Dual Role of HNF4α in Colorectal Adenocarcinoma During Carcinogenesis and Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture
2.2. HNF4α Overexpression
2.3. HNFA siRNA Transfection
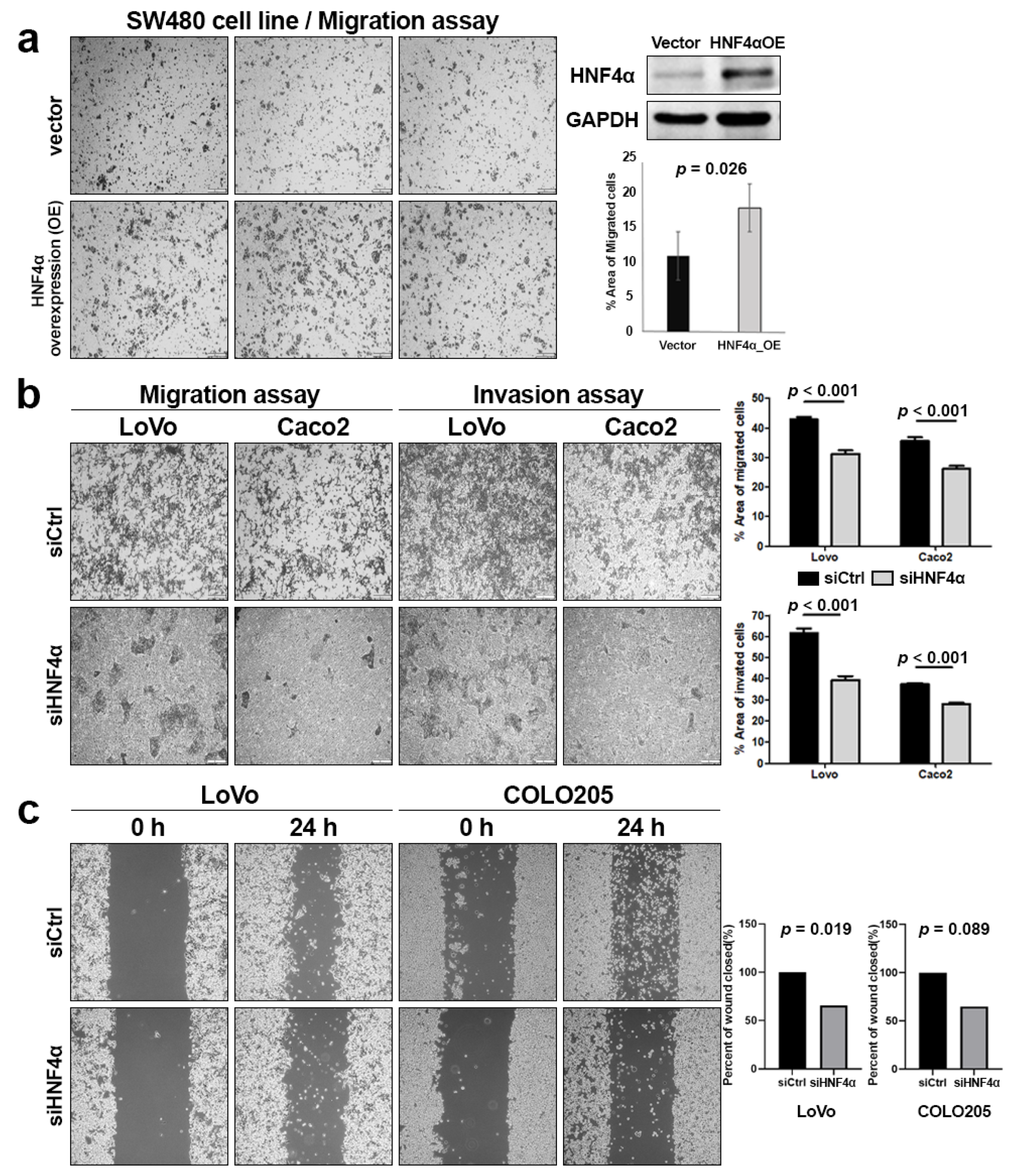
2.4. Transwell Migration and Invasion and Wound-Healing Assays
2.5. Western Blot Analysis
2.6. Immunohistochemical Staining
2.7. CRAC Patients and Specimens
2.8. Statistical Analysis
3. Results
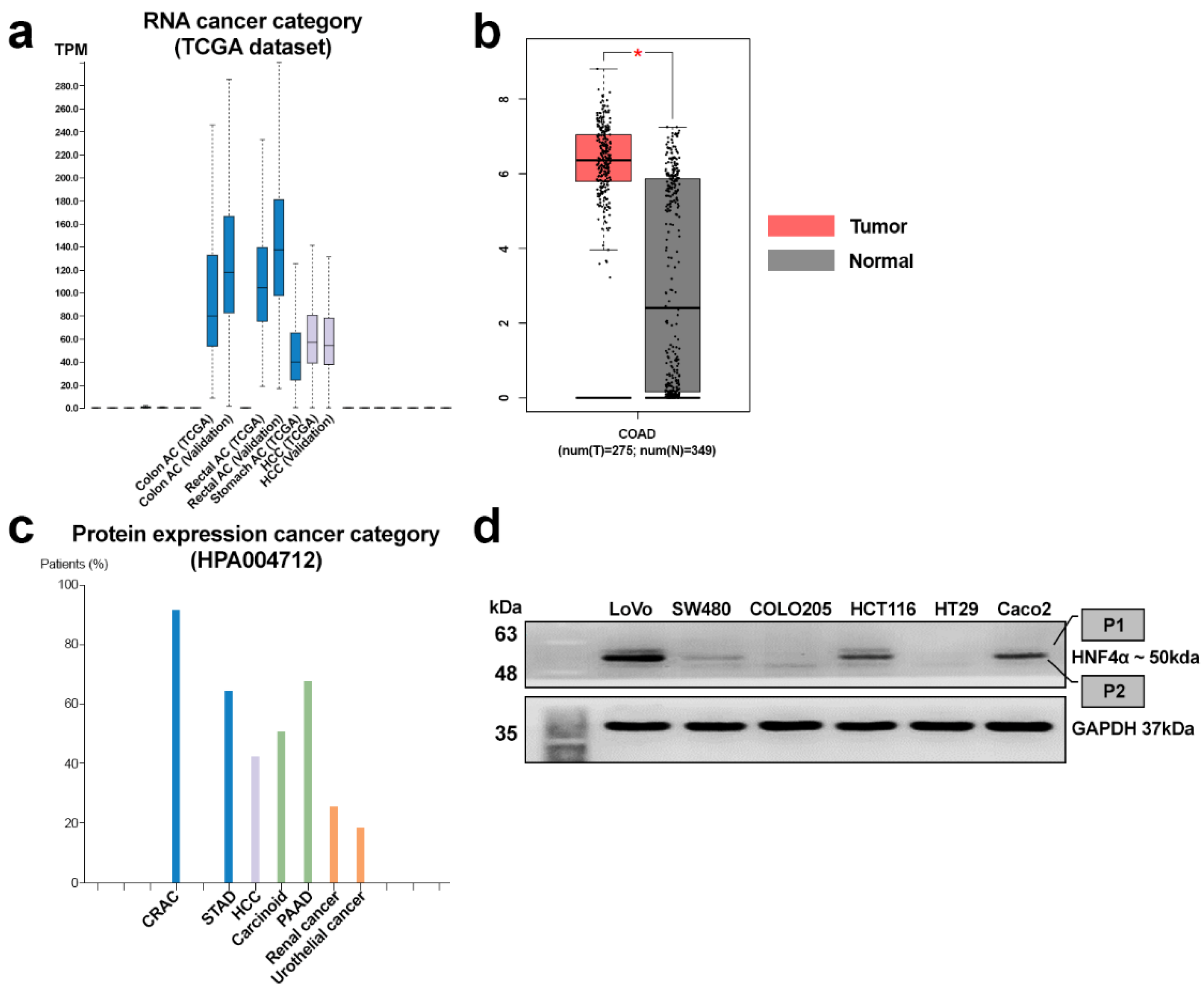
3.1. Expression Patterns of HNF4α in CRAC
3.2. Overexpression and Knockdown of HNF4α in CRAC Cell Lines
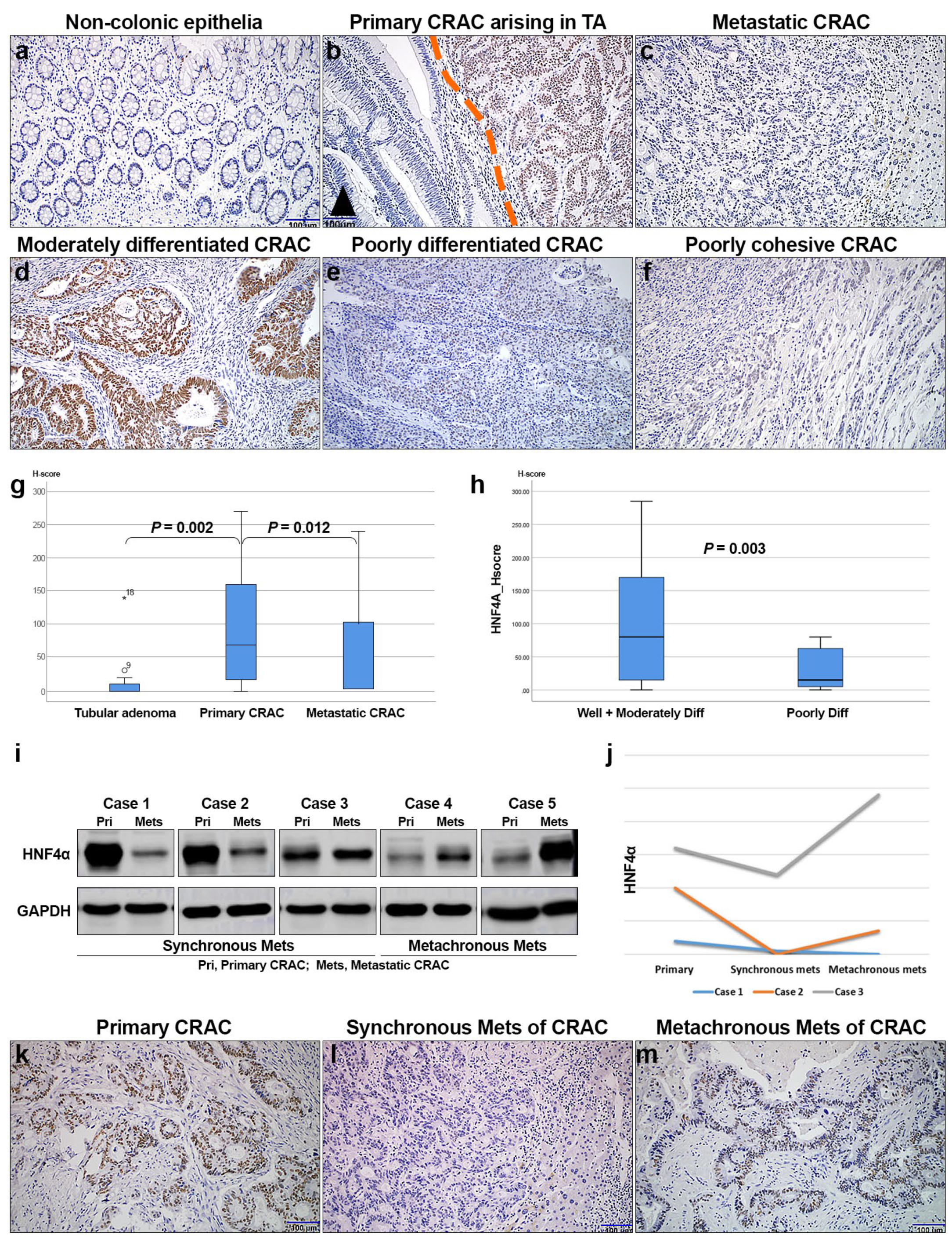
3.3. HNF4α Protein Expression Is Associated with CRAC Progression
3.4. Immunohistochemical Expression of HNF4α in Relation to Clinicopathologic Features and Survival of CRAC Patients
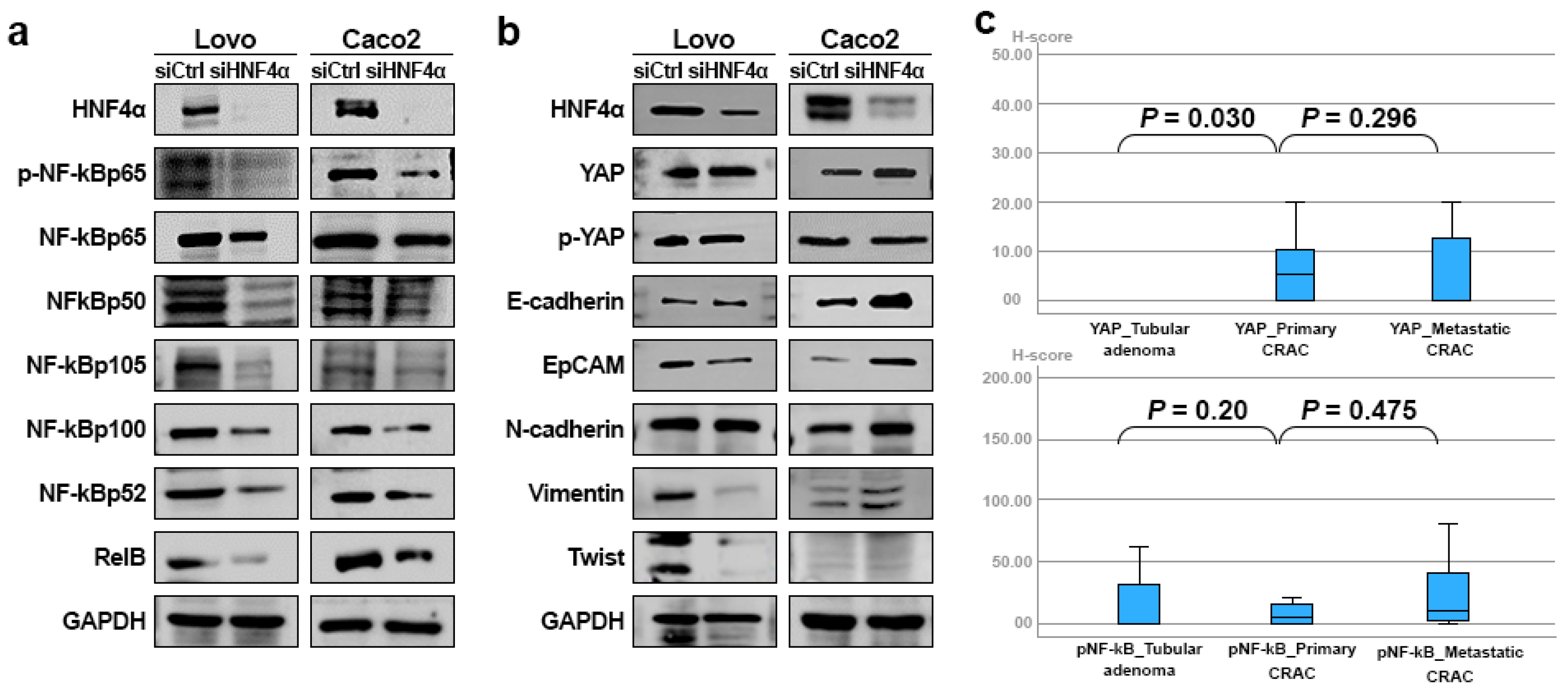
3.5. HNF4α Expression in Relation to NF-κB, YAP, and EMT Markers in CRAC
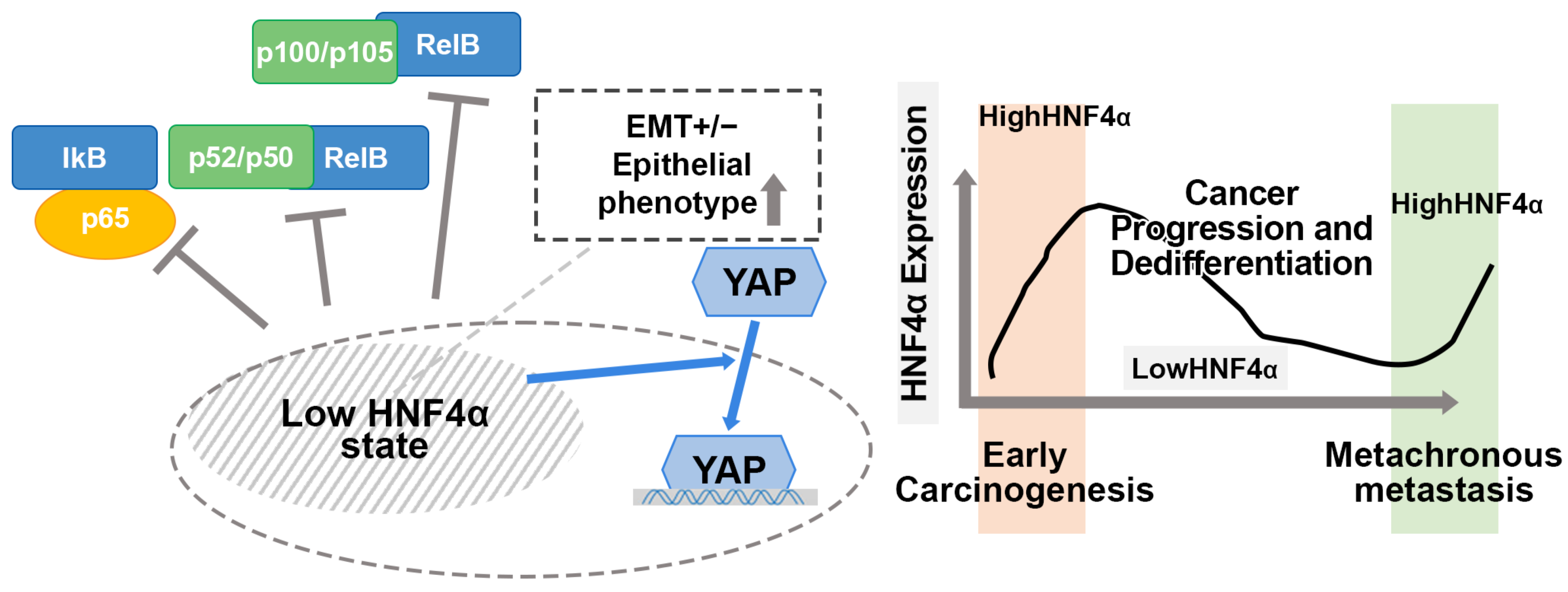
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Ning, G.; Duncan, S.A. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev. 2000, 14, 464–474. [Google Scholar] [CrossRef]
- Yang, Z.; Danzeng, A.; Liu, Q. The Role of Nuclear Receptors in the Pathogenesis and Treatment of Non-alcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2024, 20, 113–126. [Google Scholar] [CrossRef]
- Marcil, V.; Seidman, E.; Sinnett, D.; Boudreau, F.; Gendron, F.-P.; Beaulieu, J.-F.; Ménard, D.; Precourt, L.-P.; Amre, D.; Levy, E. Modification in Oxidative Stress, Inflammation, and Lipoprotein Assembly in Response to Hepatocyte Nuclear Factor HNF4α; Knockdown in Intestinal Epithelial Cells. J. Biol. Chem. 2010, 285, 40448–40460. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Luan, T.; Liu, N.; Kong, C.; Xu, L.; Yu, H.; Kang, Y.; Han, Y. Hepatocyte nuclear factor 4 a (HNF4α): A perspective in cancer. Biomed. Pharmacother. 2023, 169, 115923. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Wang, X.; Bai, W.; Shen, J.; Zeng, Y.; Sun, J. The role of hepatocyte nuclear factor 4α (HNF4α) in tumorigenesis. Front. Oncol. 2022, 12, 1011230. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Wilson, E.; Stubben, C.; Kabir, A.; Affolter, K.; Zhang, X.; Snyder, E.L. Differential control of growth and identity by HNF4α isoforms in pancreatic ductal adenocarcinoma. bioRxiv 2025. [Google Scholar] [CrossRef]
- Xu, B.-N.; Ding, C.-H.; Liu, Y.-L.; Luo, Y.-Y.; Deng, J.; Liu, S.-Q.; Xiao, M.-C.; Jiang, N.; Zhang, X.; Xu, W.-P.; et al. HNF4α inhibits the malignancy of intrahepatic cholangiocarcinoma by suppressing the Wnt signaling pathway. Transl. Oncol. 2025, 53, 102290. [Google Scholar] [CrossRef]
- Kusnik, A.; Renjithlal, S.L.M.; Chodos, A.; Shanmukhappa, S.C.; Eid, M.M.; Renjith, K.M.; Alweis, R. Trends in colorectal cancer mortality in the United States, 1999–2020. Gastroenterol. Res. 2023, 16, 217. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, J.; Choi, Y.J.; Kang, J.G. Clinical study of colorectal cancer operation: Survival analysis. Korean J. Clin. Oncol. 2020, 16, 3–8. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Väyrynen, V.; Wirta, E.-V.; Seppälä, T.; Sihvo, E.; Mecklin, J.-P.; Vasala, K.; Kellokumpu, I. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: A population-based study. BJS Open 2020, 4, 685–692. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Controversial roles of hepatocyte nuclear receptor 4 α on tumorigenesis. Oncol. Lett. 2021, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Rastinejad, F. The protein architecture and allosteric landscape of HNF4α. Front. Endocrinol. 2023, 14, 1219092. [Google Scholar] [CrossRef]
- Manickasamy, M.K.; Jayaprakash, S.; Girisa, S. Delineating the role of nuclear receptors in colorectal cancer, a focused review. Discov. Oncol. 2024, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ravi, H.; Rajeswari, V.D.; Venkatraman, G.; Ramasamy, M.; Dhanasekran, S.; Ramanathan, G. Therapeutic insight into the role of nuclear protein HNF4α in liver carcinogenesis. Adv. Protein Chem. Struct. Biol. 2025, 143, 1–37. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wu, D.; Yu, S.; Wang, Y.; Leung Chan, F. Nuclear receptor HNF4α performs a tumor suppressor function in prostate cancer via its induction of p21-driven cellular senescence. Oncogene 2020, 39, 1572–1589. [Google Scholar] [CrossRef] [PubMed]
- Hatziapostolou, M.; Koutsioumpa, M.; Zaitoun, A.M.; Polytarchou, C.; Edderkaoui, M.; Mahurkar-Joshi, S.; Vadakekolathu, J.; D’Andrea, D.; Lay, A.R.; Christodoulou, N.; et al. Promoter Methylation Leads to Hepatocyte Nuclear Factor 4A Loss and Pancreatic Cancer Aggressiveness. Gastro Hep Adv. 2024, 3, 687–702. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, X.; Wu, Z. HNF-4α promotes multidrug resistance of gastric cancer cells through the modulation of cell apoptosis. Oncol. Lett. 2017, 14, 6477–6484. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.-N.; Anjum, K.M.; Gui, L.-X.; Zhu, S.-S.; Liu, J.; Chen, J.-K.; Liu, Q.-F.; Ye, G.-D.; Wang, W.-J. A double-negative feedback loop between Wnt–β-catenin signaling and HNF4α regulates epithelial–mesenchymal transition in hepatocellular carcinoma. J. Cell Sci. 2013, 126, 5692–5703. [Google Scholar] [CrossRef]
- Ning, B.F.; Ding, J.; Liu, J.; Yin, C.; Xu, W.P.; Cong, W.M.; Zhang, Q.; Chen, F.; Han, T.; Deng, X. Hepatocyte nuclear factor 4α-nuclear factor-κB feedback circuit modulates liver cancer progression. Hepatology 2014, 60, 1607–1619. [Google Scholar] [CrossRef]
- Yao, D.; Peng, S.; Dai, C. The role of hepatocyte nuclear factor 4alpha in metastatic tumor formation of hepatocellular carcinoma and its close relationship with the mesenchymal–epithelial transition markers. BMC Cancer 2013, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qin, Z.; Bai, Y.; Shi, W.; Li, K.; Song, L.; Zhuang, A.; Ding, C. Hnf4a Functions as an Hcc Oncogene or Tumor Suppressor Depending Upon the Ampk Pathway Activity Status. 2024. Available online: https://ssrn.com/abstract=4855548 (accessed on 5 January 2024).
- Cai, S.-h.; Lu, S.-x.; Liu, L.-l.; Zhang, C.Z.; Yun, J.-p. Increased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinoma. Ther. Adv. Gastroenterol. 2017, 10, 761–771. [Google Scholar] [CrossRef]
- Babeu, J.-P.; Jones, C.; Geha, S.; Carrier, J.C.; Boudreau, F. P1 promoter-driven HNF4α isoforms are specifically repressed by β-catenin signaling in colorectal cancer cells. J. Cell Sci. 2018, 131, jcs214734. [Google Scholar] [CrossRef]
- Sang, L.; Bai, W.; Lu, C.; Dong, R.; Hao, Q.; Zhang, Y.; Sun, R.; Shen, J.; Sun, Y.; Sun, J. HNF4α is a target of the Wnt/β-catenin pathway and regulates colorectal carcinogenesis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kikuchi, A. Wnt/β-catenin signaling pathway in liver biology and tumorigenesis. Vitr. Cell. Dev. Biol.-Anim. 2024, 60, 466–481. [Google Scholar] [CrossRef]
- Ahn, S.H.; Shah, Y.M.; Inoue, J.; Morimura, K.; Kim, I.; Yim, S.; Lambert, G.; Kurotani, R.; Nagashima, K.; Gonzalez, F.J.; et al. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 908–920. [Google Scholar] [CrossRef]
- McCarty Jr, K.; Miller, L.; Cox, E.; Konrath, J.; McCarty Sr, K. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch. Pathol. Lab. Med. 1985, 109, 716–721. [Google Scholar] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathol 2019, 76, 182. [Google Scholar] [CrossRef]
- Oshima, T.; Kawasaki, T.; Ohashi, R.; Hasegawa, G.; Jiang, S.; Umezu, H.; Aoyagi, Y.; Iwanari, H.; Tanaka, T.; Hamakubo, T. Downregulated P1 promoter-driven hepatocyte nuclear factor-4α expression in human colorectal carcinoma is a new prognostic factor against liver metastasis. Pathol. Int. 2007, 57, 82–90. [Google Scholar] [CrossRef]
- Yeo, M.K.; Kim, J.M.; Suh, K.S.; Kim, S.H.; Lee, O.J.; Kim, K.H. Decreased Expression of the Polarity Regulatory PAR Complex Predicts Poor Prognosis of the Patients with Colorectal Adenocarcinoma. Transl. Oncol. 2018, 11, 109–115. [Google Scholar] [CrossRef]
- Lv, D.-D.; Zhou, L.-Y.; Tang, H. Hepatocyte nuclear factor 4α and cancer-related cell signaling pathways: A promising insight into cancer treatment. Exp. Mol. Med. 2021, 53, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Hasan, A.; Mir, S.S. Exploiting transcription factors to target EMT and cancer stem cells for tumor modulation and therapy, Semin. Cancer Biol. 2024, 100, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Staels, B.; Lefebvre, P.; Verzi, M.P.; Eeckhoute, J. Control of Cell Identity by the Nuclear Receptor HNF4 in Organ Pathophysiology. Cells 2020, 9, 2185. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Patients | HNF4α | ||
|---|---|---|---|---|
| No. (%) | Low | High | p | |
| Sex | 0.118 | |||
| Male | 93 | 54 | 39 | |
| Female | 55 | 39 | 16 | |
| Age (mean) | 63.3 | 63 | 63.8 | 0.614 |
| Tumor size (mean, cm) | 3.6 | 3.6 | 3.4 | 0.404 |
| Differentiation | 0.037 | |||
| WD + MD | 141 (95.3%) | 86 (92.5%) | 55 (100%) | |
| PD | 7 (4.7%) | 7 (7.5%) | 0 (0%) | |
| Pathologic stage | 0.008 | |||
| I–II | 87 (58.8) | 47 (50.5) | 40 (72.7) | |
| III–IV | 61 (41.2) | 46 (49.5) | 15 (27.3) | |
| Nodal metastasis | 0.003 | |||
| Absent | 90 (60.8) | 48 (51.6) | 42 (76.4) | |
| Present | 58 (39.2) | 45 (48.4) | 13 (23.6) | |
| Distant metastasis | 0.363 | |||
| Absent | 136 (91.9) | 84 (90.3) | 52 (94.5) | |
| Present | 12 (8.1) | 9 (9.7) | 3 (5.5) | |
| Radiotherapy | 0.554 | |||
| Not done | 137 (92.6) | 87 (93.5) | 50 (90.9) | |
| Done | 11 (7.4) | 6 (6.5) | 5 (9.1) | |
| Chemotherapy | 0.633 | |||
| Not done | 19 (12.8) | 11 (11.8) | 8 (14.5) | |
| Done | 128 (87.2) | 82 (88.2) | 47 (85.5) | |
| Disease-Free Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|
| p | HR | 95% CI | p | HR | 95% CI | |
| HNF4α (low vs. high) | 0.036 | 0.407 | 0.176–0.943 | 0.113 | 0.642 | 0.371–1.111 |
| Sex (male vs. female) | 0.233 | 1.547 | 0.755–3.169 | 0.216 | 0.721 | 0.430–1.211 |
| Age (under 66 vs. over 66) | 0.398 | 0.743 | 0.373–1.479 | 0.000 | 2.907 | 1.701–4.968 |
| Size (<4cm vs. ≥4cm) | 0.830 | 1.087 | 0.507–2.331 | 0.775 | 0.920 | 0.519–1.629 |
| Differentiation (WD + MD vs. PD) | 0.112 | 2.609 | 0.801–8.501 | 0.964 | 0.975 | 0.332–2.867 |
| Stage (I + II vs. III + IV) | 0.139 | 9.103 | 0.489–169.453 | 0.674 | 0.694 | 0.127–3.804 |
| Nodal metastasis (Absent vs. Present) | 0.212 | 0.163 | 0.009–2.823 | 0.185 | 3.039 | 0.587–15.743 |
| Distant metastasis (Absent vs. Present) | 0.295 | 0.337 | 0.044–2.581 | 0.007 | 3.271 | 1.393–7.682 |
| Radiotherapy (Not done vs. Done) | 0.000 | 7.789 | 0.324–18.725 | 0.009 | 2.860 | 1.294–6.325 |
| Chemotherapy (Not done vs. Done) | 0.637 | 0.789 | 0.261–2.379 | 0.651 | 0.837 | 0.387–1.810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Kim, K.-H.; Heo, J.Y.; Choi, M.K.; Yeo, M.-K. Dual Role of HNF4α in Colorectal Adenocarcinoma During Carcinogenesis and Metastasis. Cells 2025, 14, 599. https://doi.org/10.3390/cells14080599
Kim JS, Kim K-H, Heo JY, Choi MK, Yeo M-K. Dual Role of HNF4α in Colorectal Adenocarcinoma During Carcinogenesis and Metastasis. Cells. 2025; 14(8):599. https://doi.org/10.3390/cells14080599
Chicago/Turabian StyleKim, Ju Seok, Kyung-Hee Kim, Jun Young Heo, Min Kyung Choi, and Min-Kyung Yeo. 2025. "Dual Role of HNF4α in Colorectal Adenocarcinoma During Carcinogenesis and Metastasis" Cells 14, no. 8: 599. https://doi.org/10.3390/cells14080599
APA StyleKim, J. S., Kim, K.-H., Heo, J. Y., Choi, M. K., & Yeo, M.-K. (2025). Dual Role of HNF4α in Colorectal Adenocarcinoma During Carcinogenesis and Metastasis. Cells, 14(8), 599. https://doi.org/10.3390/cells14080599






