Scholar] [CrossRef]
- Wang, J.; Ding, W.; Xu, Y.; Tao, E.; Mo, M.; Xu, W.; Cai, X.; Chen, X.; Yuan, J.; Wu, X. Long non-coding RNA RHPN1-AS1 promotes tumorigenesis and metastasis of ovarian cancer by acting as a ceRNA against miR-596 and upregulating LETM1. Aging 2020, 12, 4558–4572. [Google Scholar] [CrossRef]
- Cui, S.; Li, F. RHPN1-AS1 promotes ovarian carcinogenesis by sponging miR-6884-5p thus releasing TOP2A mRNA. Oncol. Rep. 2021, 46, 221. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Li, C. RHPN1-AS1 promotes ovarian carcinogenesis by sponging miR-485-5p and releasing TPX2 mRNA. Oncol. Rep. 2021, 45, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, S.; Guo, L.; Huang, P.; Xi, J.; Feng, J.; Li, Q.; Li, Y.; Xiao, X.; Yan, R.; et al. Long Noncoding RNA RMRP Contributes to Paclitaxel Sensitivity of Ovarian Cancer by Regulating miR-580-3p/MICU1 Signaling. J. Oncol. 2022, 2022, 8301941. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Bao, A.; Hua, X.; Cao, J.; Ding, Y. RP5-1148A21.3 (lncRP5) exerts oncogenic function in human ovarian carcinoma. Acta Biochim. Biophys. Sin. 2022, 54, 209–219. [Google Scholar] [CrossRef]
- Luan, A.A.; Hou, L.L.; Zhang, F.Y. Silencing of SBF2-AS1 inhibits cell growth and invasion by sponging microRNA-338-3p in serous ovarian carcinoma. Kaohsiung J. Med. Sci. 2022, 38, 302–311. [Google Scholar] [CrossRef]
- Song, R.; Liu, Z.; Lu, L.; Liu, F.; Zhang, B. Long Noncoding RNA SCAMP1 Targets miR-137/CXCL12 Axis to Boost Cell Invasion and Angiogenesis in Ovarian Cancer. DNA Cell Biol. 2020, 39, 1041–1050. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, A.; Zhang, Z. LncRNA SDHAP1 confers paclitaxel resistance of ovarian cancer by regulating EIF4G2 expression via miR-4465. J. Biochem. 2020, 168, 171–181. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, B.; Yan, Y.; Bai, S.; Kang, H.; Zhang, J.; Ma, W.; Gao, Y.; Hui, B.; Li, R.; et al. Long non-coding RNA SNHG1 stimulates ovarian cancer progression by modulating expression of miR-454 and ZEB1. Mol. Oncol. 2021, 15, 1584–1596. [Google Scholar] [CrossRef]
- Abildgaard, C.; do Canto, L.M.; Rainho, C.A.; Marchi, F.A.; Calanca, N.; Waldstrøm, M.; Steffensen, K.D.; Rogatto, S.R. The Long Non-Coding RNA SNHG12 as a Mediator of Carboplatin Resistance in Ovarian Cancer via Epigenetic Mechanisms. Cancers 2022, 14, 1664. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Jiang, J.; Qiao, Z. The lncRNA SNHG15/miR-18a-5p axis promotes cell proliferation in ovarian cancer through activating Akt/mTOR signaling pathway. J. Cell Biochem. 2020, 121, 4699–4710. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, M.; Yuan, X.; Jiao, R.; Zhu, D.; Huang, W.; Deng, W.; Liu, Y. lncRNA SNHG15 Promotes Ovarian Cancer Progression through Regulated CDK6 via Sponging miR-370-3p. Biomed. Res. Int. 2021, 2021, 9394563. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Geng, S.; Wang, Y.; Fang, Q.; Xin, Y.; Li, Y. Tumour-derived exosome SNHG17 induced by oestrogen contributes to ovarian cancer progression via the CCL13-CCR2-M2 macrophage axis. J. Cell Mol. Med. 2024, 28, e18315. [Google Scholar] [CrossRef]
- Pan, X.; Guo, Z.; Chen, Y.; Zheng, S.; Peng, M.; Yang, Y.; Wang, Z. STAT3-Induced lncRNA SNHG17 Exerts Oncogenic Effects on Ovarian Cancer through Regulating CDK6. Mol. Ther. Nucleic. Acids. 2020, 22, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, S.; Li, W.; Hu, H.; Zou, G. Silencing of lncRNA SNHG17 inhibits the tumorigenesis of epithelial ovarian cancer through regulation of miR-485-5p/AKT1 axis. Biochem. Biophys. Res. Commun. 2022, 637, 117–126. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, Y.J. LncRNA SNHG20 promotes migration and invasion of ovarian cancer via modulating the microRNA-148a/ROCK1 axis. J. Ovarian Res. 2021, 14, 168. [Google Scholar] [CrossRef]
- Xing, X.; An, M.; Chen, T. LncRNA SNHG20 promotes cell proliferation and invasion by suppressing miR-217 in ovarian cancer. Genes. Genom. 2021, 43, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Z.; Li, H.; Lu, J.; Qin, Q. Long non-coding RNA SNHG20 promotes ovarian cancer development by targeting microRNA-338-3p to regulate MCL1 expression. Oncol. Lett. 2021, 21, 130. [Google Scholar] [CrossRef]
- Guan, N.; Zheng, H.; Wu, X.; Xie, L.; Tong, X. SP1-Regulated Non-Coding RNA SNHG22 Promotes Ovarian Cancer Growth and Glycolysis. Cancer Manag. Res. 2021, 13, 7299–7309. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, B.; Liu, M.; Qiao, H.; Zhang, S.; Qiu, J.; Ying, X. Long non-coding RNA SNHG25 promotes epithelial ovarian cancer progression by up-regulating COMP. J. Cancer 2021, 12, 1660–1668. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Wang, X.; Zhang, Y.; Huang, X.; Wu, H. lncRNA SNHG3 acts as oncogene in ovarian cancer through miR-139-5p and Notch1. Oncol. Lett. 2021, 21, 122. [Google Scholar] [CrossRef]
- Liu, E.L.; Zhou, Y.X.; Li, J.; Zhang, D.H.; Liang, F. Long-Chain Non-Coding RNA SNHG3 Promotes the Growth of Ovarian Cancer Cells by Targeting miR-339-5p/TRPC3 Axis. Onco Targets Ther. 2020, 13, 10959–10971. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, S.; Lv, Z.X.; Zhao, X.J. Promoting action of long non-coding RNA small nucleolar RNA host gene 4 in ovarian cancer. Acta Biochim. Pol. 2023, 70, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Huang, P.; Li, Q. Long noncoding RNA SNHG6 promotes the malignant phenotypes of ovarian cancer cells via miR-543/YAP1 pathway. Heliyon 2023, 9, e16291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, R.; Ye, Y. Long non-coding RNA (LncRNA) SNHG7/ Eukaryotic translation initiation factor 4 gamma 2 (EIF4G2) involves in the malignant events of ovarian cancer cells with paclitaxel resistant. Bioengineered 2021, 12, 10541–10552. [Google Scholar] [CrossRef]
- Bai, Z.; Wu, Y.; Bai, S.; Yan, Y.; Kang, H.; Ma, W.; Zhang, J.; Gao, Y.; Hui, B.; Ma, H.; et al. Long non-coding RNA SNGH7 Is activated by SP1 and exerts oncogenic properties by interacting with EZH2 in ovarian cancer. J. Cell Mol. Med. 2020, 24, 7479–7489. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Lu, T.; Liu, X.; Yin, W.; Zhang, H. LncRNA SNHG8 induces ovarian carcinoma cells cellular process and stemness through Wnt/β-catenin pathway. Cancer Biomark. 2020, 28, 459–471. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Shen, X.; Li, M.; Yue, Y.; Cheng, X.; Lu, W.; Wang, X.; Xie, X. LncRNA SPOCD1-AS from ovarian cancer extracellular vesicles remodels mesothelial cells to promote peritoneal metastasis via interacting with G3BP1. J. Exp. Clin. Cancer Res. 2021, 40, 101. [Google Scholar] [CrossRef]
- Kim, L.K.; Park, S.A.; Yang, Y.; Kim, Y.T.; Heo, T.H.; Kim, H.J. LncRNA SRA mediates cell migration, invasion, and progression of ovarian cancer via NOTCH signaling and epithelial-mesenchymal transition. Biosci. Rep. 2021, 41, BSR20210565. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, X.J.; Tang, X.Y.; Zheng, T.T.; Zhang, X.Y.; Hua, K.Q. Long noncoding RNA TC0101441 induces epithelial-mesenchymal transition in epithelial ovarian cancer metastasis by downregulating KiSS1. Int. J. Cancer 2020, 146, 2588–2598. [Google Scholar] [CrossRef]
- Ge, J.; Han, T.; Shan, L.; Na, J.; Li, Y.; Wang, J. Long non-coding RNA THOR promotes ovarian Cancer cells progression via IL-6/STAT3 pathway. J. Ovarian Res. 2020, 13, 72. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, X.; Huang, K.; Xia, B.; Bi, S.; Kong, Y. THUMPD3-AS1 inhibits ovarian cancer cell apoptosis through the miR-320d/ARF1 axis. FASEB J. 2024, 38, e23772. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lin, Y.B.; Li, L.; Liu, J. LncRNA TLR8-AS1 promotes metastasis and chemoresistance of ovarian cancer through enhancing TLR8 mRNA stability. Biochem. Biophys. Res. Commun. 2020, 526, 857–864. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, F.; Zheng, G. LncRNA TMPO-AS1 promotes LCN2 transcriptional activity and exerts oncogenic functions in ovarian cancer. FASEB J. 2020, 34, 11382–11394. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Cheng, H.; Tian, J.; Yang, S. Roles of a TMPO-AS1/microRNA-200c/TMEFF2 ceRNA network in the malignant behaviors and 5-FU resistance of ovarian cancer cells. Exp. Mol. Pathol. 2020, 115, 104481. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Wang, X.; Wang, P.; Wang, Z. LncRNA TONSL-AS1 regulates miR-490-3p/CDK1 to affect ovarian epithelial carcinoma cell proliferation. J. Ovarian Res. 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tan, X.; Abasi, A.; Dai, Y.; Wu, R.; Zhang, T.; Li, K.; Yan, M.; Huang, X. LncRNA TRPM2-AS promotes ovarian cancer progression and cisplatin resistance by sponging miR-138-5p to release SDC3 mRNA. Aging 2021, 13, 6832–6848. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Liang, J.; Liu, W.; Zou, Y.; Niu, F.; Li, M.; Zhang, H.; Li, C.; Fan, M.; Cui, G. The miRNA mir-582-3p suppresses ovarian cancer progression by targeting AKT/MTOR signaling via lncRNA TUG1. Bioengineered 2021, 12, 10771–10781. [Google Scholar] [CrossRef]
- Gu, L.; Li, Q.; Liu, H.; Lu, X.; Zhu, M. Long Noncoding RNA TUG1 Promotes Autophagy-Associated Paclitaxel Resistance by Sponging miR-29b-3p in Ovarian Cancer Cells. Onco Targets Ther. 2020, 13, 2007–2019. [Google Scholar] [CrossRef]
- Pei, Y.; Li, K.; Lou, X.; Wu, Y.; Dong, X.; Wang, W.; Li, N.; Zhang, D.; Cui, W. miR-1299/NOTCH3/TUG1 feedback loop contributes to the malignant proliferation of ovarian cancer. Oncol. Rep. 2020, 44, 438–448. [Google Scholar] [CrossRef]
- Zhan, F.L.; Chen, C.F.; Yao, M.Z. LncRNA TUG1 facilitates proliferation, invasion and stemness of ovarian cancer cell via miR-186-5p/ZEB1 axis. Cell Biochem. Funct. 2020, 38, 1069–1078. [Google Scholar] [CrossRef]
- Sonobe, R.; Yang, P.; Suzuki, M.M.; Shinjo, K.; Iijima, K.; Nishiyama, N.; Miyata, K.; Kataoka, K.; Kajiyama, H.; Kondo, Y. Long noncoding RNA TUG1 promotes cisplatin resistance in ovarian cancer via upregulation of DNA polymerase eta. Cancer Sci. 2024, 115, 1910–1923. [Google Scholar] [CrossRef]
- Wambecke, A.; Ahmad, M.; Morice, P.M.; Lambert, B.; Weiswald, L.B.; Vernon, M.; Vigneron, N.; Abeilard, E.; Brotin, E.; Figeac, M.; et al. The lncRNA ‘UCA1’ modulates the response to chemotherapy of ovarian cancer through direct binding to miR-27a-5p and control of UBE2N levels. Mol. Oncol. 2021, 15, 3659–3678. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, X.L.; Dang, Y.; Zhu, X.Z.; Zhang, Y.H.; Cai, B.X.; Zheng, L. Long non-coding RNA UCA1 promotes the progression of paclitaxel resistance in ovarian cancer by regulating the miR-654-5p/SIK2 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, H.; Tan, Y. UNC5B-AS1 promoted ovarian cancer progression by regulating the H3K27me on NDRG2 via EZH2. Cell Biol. Int. 2020, 44, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Yu, L.; Sun, Y.; Yao, N.; Ma, L. Long Non-Coding RNA USP2-AS1 Accelerates Cell Proliferation and Migration in Ovarian Cancer by Sponging miR-520d-3p and Up-Regulating KIAA1522. Cancer Manag. Res. 2020, 12, 10541–10550. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Sun, X.; Zhao, R.; Gao, K. Prognostic role of long non-coding RNA USP30-AS1 in ovarian cancer: Insights into immune cell infiltration in the tumor microenvironment. Aging 2023, 15, 13776–13798. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, T.; Xia, L.; Zhang, M. LncRNA WDFY3-AS2 promotes cisplatin resistance and the cancer stem cell in ovarian cancer by regulating hsa-miR-139-5p/SDC4 axis. Cancer Cell Int. 2021, 21, 284, Erratum in: Cancer Cell Int. 2023, 23, 176. doi: 10.1186/s12935-023-03029-y. [Google Scholar] [CrossRef]
- Xia, X.; Li, Z.; Li, Y.; Ye, F.; Zhou, X. LncRNA XIST promotes carboplatin resistance of ovarian cancer through activating autophagy via targeting miR-506-3p/FOXP1 axis. J. Gynecol. Oncol. 2022, 33, e81. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, N.; Duan, G. Long. non-coding RNA XIST regulates ovarian cancer progression via modulating miR-335/BCL2L2 axis. World J. Surg. Oncol. 2021, 19, 165. [Google Scholar] [CrossRef]
- Huang, K.C.; Rao, P.H.; Lau, C.C.; Heard, E.; Ng, S.K.; Brown, C.; Mok, S.C.; Berkowitz, R.S.; Ng, S.W. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol. Cancer Ther. 2002, 1, 769–776. [Google Scholar]
- Jiang, R.; Zhang, H.; Zhou, J.; Wang, J.; Xu, Y.; Zhang, H.; Gu, Y.; Fu, F.; Shen, Y.; Zhang, G.; et al. Inhibition of long non-coding RNA XIST upregulates microRNA-149-3p to repress ovarian cancer cell progression. Cell Death Dis. 2021, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Xu, P.; Liu, S.; Xu, S.; Xu, J.; Fu, Z.; Cao, J.; Lv, M.; Zhou, J.; Liu, G.; et al. Long noncoding RNA ZEB1-AS1 affects paclitaxel and cisplatin resistance by regulating MMP19 in epithelial ovarian cancer cells. Arch. Gynecol. Obstet. 2021, 303, 1271–1281. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhang, Z.; Xie, B.B.; Zhang, H.Y. Clinical significance of up-regulated lncRNA NEAT1 in the prognosis of ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3373–3377. [Google Scholar] [PubMed]
- Zuo, K.; Zhao, Y.; Zheng, Y.; Chen, D.; Liu, X.; Du, S.; Liu, Q. Long non-coding RNA XIST promotes malignant behavior of epithelial ovarian cancer. Onco Targets Ther. 2019, 12, 7261–7267. [Google Scholar] [CrossRef]
- Wang, C.; Qi, S.; Xie, C.; Li, C.; Wang, P.; Liu, D. Upregulation of long non-coding RNA XIST has anticancer effects on epithelial ovarian cancer cells through inverse downregulation of hsa-miR-214-3p. J. Gynecol. Oncol. 2018, 29, e99. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.R.; Zhang, B.; Zhang, L.M.; Li, C.G.; Liu, C.; Zhang, H.; Huo, Y.S.; Ma, Y.C.; Tian, P.F.; et al. LncRNA H19 downregulation confers erlotinib resistance through upregulation of PKM2 and phosphorylation of AKT in EGFR-mutant lung cancers. Cancer Lett. 2020, 486, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.; Deng, X.; Luo, M.; Wang, X.; Hu, H.; Liu, C.; Zhong, M. Long noncoding RNA H19 promotes transforming growth factor-beta-induced epithelial-mesenchymal transition by acting as a competing endogenous RNA of miR-370-3p in ovarian cancer cells. OncoTargets Ther. 2018, 11, 427–440. [Google Scholar]
- Wu, Y.; Zhou, Y.; He, J.; Sun, H.; Jin, Z. Long non-coding RNA H19 mediates ovarian cancer cell cisplatin-resistance and migration during EMT. Int. J. Clin. Exp. Pathol. 2019, 12, 2506–2515. [Google Scholar]
- An, J.; Lv, W.; Zhang, Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. OncoTargets Ther. 2017, 10, 5377–5390. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; Guo, Y. Long non-coding RNA MALAT1 is upregulated and involved in cell proliferation, migration and apoptosis in ovarian cancer. Exp. Ther. Med. 2017, 13, 3055–3060. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, X.J.; Tang, X.Y.; Zheng, T.T.; Lin, Y.Y.; Hua, K.Q. Exosomal Metastasis-Associated Lung Adenocarcinoma Transcript 1 Promotes Angiogenesis and Predicts Poor Prognosis in Epithelial Ovarian Cancer. Int. J. Biol. Sci. 2018, 14, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.R.; Zhao, M.Y.; Sun, L.; Yang, B.C.; Hei, K.W.; Du, X.; Li, Y.M. Expression of IncRNA UCA1 in ovarian cancer and its clinical significance. Eur. J. Gynaecol. Oncol. 2017, 38, 191–195. [Google Scholar] [PubMed]
- Wang, F.; Zhou, J.; Xie, X.; Hu, J.; Chen, L.; Hu, Q.; Guo, H.; Yu, C. Involvement of SRPK1 in cisplatin resistance related to long non-coding RNA UCA1 in human ovarian cancer cells. Neoplasma. 2015, 62, 432–438. [Google Scholar] [CrossRef]
- Li, Z.; Niu, H.; Qin, Q.; Yang, S.; Wang, Q.; Yu, C.; Wei, Z.; Jin, Z.; Wang, X.; Yang, A.; et al. lncRNA UCA1 Mediates Resistance to Cisplatin by Regulating the miR-143/FOSL2-Signaling Pathway in Ovarian Cancer. Mol. Ther. Nucleic Acids 2019, 17, 92–101. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, Y.Y.; Ye, L.C.; Ding, J.X.; Feng, W.W.; Jin, H.Y.; Zhang, Y.; Li, Q.; Hua, K.Q. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Song, T.; Zou, Y.; Jiang, J.; Fang, L.; Li, P. HOTAIR is a potential target for the treatment of cisplatin-resistant ovarian cancer. Mol. Med. Rep. 2015, 12, 2211–2216. [Google Scholar] [CrossRef]
- Teschendorff, A.E.; Lee, S.H.; Jones, A.; Fiegl, H.; Kalwa, M.; Wagner, W.; Chindera, K.; Evans, I.; Dubeau, L.; Orjalo, A.; et al. HOTAIR and its surrogate DNA methylation signature indicate carboplatin resistance in ovarian cancer. Genome Med. 2015, 7, 108. [Google Scholar] [CrossRef]
- Wu, H.; Shang, X.; Shi, Y.; Yang, Z.; Zhao, J.; Yang, M.; Li, Y.; Xu, S. Genetic variants of lncRNA HOTAIR and risk of epithelial ovarian cancer among Chinese women. Oncotarget 2016, 7, 41047–41052. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumor models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- Croft, P.K.; Sharma, S.; Godbole, N.; Rice, G.E.; Salomon, C. Ovarian-Cancer-Associated Extracellular Vesicles: Microenvironmental Regulation and Potential Clinical Applications. Cells 2021, 10, 2272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arriaga-Canon, C.; Contreras-Espinosa, L.; Aguilar-Villanueva, S.; Bargalló-Rocha, E.; García-Gordillo, J.A.; Cabrera-Galeana, P.; Castro-Hernández, C.; Jiménez-Trejo, F.; Herrera, L.A. The Clinical Utility of lncRNAs and Their Application as Molecular Biomarkers in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7426. [Google Scholar] [CrossRef] [PubMed]
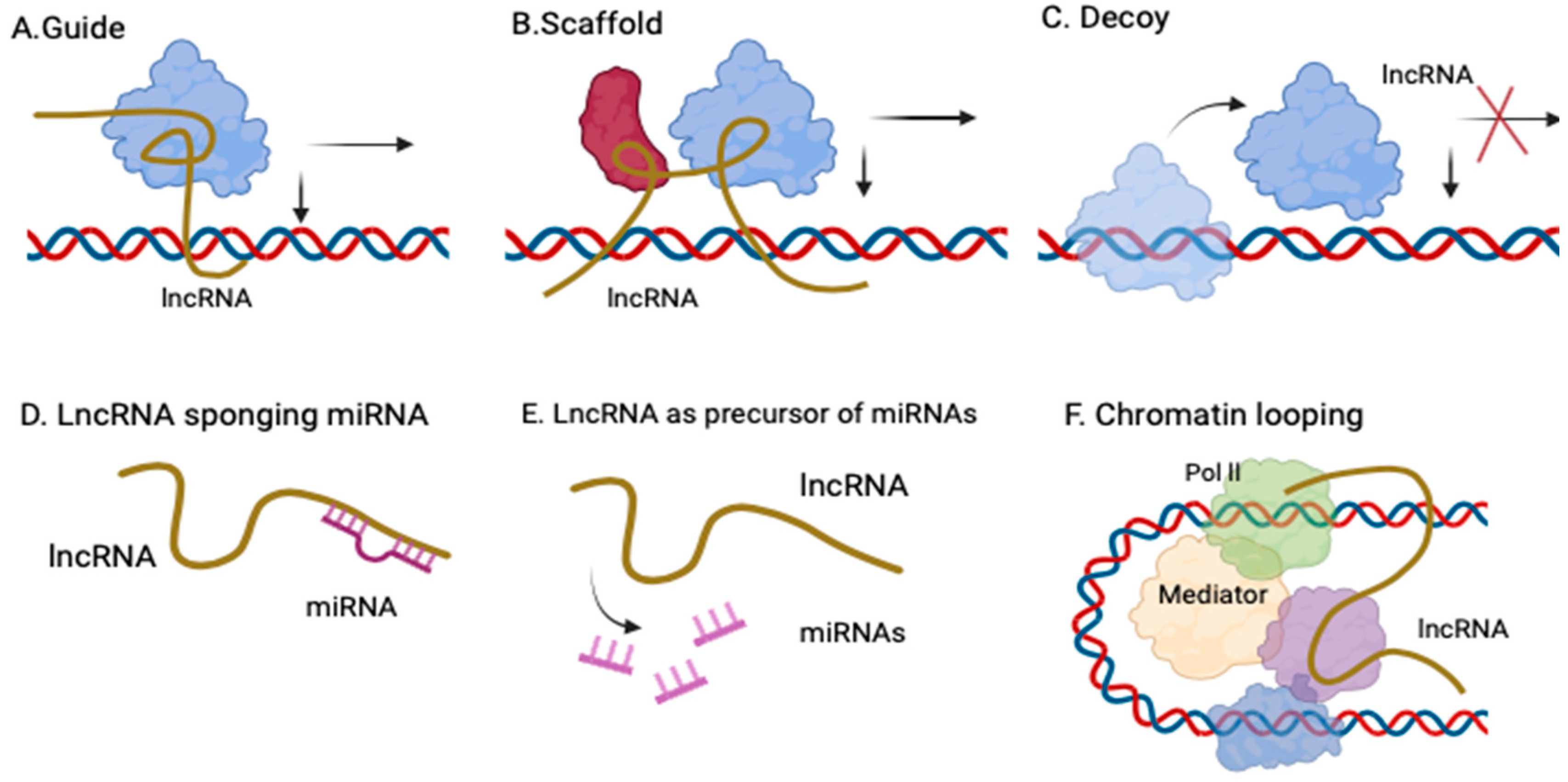
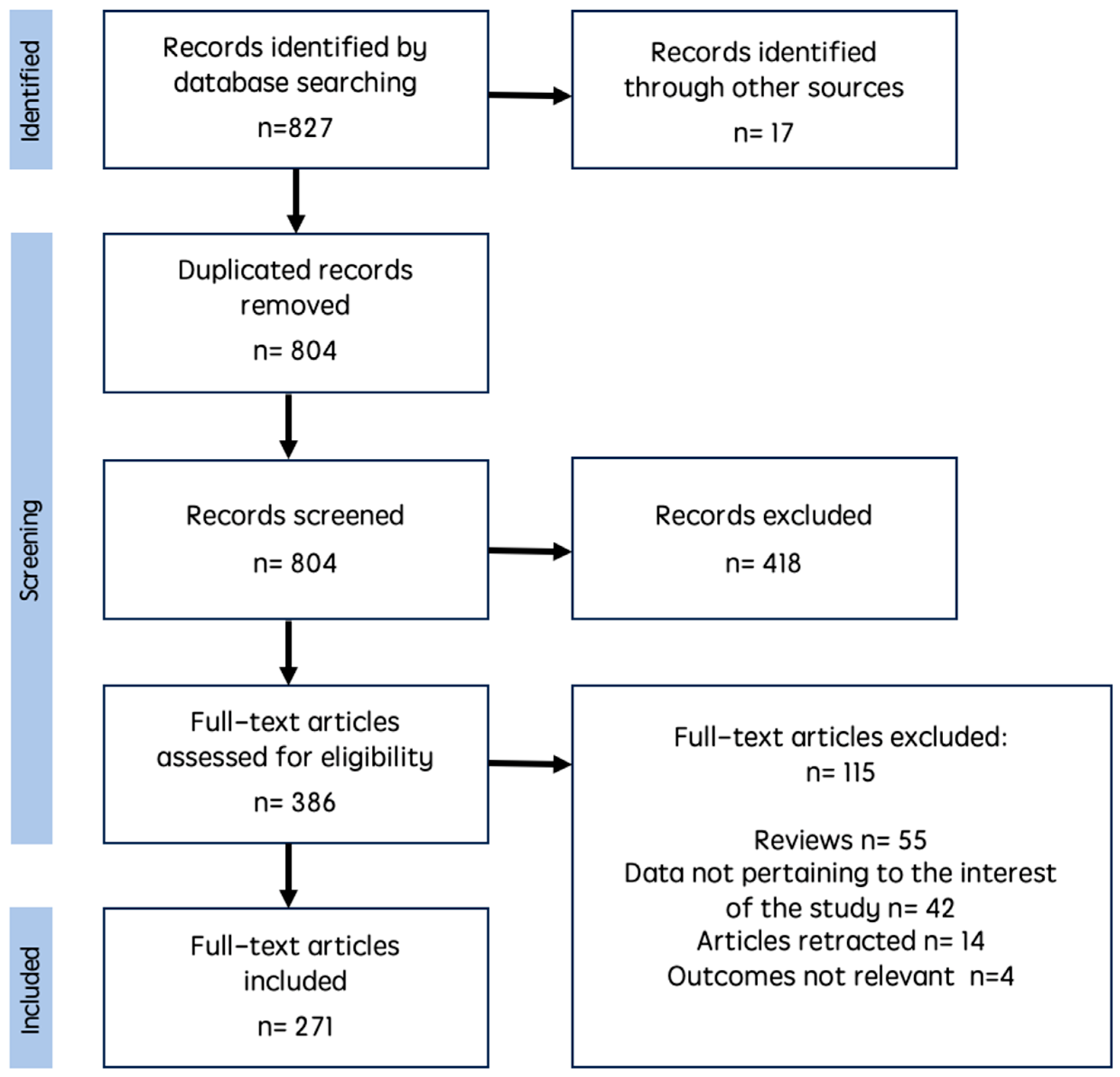
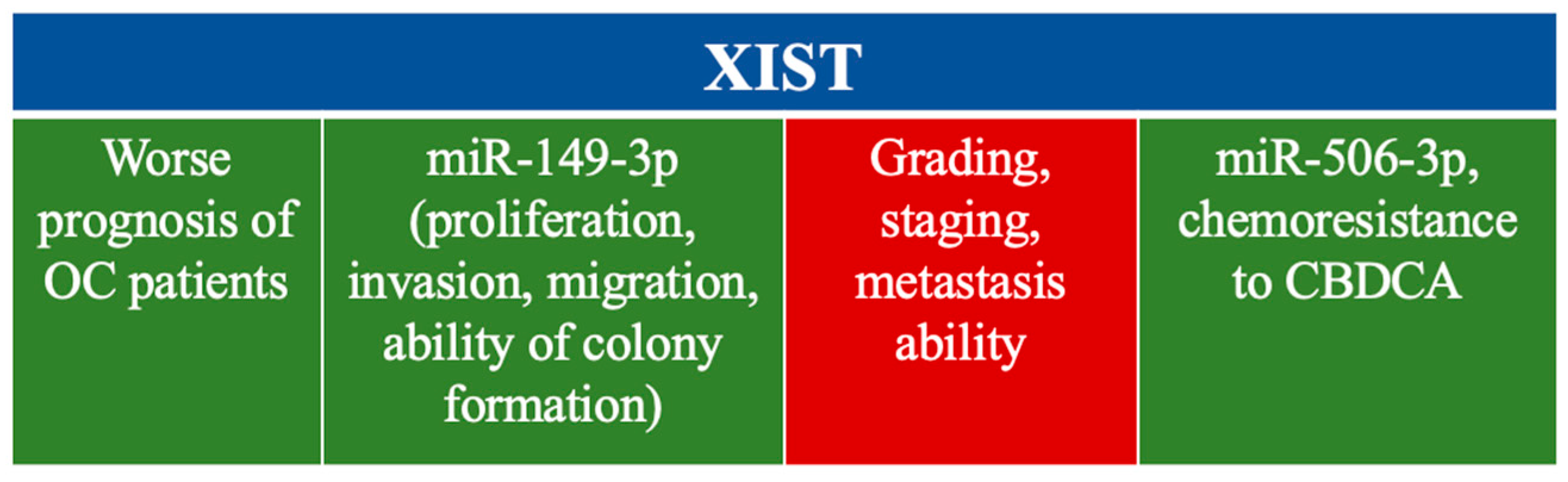
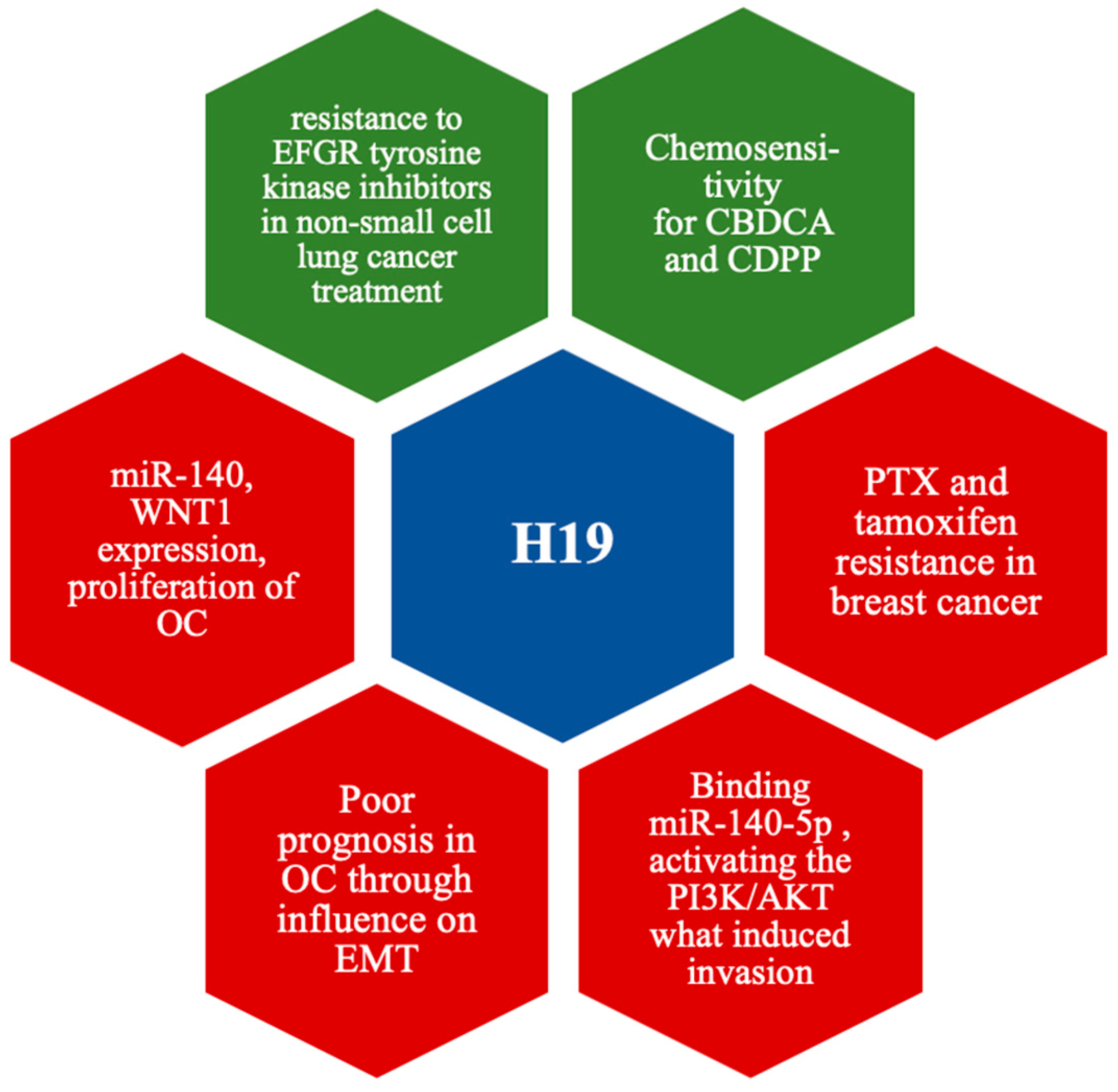
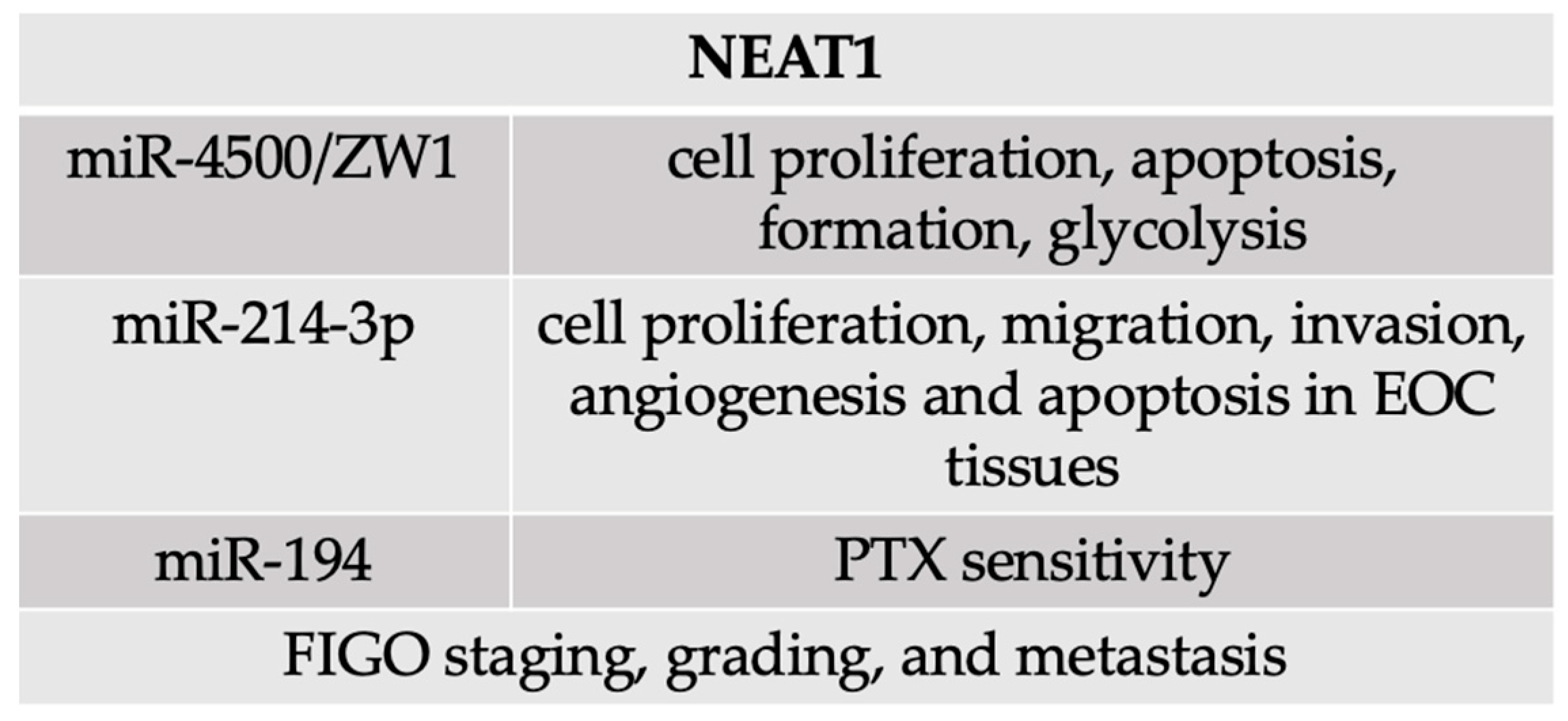
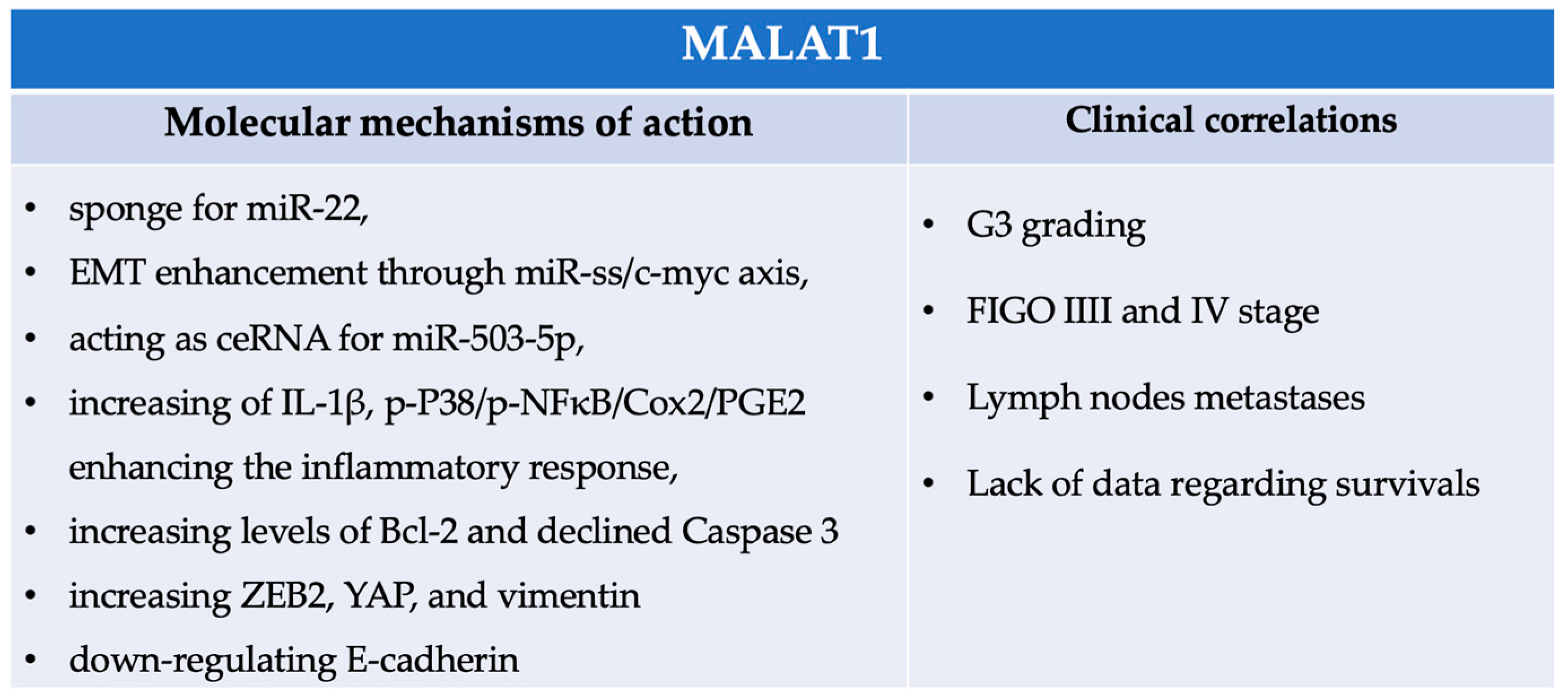


| Type of Ovarian Cancer | LncRNA | Target | Functions | Reference |
|---|---|---|---|---|
| EOC | AOC4P | FIGO stage, migration, invasion (+) | Lin et al. [33] | |
| EOC | ASMTL-AS1 | miR-1228-3p | FIGO stage, ascites cytology, lymph node (+) proliferation, migration, invasion (−) | Xu et al. [34] |
| EOC | CTBP1-AS2 | miR-216a/PTEN | poor survival (+) | Cui et al. [35] |
| EOC | EPB41L4A-AS2 | miR-103a | cell proliferation, migration, invasion (+) | Sun et al. [36] |
| EOC | FAM225B | DDX17/PDIA4 | progression, migration, invasion, apoptosis (+) | Yao et al. [37] |
| EOC HGSOC | FGD5-AS1 | miR-107/RBBP6 | proliferation, angiogenesis (+) | Zhang et al. [38] |
| EOC OCCC | GAS5 | miR-96-5p/PTEN | proliferation, invasion | Dong et al. [39] |
| miR-23a/WT1 | migration, proliferation, poor prognosis (+) apoptosis (−) | Zhou et al. [40] | ||
| miR-31-5p/ARID1A | viability, invasion (+) | Zhang et al. [41] | ||
| PI3K/AKT/mTOR pathway, hnRNPK | low survival, migration, invasion, proliferation (+) apoptosis (−) | |||
| EOC | GAS8-AS1 | viability, migration, invasion (−) | Fang et al. [42] | |
| EOC, HGSOC | HAND2-AS1 | viability, migration (−) | Gokulnath et al. [43] | |
| miR106a/PTEN | CDDP-resistance (−) | Li et al. [44] | ||
| EOC | HCG11 | miR-1270/PTEN; AKT/mTOR pathway | proliferation, migration, EMT (−) | Chen et al. [45] |
| EOC | HOTAIRM1 | miR-106a-5p, ARHGAP24 | proliferation, invasion (+) apoptosis (−) | Chao et al. [46] |
| EOC | LEMD1-AS1 | OS, FIGO stage (−) | Yang et al. [47] | |
| miR-183-5p/TP53 | proliferation, migration, invasion (+) | Guo et al. [48] | ||
| EOC | LIFR-AS1 | tumor size, grade, metastasis, poor prognosis (+) | Liu et al. [49] | |
| EOC | LINC-PINT | miR-374a-5p | apoptosis (−) proliferation, migration, invasion, EMT (+) | Hao et al. [50] |
| EOC, HGSOC | LINC00629 | LINC00629/c-Myc | proliferation, poor survival (+) | Liu et al. [51] |
| EOC | LINC00936 | miR-221-3p | proliferation, migration, invasion, angiogenesis | Shu et al. [52] |
| EOC | LINC01296 | miR-29c-3p | proliferation, invasion and migration (+) | Xu et al. [53] |
| EOC | LINC01508 | Hippo/YAP | CDDP-resistance (+) | Xiao et al. [54] |
| EOC | LINC01554 | FIGO stage, metastasis (+) | Luo et al. [55] | |
| EOC HGSOC | MAGI2-AS3 | miR-525-5p/MXD1 | cell proliferation, cell cycle, migration, invasion (+) | Chang et al. [56] |
| EOC | MALAT1 | miR-503-5p | proliferation (+) apoptosis (−) | Sun et al. [57] |
| HGSOC EOC | MEG3 | PTEN | migration, invasion, growth, proliferation, spheroid growth in ECM (+) | Butarelli et al. [31] |
| miR-30e-3p, LAMA4 | angiogenesis (+) | Liu et al. [58] | ||
| miR-376a/RASA1 | proliferation (+) apoptosis (−) | Li et al. [59] | ||
| EOC | MIR503HG | proliferation, invasion (+) apoptosis (−) | Tian et al. [60] | |
| EOC | MSC-AS1 | miR-425-5p | proliferation (+) apoptosis (−) | Zhao et al. [61] |
| EOC | NPBWR1-2 | viability, proliferation, migration, invasion (+) apoptosis (−) | Liu et al. [62] | |
| EOC | PAXIP1-AS1 | tumor grade, invasion, | Chen et al. [63] | |
| immune infiltration (+) | ||||
| EOC | PITPNA-AS1 | miR-223-3p/RHOB | apoptosis (−) | Zhang et al. [64] |
| migration, proliferation, EMT (+) | ||||
| HGSOC | PLADE | HNRNPD | proliferation, migration, invasion (−) | Liu et al. [65] |
| CDDP sensitivity, apoptosis (+) | ||||
| EOC | RFPL1S-202 | IFN/STAT1 | CDDP, PTX cytotoxicity (+) proliferation, invasion, migration (−) | Liu et al. [66] |
| EOC | RP11-499E18.1 | PAK2-SOX2 | proliferation, migration, EMT (+) | Yang et al. [67] |
| EOC | SDCBP2-AS1 | miR-100-5p, EPDR1 | viability, migration, invasion (+) apoptosis (−) | Liu et al. [68] |
| EOC | SLC25A21-AS1 | KCNK4, EZH2 | sensitivity to PTX, CDDP (+) proliferation, invasion, migration (−) | Huang et al. [69] |
| PTBP3 | proliferation, metastasis (+) | Li et al. [70] | ||
| EOC | SNHG10 | miR-200a-3p/BIN1 | proliferation, colony formation, migration, invasion (−) | Lv et al. [71] |
| EOC | SNHG5 | miR-23a | PTX sensitivity (+) | Lin et al. [72] |
| EOC | SNHG9 | miR-214-5p | FIGO stage, metastasis, migration, proliferation, invasion (+) | Chen et al. [73] |
| EOC | TTN-AS1 | miR-15b-5p/FBXW7 | proliferation (+) apoptosis (−) | Miao et al. [74] |
| EOC | TUSC7 | miR-616-5p/GSK3β | poor prognosis, proliferation, invasion, migration (+) | Zhu et al. [75] |
| Type of Ovarian Cancer | LncRNA | Target | Function | Reference |
|---|---|---|---|---|
| EOC | AC005224.4 | miR-140-3p/SNAI2 | proliferation, migration, invasion, EMT (+) | Xiong et al. [76] |
| EOC | ACTA2-AS1 | miR-378a-3p/Wnt5a | CDDP-resistance | Lin et al. [77] |
| miR-532-5p, CXCL2 | proliferation and invasion (+) apoptosis (−) | Li et al. [78] | ||
| EOC | ADAMTS9-AS1 | miR-587/SLC7A11 | ferroptosis (+) | Cai et al. [79] |
| EOC | AFAP1-AS1 | FIGO stage, tumor size, proliferation, metastasis | Zhou et al. [80] | |
| miR-107/PDK4 | migration, invasion (+) | Liu et al. [81] | ||
| EOC | ARAP1-AS1 | miR-4735-3p/PLAGL2 | proliferation, migration, invasion (+) | Li et al. [82] |
| EOC | ASAP1IT1 | miR-2278/LATS2 | proliferation (+) apoptosis (−) | Wang et al. [83] |
| EOC | ASB16-AS1 | miR-3918 | proliferation, migration, invasion (+) apoptosis (−) | Fan et al. [84] |
| EOC | ATB | miR-204-3p | proliferation, invasion, migration (+) apoptosis (−) | Yuan et al. [85] |
| EZH2 | proliferation, invasion, migration (+) | Chen et al. [86] | ||
| miR-204-3p/TGFβR2 | viability, angiogenesis, migration (+) | Yuan et al. [87] | ||
| EOC | BBOX1-AS1 | miR-361-3p/PODXL | proliferation (+) apoptosis (−) | Yao et al. [88] |
| EOC | BC041954 | miR-197-3p; miR-23b-3p; miR-149-3pl; | FIGO stage, metastasis (+) | Lu et al. [89] |
| miR-193a | ||||
| EOC | BLACAT1 | miRNA-519d-3p | proliferation, migration, and invasion (+) | Yang et al. [90] |
| EOC | CACNA1G-AS1 | FTH1-IGF2BP1 | ferroptosis/ferritinophagy (−) | Jin et al. [91] |
| proliferation, migration (+) | ||||
| EOC HGSOC | CASC10 | apoptosis, proliferation, invasion (−) | Noriega-Rivera et al. [92] | |
| CDDP sensitivity (+) | ||||
| EOC OCCC | CASC15 | miR-23b-3p/miR-24-3p; TGF-β/SMAD3 | OS (−) migratiOn, invasion, EMT (+) | Lin et al. [93] |
| EOC | CCAT1 | miR-454/survivin | CDDP-resistance (−) | Wang et al. [94] |
| EOC | CCNG1 | miR-488-3p | proliferation, migration, invasion (+) | Sun et al. [95] |
| EOC | CDKN2A-AS1 | SOSTDC1 | proliferation, migration, invasion (+) | Zhao et al. [96] |
| miR-143-3p/SMAD3 | proliferation, invasion, migration (+) apoptosis (−) | Xu et al. [97] | ||
| EOC | CDKN2BAS | GAS6 | proliferation, migration (+) | Wang et al. [98] |
| EOC | CRNDE | miR-423-5p/FSCN1 | proliferation, invasion, migration (+) | Wang et al. [99] |
| SRSF1/TIA1 | CDDP-resistance (+) | Wu et al. [100] | ||
| HGSOC | CTBP1-DT | miR-188-5p/MAP3K3 | proliferation, migration, invasion (+) | Liu et al. [101] |
| EOC | DDUP | Ren et al. [102] | ||
| EOC | CTD-2288O8 | EGFR/AKT | CDDP-resistance, viability, proliferation, invasion (+) | Liu et al. [103] |
| EOC | CTSLP8 | PKM2/c-Myc | proliferation, CDDP-resistance, glycolysis | Li et al. [104] |
| miR-199a-5p, CTSL1 | autophagy, EMT (+) | Wang et al. [105] | ||
| EOC | DANCR | miR-214 | viability, migration, and invasion (+) | Huang et al. [106] |
| EOC | DARS-AS1 | miR-194-5p/RBX1 | proliferation, migration, apoptosis (+) | Zhou et al. [107] |
| EOC | DATOC-1 | miR-7 | proliferation, invasion (+) | Qin et al. [108] |
| EOC | DLEU1 | miR-429/TFAP2A | proliferation, migration, invasion (+) | Xu et al. [109] |
| EOC OCCC | DLX6-AS1 | miR-195-5p | proliferation, migration, invasion (+) apoptosis (−) | Kong et al. [110] |
| EOC OCCC | DNM3 | miR-193a-3p/MAP3K3 | proliferation, migration, invasion (+) | He et al. [111] |
| EOC | DSCR8 | miR-98-5p/STAT3/HIF-α | poor survival, proliferation, invasion, EMT (+) apoptosis (−) | Dong et al. [112] |
| EOC OCCC | DUXAP8 | microRNA-29a-3p | poor prognosis, proliferation, migration | Li et al. [113] |
| miR-590-5p | proliferation (+) apoptosis (−) | Meng et al. [114] | ||
| EOC | ELFN1-AS1 | miR-497-3p/CLDN4 | poor prognosis, proliferation, invasion, migration (+) | Jie et al. [115] |
| EOC | FAM83H-AS1 | OS | Yuan et al. [116] | |
| EOC | FEZF1-AS1 | miR-130a-5p/SOX4 | proliferation, migration, invasion (+) | Sun et al. [117] |
| EOC | FGD5-AS1 | miR-142-5p/PD-L1 | proliferation, migration, invasion (+) | Aichen et al. [118] |
| EOC | FGFR3-AS1 | tumor grade, FIGO stage, poor prognosis, cell growth, proliferation (+) apoptosis (−) | Zhang et al. [119] | |
| EOC | FLVCR1-AS1 | miR-513/YAP1 | growth, migration, invasion, and EMT (+)apoptosis (−) | Yan et al. [120] |
| EOC | FOXD2-AS1 | miR-4492 | proliferation, invasion (+) | Gao et al. [121] |
| EOC | FTX | miR-7515/TPD52 | migration, invasion, EMT (+) | Li et al. [122] |
| EOC | GClnc1 | p53 | proliferation, migration | Li et al. [123] |
| NOTCH1/NF-κB/Snail | proliferation, EMT (+) | Wu et al. [124] | ||
| EOC | H19 | migration, invasion | Ma et al. [125] | |
| miR-29b-3p | CBDCA-resistance | Tian et al. [126] | ||
| miRNA-140/Wnt1 | proliferation, migration | Wang et al. [127] | ||
| miR-140-5p | proliferation, invasion, migration, EMT (+) | Xu et al. [128] | ||
| EOC | HAGLROS | miRNA-26b-5p | proliferation (+) apoptosis (−) | Zhu et al. [129] |
| EOC | HCG11 | miR-144-3p/PBX3 | viability, metastasis (+) | Li et al. [130] |
| EOC | HCG18 | miR-29a/b/TRAF4/5 | proliferation, migration, EMT (+) | Zhang et al. [131] |
| EOC | miR-525-5p/PRC1 | proliferation, invasion, migration, EMT (+) | Wang et al. [132] | |
| HGSOC | HCP5 | PTBP1 | apoptosis (−) proliferation, migration (+) | Shou et al. [133] |
| EOC | HEIH | miR-3619-5p/CTTNBP2 | poor prognosis, proliferation, | Si et al. [134] |
| migration, invasion (+) | ||||
| EOC | HIF1A-AS3 | proliferation (+) | Xie et al. [135] | |
| miR-21-3p/PEG3 | Fang et al. [136] | |||
| EOC | HOTAIR | miR-222-3p/CDK19 | proliferation, migration, metastasis (+) | Fan et al. [137] |
| ZEB1 and TGF-β1 | Zhou et al. [138] | |||
| CHEK1 | Jiang et al. [139] | |||
| EZH2, H3K27 | anoikis resistance (+) | Dai et al. [140] | ||
| miR-138-5p, EZH2 | CDDP-resistance | Zhang et al. [141] | ||
| miR-206/TBX3 | steamness of stem cells (+) | Zhang et al. [142] | ||
| EOC | HOTAIRM1 | proliferation (+) apoptosis (−) | Ye et al. [143] | |
| EOC | HOTTIP | interaction with HIF-1α | migration, invasion, viability, apoptosis | Zhang et al. [144] |
| MEK/ERK | proliferation, migration (+) | Liu et al. [145] | ||
| miR-615-3p/SMARCE1 | migration, invasion (+) | |||
| miR-148a-3p/AKT2 | Tan et al. [146] | |||
| miR-205/ZEB2 | CDDP-resistance (+) | Dong et al. [147] | ||
| EOC | HOXAAS2 | miR372 | metastasis. FIGO stage, proliferation (+) apoptosis (−) | Wang et al. [148] |
| metastasis, proliferation, migration (+) | Eoh et al. [149] | |||
| EOC | HOXA11-AS | viability, migration, invasion (+) | Chen et al. [150] | |
| apoptosis, DDP sensitivity (−) | ||||
| EOC | HOXB-AS3 | miR-378a-3p | proliferation, invasion, migration (+) | Xu et al. [151] |
| EOC | HOXC-AS3 | miR-96 | proliferation (+) | Yang et al. [152] |
| EOC HGSOC | HULC | miR-137/ITGB8 | tumor growth, PTX-resistance (+) | Huang et al. [153] |
| EOC | IDH1-AS1 | miR-518c-5p/RBM47 | poor prognosis, OS, progression (+) | Zhou et al. [154] |
| EOC | IL21-AS1 | miR-561-5p/CD24 | proliferation (+) apoptosis, phagocytosis (−) | Liu et al. [155] |
| EOC | KCNQ1OT1 | miR-142-5p/CAPN10 | proliferation, metastasis (+) | Liu et al. [156] |
| miR-125b-5p/CD147 | Chen et al. [157] | |||
| EIF2B5 | He et al. [158] | |||
| EOC | KHDRBS3 | miR17H/CLDN6 | PTX sensitivity, cell proliferation, colony formation, glycolysis (+) | Wu et al. [159] |
| EOC | KTN1-AS1 | miR-505-3p/ZNF326 | proliferation, invasion, migration (+) | Xie et al. [160] |
| EOC | LBX2-AS1 | miR-4784/KDM5C | cell proliferation, migration, stemness (+) apoptosis (−) | Gu et al. [161] |
| EOC | LEF1-AS1 | miR-1285-3p | metastasis, FIGO stage, cell proliferation, migration, invasion (+) | Zhang et al. [162] |
| EOC | LINC00152 | BCL6 Bcl-2, Bax Caspase-3 | proliferation, invasion (+)CDDP chemonseitivity (−) | Wang et al. [163] Zou et al. [164] |
| EOC | LINC00176 | BCL3 | EMT (+) | Dai et al. [165] |
| EOC | LINC00273 | proliferation, invasion (−) | Shu et al. [166] | |
| EOC | LINC00452 | miR-501-3p | viability, migration, invasion (+) | Yang et al. [167] |
| EOC | LINC00494 | NFκB1, FBXO32 | migration, invasion (+) | Shu et al. [168] |
| EOC | LINC00504 | miR-1244 | proliferation, apoptosis (+) | Liu et al. [169] |
| EOC | LINC00662 | miR-375 | OS (−) | Tao et al. [170] |
| LINC00665 | miR-148b-3p/KLF5 | cell proliferation, invasion, migration (+) apoptosis (−) | Wang et al. [171] | |
| EOC | LINC00665 | miR-181a-5p/FHDC | viability, proliferation, migration (+) | Wang et al. [172] |
| EOC | LINC00707 | miR-382-5p/LRRK2 | Zhao et al. [173] | |
| LINC00852 | miR-140-3p/AGTR1 | proliferation, invasion (+) | Qiao et al. [174] | |
| EOC | LINC00857 | miR-486-5p | proliferation, migration, invasion, glycolysis (+) apoptosis (−) | Lin et al. [175] |
| EOC | LINC00858 | miR-134-5p/RAD18 | proliferation, motility, EM (+), apoptosis (−) | Xue et al. [176] |
| miR-134-5p/TRIM44 | proliferation, migration, invasion (+) | Li et al. [177] | ||
| EOC | LINC00909 | miR-23b-3p/MRC2 | proliferation, migration, invasion (+) | Yang et al. [178] |
| EOC | LINC00922 | miR-361-3p | proliferation, migration, invasion, EMT (+) | Wang et al. [179] |
| EOC | LINC00958 | Wnt/β-catenin | proliferation, migration, invasion (+) | Xie et al. [180] |
| EOC HGSOC OCCC | LINC01094 | miR-577 | FIGO stage, lymph node metastasis, cell proliferation, migration, invasion and EMT (+) | Xu et al. [181] |
| EOC | LINC01123 | miR-516b-5p | proliferation, metastasis (+) apoptosis (−) | Dong et al. [182] |
| EOC | LINC01132 | miRNA4315p/SOX9 | proliferation, migration, invasion (+) apoptosis (−) | Zhu et al. [183] |
| EOC | LINC01133 | miR-495-3p | migration, invasion (+) | Liu et al. [184] |
| EOC | LINC01215 | RUNX3 | proliferation, migration, invasion, EMT, metastasis (+) | Liu et al. [185] |
| EOC | LINC01342 | miRNA-30c-2-3p | proliferation, metastasis (+) | Zhang et al. [186] |
| EOC | LINC01503 | miR-766-5p/PD-L1 | CBDCA-resistance | Li et al. [187] |
| EOC HGSOC | LINC01605 | mut_p53 | migration (+) | Coan et al. [188] |
| EOC | LINC01969 | miR-144-5p/LARP1 | migration, invasion, proliferation (+) | Chen et al. [189] |
| EOC | LINC02323 | miR-1343-3p | proliferation, migration (+) | Li et al. [190] |
| EOC | LINK-A | Maleki et al. [191] | ||
| EOC | LNC00115 | miR-7/ERK | CDDP-resistance, invasion, migration (+) | Jang et al. [192] |
| EOC | LOC102724169 | cell viability, CDDP efficacy (+) apoptosis (−) | Zhou et al. [193] | |
| EOC | LOC285194 | proliferation, migration, poor prognosis (+) apoptosis (−) | Yim et al. [194] | |
| EOC | LOC642852 | invasion (+) | Filippov-Levy et al. [195] | |
| EOC | LOC646029 | miR-627-3p/SPRED1 | proliferation, invasion, metastasis, poor prognosis (+) | Zhao et al. [196] |
| EOC | LOXL1-AS1 | miR-18b-5p/VMA21 | proliferation, migration, invasion, metastasis (+) | Xue et al. [197] |
| LUCAT1 | miR-199a-5p | proliferation, apoptosis (+) | Liu et al. [198] | |
| EOC | MAFG-AS1 | miR-339-5p | tumor stage, size, lymph node metastasis, invasion, EMT, migration (+) | |
| Bai et al. [199] | ||||
| EOC | MALAT1 | migration, invasion (+) apoptosis (−) | Mao et al. [200] | |
| miRNA-22 | proliferation, migration, invasion (+) | Pei et al. [201] | ||
| miR-503-5p | proliferation (+) apoptosis (−) | Sun et al. [59] | ||
| EOC | MCF2L-AS1 | IGF2BP1/IGF2/ MEK/ERK axis | PTX-resistance (+) | Zhu et al. [202] |
| EOC | MIAT | miR-150-5p | cell growth, migration, invasion, EMT (+) | Zhou et al. [203] |
| EOC | MIF-AS1 | miRNA-31-5p | proliferation, migration, invasion (+) | Fan et al. [204] |
| EOC | MIR210HG | EMT, angiogenesis (+) | Liu et al. [205] | |
| EOC | MIR4435-2HG | miR-128-3p/CDK14 | proliferation, invasion, migration (+), apoptosis (−) | Zhu et al. [206] |
| EOC | MNX1-AS1 | miR-4697-3p/HOXB13 | CBDCA-resistance | Wu et al. [207] |
| miR-744-5p/SOX12 | cell proliferation, migration, invasion (+) apoptosis (−) | Shen et al. [208] | ||
| EOC | MYCN | miR-152/MKK7 | poor survival, proliferation (+) | Zhang et al. [209] |
| EOC | MYU | miR-6827-5p | FIGO stage, metastasis, proliferation (+) | Wang et al. [210] |
| EOC | NCK1-AS1 | miR-137 | cell proliferation, migration, invasion, chemoresistance (+) | Chang et al. [211] |
| EOC OCC HGSOC | NEAT1 | CSTF3 | CDDP sensitivity (−) | Luo et al. [212] |
| miR-214-3p | proliferation, migration, invasion, angiogenesis (+) apoptosis (−) | Liu et al. [213] | ||
| miR-770-5p/PARP1 | CDDP-resistance, viability (+) apoptosis (−) | Zhu et al. [214] | ||
| miR-365 | proliferation, angiogenesis | Yaun et al. [215] | ||
| miR4500/BZW1 | proliferation, colony formation, migration, invasion, glycolysis (+) apoptosis (−) | Xu et al. [216] | ||
| let-7 g/MEST/ATGL | proliferation, migration, invasion (+) | Yin et al. [217] | ||
| miR1321 | migration, invasion (+) | Luo et al. [218] | ||
| EOC | NORAD | miR-199a-3p | proliferation, invasion, migration, EMT (+) | Xu et al. [219] |
| EOC | NRSN2-AS1 | miR-744-5p/PRKX | migration, invasion (+) | Chen et al. [220] |
| PTK2/β-catenin | proliferation, metastasis (+) | Wu et al. [221] | ||
| EOC | OIP5-AS1 | miR-92a | EMT, viavbility, proliferation, migration, invasion (+) apoptosis (−) | Wang et al. [222] |
| miR-324-3p/NFIB | viability, invasion, migration (+) | Liu et al. [223] | ||
| miR-137/ZNF217 | proliferation, migration, invasion, EMT (+) | Guo et al. [224] | ||
| miR-34a | invasion, migration (+) | Jiang et al. [225] | ||
| miR1283p/CCNG1 | cell viability, migration, invasion, glycolysis (+) apoptosis (−) | Liu et al. [226] | ||
| EOC | OR3A4 | KLF6 | proliferation and migration (+) | Guo et al. [227] |
| EOC | PART1 | miR-150-5p/MYB | proliferation, migration, invasion (+) | Wang et al. [228] |
| miR-6884-5p/RACGAP1/RRM2 | proliferation, invasion, migration (+) | Li et al. [229] | ||
| miR-503-5p | cell viability, migration, invasion (−) apoptosis (+) | Li et al. [230] | ||
| EOC | PCA3 | miR-106b-5p | cell proliferation, migration, invasion.(+) protein expression (+) | Liu et al. [231] |
| EOC | PLAC2 | CDK2 | proliferation (+) | He et al. [232] |
| EOC | PRLB | miR-150-5p, PRLB/RSF1 | apoptosis (−) PTX-resistance (+) | Zhao et al. [233] |
| EOC | PRNCR1 | miR-653-5p/ELF2 | invasion, migration, proliferation (+) | Qi et al. [234] |
| EOC | PRPF6 | SNHG16-L/CEBPB/GATA3 | FIGO stage, metastasis, PTX-resistance progression (+) | Wang et al. [235] |
| EOC | PSMA3-AS1 | miR-378a-3p/GALNT3 | proliferation, migration, invasion (+) | Xu et al. [236] |
| EOC | PTAL | miR-101/fn1 | invasion, migration (+) | Liang et al. [237] |
| EOC | PURPL | miR-338-3p | FIGO stage, metastasis (+) | Zhang et al. [238] |
| EOC OCCC | PVT1 | CTGF | proliferation, migration, invasion, EMT (+) | Dong et al. [239] |
| miR-148a/AGO1 | FIGO stage, poor survival rate, metastasis, cell viability, proliferation (+) apoptosis (−) | Wu et al. [240] | ||
| miR-149-5p/FOXM1 | proliferation, migration, invasion (+) | Li et al. [241] | ||
| miR-370, miR-576, FOXM1 | migration, invasion, proliferation, | Yi et al. [242] | ||
| chemoresistance (+) | ||||
| miR-543/SERPINI1 | proliferation, migration, invasion (+) apoptosis (−) | Qu et al. [243] | ||
| cell survival, growth, migration, chemoresistance (+) | Tabury et al. [244] | |||
| EOC | RAD51-AS1 | miR-140-3p/EIF5A2 | poor prognosis (+) | Zhao et al. [245] |
| EOC | RBAT1 | FIGO stage, metastasis (+) | Luo et al. [246] | |
| EOC | RHPN1-AS1 | PI3K/AKT | viability, migration, invasion, tumor growth (+) | Cui et al. [247] |
| miR-1299 | proliferation, migration, invasion, low survival rate (+) | Zhao et al. [248] | ||
| miR-665/Akt3 | cell proliferation, migration, invasion (+) | Zhao et al. [249] | ||
| miR-596/LETM1 | proliferation, invasion, viability (+) | Wang et al. [250] | ||
| miR-6884-5p | proliferation, viability, adhesion, migration (+) apoptosis (−) | Cui et al. [251] | ||
| miR4855p | proliferation, apoptosis, adhesion, migration (+) | Cui et al. [252] | ||
| EOC HGSOC | RMRP | miR-580-3p | proliferation (+) apoptosis (−) PTX-resistance (+) | Li et al. [253] |
| EOC | RP5 | miR-545-5p/PTP4A1 | proliferation, metastasis (+) | Sun et al. [254] |
| EOC | SBF2-AS1 | miR-338-3p | proliferation, invasion (+) | Luan et al. [255] |
| EOC | SCAMP1 | miR-137/CXCL12 | invasion, angiogenesis (+) | Song et al. [256] |
| EOC | SDHAP1 | miR-4465/EIF4G2 | PTX-resistance (+) | Zhao et al. [257] |
| EOC OCCC | SNHG1 | miR-454/ZEB1 | proliferation (+) | Wu et al. [258] |
| EOC HGSOC | SNHG12 | CBDCA sensitivity (+) | Abildgaard et al. [259] | |
| EOC OCCC | SNHG17 | miR-18a-5p | migration and invasion (+) | Zhang et al. [260] |
| miR-370-3p/CDK6 | poor prognosis, migration, proliferation (+) | Wang et al. [261] | ||
| CCL13-CCR2 | proliferation, invasion, migration, EMT, metastasis (+) | Liang et al. [262] | ||
| miR-214-3p | poor prognosis, proliferation (+) apoptosis (−) | Pan et al. [263] | ||
| miR-485-5p/AKT1 | proliferation (+) apoptosis (−) | Wang et al. [264] | ||
| EOC | SNHG20 | miR-148a/ROCK1 | invasion, migration, proliferation (+) apoptosis (−) | Yang et al. [265] |
| miR-217 | cell proliferation, invasion (+) | Xing et al. [266] | ||
| miR-338-3p/MCL1 | proliferation, migration, invasion, EMT (+) apoptosis (−) | Wang et al. [267] | ||
| EOC | SNHG22 | SP1 | poor prognosis, glycolysis, proliferation (+) | Guan et al. [268] |
| EOC | SNHG25 | COMP | proliferation, migration, invasion (+) apoptosis (−) | Liu et al. [269] |
| EOC | SNHG3 | miR-139-5p | proliferation, migration (+) | Zhang et al. [270] |
| HGSOC | miR-339-5p/TRPC3 | poor prognosis, apoptosis (+) | Liu et al. [271] | |
| EOC | SNHG4 | miR-98-5p/TMED5 | proliferation, migration, invasion (+) apoptosis (−) | Liu et al. [272] |
| EOC | SNHG6 | miR-543/YAP1 | proliferation, migration, invasion, EMT | Su et al. [273] |
| EOC HGSOC OCCC | SHNG7 | EIF4G2 | cell viability, migration, invasion, PTX-resistance (+) | Zhang et al. [274] |
| KLF2, EZH2 | invasion and proliferation (+) | Bai et al. [275] | ||
| EOC | SNHG8 | Wnt/β-catenin | proliferation, migration, EMT (+) | Miao et al. [276] |
| EOC HGSOC | SPOCD1-AS | G3BP1 | Wang et al. [277] | |
| EOC | SRA | proliferation, invasion, migration (+) | Kim et al. [278] | |
| EOC | TC0101441 | KiSS1 | invasion, migration, metastasis, EMT (+) | Qiu et al. [279] |
| EOC HGSOC | THOR | IL-6/STAT3 | poor prognosis, proliferation, invasion (+) | Ge et al. [280] |
| EOC HGSOC | THUMPD3-AS1 | miR-320d/ARF1 | apoptosis (−) viability (+) | Mu et al. [281] |
| EOC | TLR8-AS1 | metastasis, chemoresistance (+) | Xu et al. [282] | |
| EOC | TMPO-AS1 | LCN2 | migration, proliferation, invasion, angiogenesis (+) | Zhao et al. [283] |
| miR-200c/TMEFF2 | invasion, migration, and 5-Fu resistance, EMT (+) | Li et al. [284] | ||
| EOC | TONSL-AS1 | miR-490-3p/CDK1 | proliferation (+) | Liu et al. [285] |
| EOC HGSOC | TRPM2-AS1 | miR-138-5p/SDC3 | proliferation, invasion, migration, PTX-resistance (+) apoptosis (−) | Ding et al. [286] |
| EOC | TUG1 | miR-582-3p | viability and migration (+) | Dai et al. [287] |
| miR-29b-3p | PTX-resistance (+) | Gu et al. [288] | ||
| miR-1299/NOTCH3 | proliferation (+) | Pei et al. [289] | ||
| miR-186-5p/ZEB1 | proliferation, invasion (+) | Zhan et al. [290] | ||
| miR-4687-3p, miR-6088 | CDDP sensitivity (+) | Sonobe et al. [291] | ||
| EOC | UCA1 | miR-27a-5p/UBE2N | CDDP-resistance | Wambecke et al. [292] |
| EOC HGSOC | miR-654-5p/SIK2 | proliferation, migration, invasion, PTX-resistance (+) apoptosis (−) | Li et al. [293] | |
| EOC | UNC5B-AS1 | H3K27me, NDRG2 | proliferation (+) apoptosis (−) | Wang et al. [294] |
| EOC OCCC | USP2-AS1 | miR-520d-3p | proliferation, migration (+) | Guo et al. [295] |
| EOC | USP30-AS1 | Xiong et al. [296] | ||
| EOC | WDFY3-AS2 | miR-139-5p/SDC4 | CDDP-resistance, cell proliferation, migration, invasion (+) apoptosis (−) | Wu et al. [297] |
| EOC | XIST | miR-506-3p/FOXP | apoptosis (−) CBDCA -resistance, autophagy (+) | Xia et al. [298] |
| OCCC | miR-335/BCL2L2 | proliferation invasion, migration (+) | Meng et al. [299] | |
| miR-93-5p/KMT2C | PTX resistance (+) | Huang et al. [300] | ||
| miR-149-3p | poor prognosis, proliferation, invasion, migration (+) apoptosis (−) | Jiang et al. [301] | ||
| EOC | ZEB1-AS1 | MMP19 | PTX, CDDP-resistance (−) | Dai et al. [302] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwas, K.; Szubert, M.; Wilczyński, J.R. Latest Update on lncRNA in Epithelial Ovarian Cancer—A Scoping Review. Cells 2025, 14, 555. https://doi.org/10.3390/cells14070555
Kwas K, Szubert M, Wilczyński JR. Latest Update on lncRNA in Epithelial Ovarian Cancer—A Scoping Review. Cells. 2025; 14(7):555. https://doi.org/10.3390/cells14070555
Chicago/Turabian StyleKwas, Katarzyna, Maria Szubert, and Jacek Radosław Wilczyński. 2025. "Latest Update on lncRNA in Epithelial Ovarian Cancer—A Scoping Review" Cells 14, no. 7: 555. https://doi.org/10.3390/cells14070555
APA StyleKwas, K., Szubert, M., & Wilczyński, J. R. (2025). Latest Update on lncRNA in Epithelial Ovarian Cancer—A Scoping Review. Cells, 14(7), 555. https://doi.org/10.3390/cells14070555






