Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells
Abstract
:1. Introduction
2. Cell-Depleting Therapies
2.1. Anti-CD20 Monoclonal Antibodies
2.1.1. Mode of Action and Efficacy
Rituximab
Ocrelizumab
Ofatumumab
Ublituximab
2.1.2. Adverse Events
Hypogammaglobulinemia
Infections
Derisking Strategies
Vaccines
Other AEs
2.2. Alemtuzumab
2.2.1. Mode of Action and Efficacy
2.2.2. Adverse Events
Autoimmunity
Infections
Other AEs
Vaccines
2.3. Cladribine
2.3.1. Mode of Action and Efficacy
2.3.2. Adverse Events
Infections
Malignancies
Vaccines
3. Sequestering Therapies
3.1. Natalizumab
3.1.1. Mode of Action and Efficacy
3.1.2. Adverse Events
Infections
Vaccines
3.2. Sphingosine-1-Phosphate Receptor Modulators
3.2.1. Mode of Action and Efficacy
3.2.2. Adverse Events
Infections
Other AEs
Vaccines
4. Future Perspectives
4.1. Bruton’s Tyrosine Kinase Inhibitors
4.2. Future Advancements on Ocrelizumab
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in multiple sclerosis. WIREs Mech. Dis. 2023, 15, e1583. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Ebers, G. Multiple sclerosis: The environment and causation. Curr. Opin. Neurol. 2007, 20, 261. [Google Scholar] [CrossRef]
- Selter, R.C.; Hemmer, B. Update on immunopathogenesis and immunotherapy in multiple sclerosis. Immunotargets Ther. 2013, 2, 21–30. [Google Scholar] [CrossRef]
- O’Connor, K.C.; Bar-Or, A.; Hafler, D.A. The neuroimmunology of multiple sclerosis: Possible roles of T and B lymphocytes in immunopathogenesis. J. Clin. Immunol. 2001, 21, 81–92. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Kaskow, B.J.; Baecher-Allan, C. Effector T Cells in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029025. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H.G. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef]
- Steinman, L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron 1999, 24, 511–514. [Google Scholar] [CrossRef]
- Dörner, T.; Jacobi, A.M.; Lipsky, P.E. B cells in autoimmunity. Arthritis Res. Ther. 2009, 11, 247. [Google Scholar] [CrossRef]
- Holloman, J.P.; Axtell, R.C.; Monson, N.L.; Wu, G.F. The Role of B Cells in Primary Progressive Multiple Sclerosis. Front. Neurol. 2021, 12, 680581. [Google Scholar] [CrossRef] [PubMed]
- Meinl, E.; Krumbholz, M.; Hohlfeld, R. B lineage cells in the inflammatory central nervous system environment: Migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 2006, 59, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Meinl, E. B cells in multiple sclerosis: Good or bad guys?: An article for 28 May 2014-World MS Day 2014. Eur. J. Immunol. 2014, 44, 1247–1250. [Google Scholar] [CrossRef]
- Link, H.; Huang, Y.-M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: An update on methodology and clinical usefulness. J. Neuroimmunol. 2006, 180, 17–28. [Google Scholar] [CrossRef]
- Silva, B.A.; Miglietta, E.; Ferrari, C.C. Insights into the role of B cells in the cortical pathology of Multiple sclerosis: Evidence from animal models and patients. Mult. Scler. Relat. Disord. 2021, 50, 102845. [Google Scholar] [CrossRef]
- Arneth, B.M. Impact of B cells to the pathophysiology of multiple sclerosis. J. Neuroinflamm. 2019, 16, 128. [Google Scholar] [CrossRef]
- Fraussen, J.; de Bock, L.; Somers, V. B cells and antibodies in progressive multiple sclerosis: Contribution to neurodegeneration and progression. Autoimmun. Rev. 2016, 15, 896–899. [Google Scholar] [CrossRef]
- Angelini, G.; Bani, A.; Constantin, G.; Rossi, B. The interplay between T helper cells and brain barriers in the pathogenesis of multiple sclerosis. Front. Cell Neurosci. 2023, 17, 1101379. [Google Scholar] [CrossRef]
- Lund, F.E.; Randall, T.D. Effector and regulatory B cells: Modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 2010, 10, 236–247. [Google Scholar] [CrossRef]
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. 2018, 24, 96–120. [Google Scholar] [CrossRef] [PubMed]
- Samjoo, I.A.; Worthington, E.; Drudge, C.; Zhao, M.; Cameron, C.; Häring, D.A.; Stoneman, D.; Klotz, L.; Adlard, N. Efficacy classification of modern therapies in multiple sclerosis. J. Comp. Eff. Res. 2021, 10, 495–507. [Google Scholar] [CrossRef]
- Filippi, M.; Amato, M.P.; Centonze, D.; Gallo, P.; Gasperini, C.; Inglese, M.; Patti, F.; Pozzilli, C.; Preziosa, P.; Trojano, M. Early use of high-efficacy disease—Modifying therapies makes the difference in people with multiple sclerosis: An expert opinion. J. Neurol. 2022, 269, 5382–5394. [Google Scholar] [CrossRef]
- Kappos, L.; Li, D.; Calabresi, P.A.; O’Connor, P.; Bar-Or, A.; Barkhof, F.; Yin, M.; Leppert, D.; Glanzman, R.; Tinbergen, J.; et al. Ocrelizumab in relapsing-remitting multiple sclerosis: A phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011, 378, 1779–1787. [Google Scholar] [CrossRef]
- Sorensen, P.S.; Lisby, S.; Grove, R.; Derosier, F.; Shackelford, S.; Havrdova, E.; Drulovic, J.; Filippi, M. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: A phase 2 study. Neurology 2014, 82, 573–581. [Google Scholar] [CrossRef]
- Hawker, K.; O’Connor, P.; Freedman, M.S.; Calabresi, P.A.; Antel, J.; Simon, J.; Hauser, S.; Waubant, E.; Vollmer, T.; Panitch, H.; et al. Rituximab in patients with primary progressive multiple sclerosis: Results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009, 66, 460–471. [Google Scholar] [CrossRef]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Li, R.; Patterson, K.R.; Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 2018, 19, 696–707. [Google Scholar] [CrossRef]
- von Essen, M.R.; Ammitzbøll, C.; Hansen, R.H.; Petersen, E.R.S.; McWilliam, O.; Marquart, H.V.; Damm, P.; Sellebjerg, F. Proinflammatory CD20+ T cells in the pathogenesis of multiple sclerosis. Brain 2019, 142, 120–132. [Google Scholar] [CrossRef]
- Cragg, M.S.; Morgan, S.M.; Chan, H.T.C.; Morgan, B.P.; Filatov, A.V.; Johnson, P.W.M.; French, R.R.; Glennie, M.J. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood 2003, 101, 1045–1052. [Google Scholar] [CrossRef]
- Beers, S.A.; Chan, C.H.T.; French, R.R.; Cragg, M.S.; Glennie, M.J. CD20 as a target for therapeutic type I and II monoclonal antibodies. Semin. Hematol. 2010, 47, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Boross, P.; Leusen, J.H.W. Mechanisms of action of CD20 antibodies. Am. J. Cancer Res. 2012, 2, 676–690. [Google Scholar] [PubMed]
- de Sèze, J.; Maillart, E.; Gueguen, A.; Laplaud, D.A.; Michel, L.; Thouvenot, E.; Zephir, H.; Zimmer, L.; Biotti, D.; Liblau, R. Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic. Front. Immunol. 2023, 14, 1004795. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Lammens, A.; Schäfer, W.; Georges, G.; Schwaiger, M.; Mössner, E.; Hopfner, K.-P.; Umaña, P.; Niederfellner, G. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs 2013, 5, 22–33. [Google Scholar] [CrossRef]
- Delgado, S.R.; Faissner, S.; Linker, R.A.; Rammohan, K. Key characteristics of anti-CD20 monoclonal antibodies and clinical implications for multiple sclerosis treatment. J. Neurol. 2024, 271, 1515–1535. [Google Scholar] [CrossRef]
- Le Garff-Tavernier, M.; Herbi, L.; De Romeuf, C.; Nguyen-Khac, F.; Davi, F.; Grelier, A.; Boudjoghra, M.; Maloum, K.; Choquet, S.; Urbain, R.; et al. Antibody-dependent cellular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia 2014, 28, 230–233. [Google Scholar] [CrossRef]
- Roll, P.; Palanichamy, A.; Kneitz, C.; Dorner, T.; Tony, H.-P. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2377–2386. [Google Scholar] [CrossRef]
- Leandro, M.J. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res. Ther. 2013, 15 (Suppl. 1), S3. [Google Scholar] [CrossRef]
- Nissimov, N.; Hajiyeva, Z.; Torke, S.; Grondey, K.; Brück, W.; Häusser-Kinzel, S.; Weber, M.S. B cells reappear less mature and more activated after their anti-CD20-mediated depletion in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 25690–25699. [Google Scholar] [CrossRef]
- Rodriguez-Mogeda, C.; van Lierop, Z.Y.G.J.; van der Pol, S.M.A.; Coenen, L.; Hogenboom, L.; Kamermans, A.; Rodriguez, E.; van Horssen, J.; van Kempen, Z.L.E.; Uitdehaag, B.M.J.; et al. Extended interval dosing of ocrelizumab modifies the repopulation of B cells without altering the clinical efficacy in multiple sclerosis. J. Neuroinflamm. 2023, 20, 215. [Google Scholar] [CrossRef]
- Cotchett, K.R.; Dittel, B.N.; Obeidat, A.Z. Comparison of the Efficacy and Safety of Anti-CD20 B Cells Depleting Drugs in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 49, 102787. [Google Scholar] [CrossRef] [PubMed]
- The Role of CD19 in Monitoring B-Cell Depletion Therapy with Rituximab. Available online: https://www.sciqst.com/The%20Role%20of%20CD19%20in%20Monitoring%20B-Cell%20Depletion%20Therapy%20with%20Rituximab (accessed on 19 March 2025).
- Ellrichmann, G.; Bolz, J.; Peschke, M.; Duscha, A.; Hellwig, K.; Lee, D.-H.; Linker, R.A.; Gold, R.; Haghikia, A. Peripheral CD19+ B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J. Neurol. 2019, 266, 57–67. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Tavakolpour, S.; Alesaeidi, S.; Darvishi, M.; GhasemiAdl, M.; Darabi-Monadi, S.; Akhlaghdoust, M.; Elikaei Behjati, S.; Jafarieh, A. A comprehensive review of rituximab therapy in rheumatoid arthritis patients. Clin. Rheumatol. 2019, 38, 2977–2994. [Google Scholar] [CrossRef]
- Bar-Or, A.; Calabresi, P.A.J.; Arnold, D.; Markowitz, C.; Shafer, S.; Kasper, L.H.; Waubant, E.; Gazda, S.; Fox, R.J.; Panzara, M.; et al. Rituximab in relapsing-remitting multiple sclerosis: A 72-week, open-label, phase I trial. Ann. Neurol. 2008, 63, 395–400. [Google Scholar] [CrossRef]
- Kaplan, B.; Kopyltsova, Y.; Khokhar, A.; Lam, F.; Bonagura, V. Rituximab and immune deficiency: Case series and review of the literature. J. Allergy Clin. Immunol. Pract. 2014, 2, 594–600. [Google Scholar] [CrossRef]
- OCREVUS® (ocrelizumab) | Multiple Sclerosis (MS) Treatment. Available online: https://www.ocrevus.com/ (accessed on 6 October 2024).
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Wolinsky, J.S.; Arnold, D.L.; Brochet, B.; Hartung, H.P.; Montalban, X.; Naismith, R.T.; Manfrini, M.; Overell, J.; Koendgen, H.; Sauter, A.; et al. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: A post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020, 19, 998–1009. [Google Scholar] [CrossRef]
- Ocrevus® (Ocrelizumab). 2021. Available online: https://www.gene.com/download/pdf/ocrevus_prescribing.pdf (accessed on 23 May 2022).
- Kesimpta | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kesimpta (accessed on 6 October 2024).
- Food and Drug Administration. Highlights of Prescribing Information. Kesimpta (Ofatumumab) Injection, for Subcutaneous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf (accessed on 11 January 2023).
- Gärtner, J.; Hauser, S.L.; Bar-Or, A.; Montalban, X.; Cohen, J.A.; Cross, A.H.; Deiva, K.; Ganjgahi, H.; Häring, D.A.; Li, B.; et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: Results from ASCLEPIOS I and II. Mult. Scler. 2022, 28, 1562–1575. [Google Scholar] [CrossRef]
- Kira, J.-I.; Nakahara, J.; Sazonov, D.V.; Kurosawa, T.; Tsumiyama, I.; Willi, R.; Zalesak, M.; Pingili, R.; Häring, D.A.; Ramanathan, K.; et al. Effect of ofatumumab versus placebo in relapsing multiple sclerosis patients from Japan and Russia: Phase 2 APOLITOS study. Mult. Scler. 2022, 28, 1229–1238. [Google Scholar] [CrossRef]
- Bar-Or, A.; Wiendl, H.; Montalban, X.; Alvarez, E.; Davydovskaya, M.; Delgado, S.R.; Evdoshenko, E.P.; Giedraitiene, N.; Gross-Paju, K.; Haldre, S.; et al. Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study. Mult. Scler. 2022, 28, 910–924. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; O’Brien, S.M.; Sweeney, M.L.; Fox, E.J.; Cohen, J.A. Clinical Perspectives on the Molecular and Pharmacological Attributes of Anti-CD20 Therapies for Multiple Sclerosis. CNS Drugs 2021, 35, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Savelieva, M.; Kahn, J.; Bagger, M.; Meier, D.P.; Tomic, D.; Leppert, D.; Wallström, E. Comparison of the B-cell recovery time following discontinuation of anti-CD20 therapies. In Proceedings of the ECTRIMS 2017, Paris, France, 25–28 October 2017. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Product Character istics (Kesimpta). Available online: https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf (accessed on 13 January 2023).
- Yu, H.; Graham, G.; David, O.J.; Kahn, J.M.; Savelieva, M.; Pigeolet, E.; Das Gupta, A.; Pingili, R.; Willi, R.; Ramanathan, K.; et al. Population Pharmacokinetic-B Cell Modeling for Ofatumumab in Patients with Relapsing Multiple Sclerosis. CNS Drugs 2022, 36, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Grove, R.A.; Austin, D.J.; Tolson, J.M.; VanMeter, S.A.; Lewis, E.W.; Derosier, F.J.; Lopez, M.C.; Kavanagh, S.T.; Miller, A.E.; et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study. Neurology 2018, 90, e1805–e1814. [Google Scholar] [CrossRef]
- Steinman, L.; Fox, E.; Hartung, H.-P.; Alvarez, E.; Qian, P.; Wray, S.; Robertson, D.; Huang, D.; Selmaj, K.; Wynn, D.; et al. Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2022, 387, 704–714. [Google Scholar] [CrossRef]
- Lovett-Racke, A.E.; Yang, Y.; Liu, Y.; Gormley, M.; Kraus, E.; Graham, C.; Wray, S.; Racke, M.K.; Alvarez, E.; Bass, A.; et al. B cell depletion changes the immune cell profile in multiple sclerosis patients: One-year report. J. Neuroimmunol. 2021, 359, 577676. [Google Scholar] [CrossRef]
- Fox, E.; Lovett-Racke, A.E.; Gormley, M.; Liu, Y.; Petracca, M.; Cocozza, S.; Shubin, R.; Wray, S.; Weiss, M.S.; Bosco, J.A.; et al. A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult. Scler. 2021, 27, 420–429. [Google Scholar] [CrossRef]
- Margoni, M.; Preziosa, P.; Filippi, M.; Rocca, M.A. Anti-CD20 therapies for multiple sclerosis: Current status and future perspectives. J. Neurol. 2022, 269, 1316–1334. [Google Scholar] [CrossRef]
- Athni, T.S.; Barmettler, S. Hypogammaglobulinemia, late-onset neutropenia, and infections following rituximab. Ann. Allergy Asthma Immunol. 2023, 130, 699–712. [Google Scholar] [CrossRef]
- Alvarez, E.; Longbrake, E.E.; Rammohan, K.W.; Stankiewicz, J.; Hersh, C.M. Secondary hypogammaglobulinemia in patients with multiple sclerosis on anti-CD20 therapy: Pathogenesis, risk of infection, and disease management. Mult. Scler. Relat. Disord. 2023, 79, 105009. [Google Scholar] [CrossRef]
- Vollmer, B.L.; Wallach, A.I.; Corboy, J.R.; Dubovskaya, K.; Alvarez, E.; Kister, I. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann. Clin. Transl. Neurol. 2020, 7, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Saidha, S.; Bell, J.; Harold, S.; Belisario, J.M.; Hawe, E.; Shao, Q.; Wyse, K.; Maiese, E.M. Systematic literature review of immunoglobulin trends for anti-CD20 monoclonal antibodies in multiple sclerosis. Neurol. Sci. 2023, 44, 1515–1532. [Google Scholar] [CrossRef]
- Tallantyre, E.C.; Robertson, N.P.; Jolles, S. Secondary antibody deficiency in neurology. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 481–488. [Google Scholar] [CrossRef]
- Mears, V.; Jakubecz, C.; Seeco, C.; Woodson, S.; Serra, A.; Abboud, H. Predictors of hypogammaglobulinemia and serious infections among patients receiving ocrelizumab or rituximab for treatment of MS and NMOSD. J. Neuroimmunol. 2023, 377, 578066. [Google Scholar] [CrossRef]
- Casulo, C.; Maragulia, J.; Zelenetz, A.D. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin. Lymphoma Myeloma Leuk. 2013, 13, 106–111. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, N.Y.; Kim, K.H.; Hyun, J.-W.; Kim, H.J. Rituximab-Induced Hypogammaglobulinemia and Risk of Infection in Neuromyelitis Optica Spectrum Disorders: A 14-Year Real-Life Experience. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1179. [Google Scholar] [CrossRef]
- Irie, E.; Shirota, Y.; Suzuki, C.; Tajima, Y.; Ishizawa, K.; Kameoka, J.; Harigae, H.; Ishii, T. Severe hypogammaglobulinemia persisting for 6 years after treatment with rituximab combined chemotherapy due to arrest of B lymphocyte differentiation together with alteration of T lymphocyte homeostasis. Int. J. Hematol. 2010, 91, 501–508. [Google Scholar] [CrossRef]
- Marco, H.; Smith, R.M.; Jones, R.B.; Guerry, M.-J.; Catapano, F.; Burns, S.; Chaudhry, A.N.; Smith, K.G.C.; Jayne, D.R.W. The effect of rituximab therapy on immunoglobulin levels in patients with multisystem autoimmune disease. BMC Musculoskelet. Disord. 2014, 15, 178. [Google Scholar] [CrossRef]
- McNulty, C.M.; Isikwei, E.A.; Shrestha, P.; Snyder, M.R.; Kabat, B.F.; Rabe, K.G.; Slager, S.L.; Parikh, S.A.; Joshi, A.Y. Risk factors for hypogammaglobulinemia in chronic lymphocytic leukemia patients treated with anti-CD20 monoclonal antibody-based therapies. J. Hematopathol. 2020, 13, 221–229. [Google Scholar] [CrossRef]
- Elgenidy, A.; Abdelhalim, N.N.; Al-Kurdi, M.A.; Mohamed, L.A.; Ghoneim, M.M.; Fathy, A.W.; Hassaan, H.K.; Anan, A.; Alomari, O. Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with anti-CD20 treatments: A systematic review and meta-analysis of 19,139 multiple sclerosis patients. Front. Neurol. 2024, 15, 1380654. [Google Scholar] [CrossRef]
- Smolik, K.; Camilli, F.; Panzera, I.; Fiore, A.; Franceschini, A.; Foschi, M.; Surcinelli, A.; Pesci, I.; Ferri, C.; Bazzurri, V.; et al. Hypogammaglobulinemia and severe infections in Multiple Sclerosis patients on anti-CD20 agents: A multicentre study. Mult. Scler. Relat. Disord. 2024, 93, 106191. [Google Scholar] [CrossRef] [PubMed]
- Evertsson, B.; Hoyt, T.; Christensen, A.; Nimer, F.A.; Foley, J.; Piehl, F. A comparative study of tolerability and effects on immunoglobulin levels and CD19 cell counts with ocrelizumab vs low dose of rituximab in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320964505. [Google Scholar] [CrossRef]
- Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Brochet, B.; Naismith, R.T.; Traboulsee, A.; Wolinsky, J.S.; Belachew, S.; Koendgen, H.; et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology 2020, 95, e1854–e1867. [Google Scholar] [CrossRef] [PubMed]
- Habek, M.; Piskač, D.; Gabelić, T.; Barun, B.; Adamec, I.; Krbot Skorić, M. Hypogammaglobulinemia, infections and COVID-19 in people with multiple sclerosis treated with ocrelizumab. Mult. Scler. Relat. Disord. 2022, 62, 103798. [Google Scholar] [CrossRef]
- Disanto, G.; Ripellino, P.; Riccitelli, G.C.; Sacco, R.; Scotti, B.; Fucili, A.; Pravatà, E.; Kuhle, J.; Gobbi, C.; Zecca, C. De-escalating rituximab dose results in stability of clinical, radiological, and serum neurofilament levels in multiple sclerosis. Mult. Scler. 2021, 27, 1230–1239. [Google Scholar] [CrossRef]
- Hauser, S.L.; Kappos, L.; Montalban, X.; Craveiro, L.; Chognot, C.; Hughes, R.; Koendgen, H.; Pasquarelli, N.; Pradhan, A.; Prajapati, K.; et al. Safety of Ocrelizumab in Patients with Relapsing and Primary Progressive Multiple Sclerosis. Neurology 2021, 97, e1546–e1559. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; de Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cross, A.H.; Winthrop, K.; Wiendl, H.; Nicholas, J.; Meuth, S.G.; Giacomini, P.S.; Saccà, F.; Mancione, L.; Zielman, R.; et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult. Scler. 2022, 28, 1576–1590. [Google Scholar] [CrossRef]
- Perriguey, M.; Maarouf, A.; Stellmann, J.-P.; Rico, A.; Boutiere, C.; Demortiere, S.; Durozard, P.; Pelletier, J.; Audoin, B. Hypogammaglobulinemia and Infections in Patients with Multiple Sclerosis Treated with Rituximab. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1115. [Google Scholar] [CrossRef]
- Schuckmann, A.; Steffen, F.; Zipp, F.; Bittner, S.; Pape, K. Impact of extended interval dosing of ocrelizumab on immunoglobulin levels in multiple sclerosis. Med 2023, 4, 361–372.e3. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; López Acevedo, S.N.; Cuziol, C.; De Tavernier, E.; Fahad, A.S.; Longjam, P.S.; Rao, S.P.; Aguilera-Rodríguez, D.; Rezé, M.; Bricault, C.A.; et al. Large-scale antibody immune response mapping of splenic B cells and bone marrow plasma cells in a transgenic mouse model. Front. Immunol. 2023, 14, 1137069. [Google Scholar] [CrossRef] [PubMed]
- GlaxoSmithKline A Randomized, Double-blind, Placebo-controlled, Parallel-Group, Dose-Ranging Study to Investigate the MRI Efficacy and Safety of Six Months’ Administration of Ofatumumab in Subjects with Relapsing-Remitting Multiple Sclerosis (RRMS); clinicaltrials.gov. 2018. Available online: https://onderzoekmetmensen.nl/en/trial/39973 (accessed on 24 October 2024).
- Peters, J.; Longbrake, E.E. Infection risk in a real-world cohort of patients treated with long-term B-cell depletion for autoimmune neurologic disease. Mult. Scler. Relat. Disord. 2022, 68, 104400. [Google Scholar] [CrossRef]
- Seery, N.; Sharmin, S.; Li, V.; Nguyen, A.-L.; Meaton, C.; Atvars, R.; Taylor, N.; Tunnell, K.; Carey, J.; Marriott, M.P.; et al. Predicting Infection Risk in Multiple Sclerosis Patients Treated with Ocrelizumab: A Retrospective Cohort Study. CNS Drugs 2021, 35, 907–918. [Google Scholar] [CrossRef]
- Kridin, K.; Ahmed, A.R. Post-rituximab immunoglobulin M (IgM) hypogammaglobulinemia. Autoimmun. Rev. 2020, 19, 102466. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Luna, G.; Alping, P.; Burman, J.; Fink, K.; Fogdell-Hahn, A.; Gunnarsson, M.; Hillert, J.; Langer-Gould, A.; Lycke, J.; Nilsson, P.; et al. Infection Risks Among Patients with Multiple Sclerosis Treated with Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2020, 77, 184–191. [Google Scholar] [CrossRef]
- Md Yusof, M.Y.; Vital, E.M.; McElvenny, D.M.; Hensor, E.M.A.; Das, S.; Dass, S.; Rawstron, A.C.; Buch, M.H.; Emery, P.; Savic, S. Predicting Severe Infection and Effects of Hypogammaglobulinemia During Therapy with Rituximab in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 2019, 71, 1812–1823. [Google Scholar] [CrossRef]
- Alvarez, E.; Nair, K.V.; Sillau, S.; Shelton, I.; Seale, R.; Selva, S.; Corboy, J.; Vollmer, T.L. Tolerability and Safety of Switching from Rituximab to Ocrelizumab: Evaluating Factors Associated with Infusion Related Reactions. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 20552173211069360. [Google Scholar] [CrossRef]
- ECTRIMS 2019—Oral Presentations: Serum Immuno Globulin Levels and Risk of Serious Infections in the Pivota. Available online: https://journals.sagepub.com/doi/pdf/10.1177/1352458519868070 (accessed on 19 March 2025).
- Derfuss, T.; Bermel, R.; Lin, C.-J.; Hauser, S.L.; Kappos, L.; Vollmer, T.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Weber, M.S.; et al. Long-term analysis of infections and associated risk factors in patients with multiple sclerosis treated with ocrelizumab: Pooled analysis of 13 interventional clinical trials. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241277736. [Google Scholar] [CrossRef]
- Cohen, J.; Hauser, S.; Cross, A.; Winthrop, K.; Wiendl, H.; Nicholas, J.; Meuth, S.; Giacomini, P.; Sacca, F.; Zielman, R.; et al. Five-Year Safety of Ofatumumab in People Living with Relapsing Multiple Sclerosis (P8-3.004). Neurology 2023, 100, 2942. [Google Scholar] [CrossRef]
- Liu, N.; Yu, W.; Sun, M.; Zhang, W.; Zhou, D.; Sun, J.; Wang, M. Outcome of COVID-19 Infection in Patients with Multiple Sclerosis Who Received Disease-Modifying Therapies: A Systematic Review and Meta-Analysis. J. Clin. Neurol. 2023, 19, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Sormani, M.P.; De Rossi, N.; Schiavetti, I.; Carmisciano, L.; Cordioli, C.; Moiola, L.; Radaelli, M.; Immovilli, P.; Capobianco, M.; Trojano, M.; et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann. Neurol. 2021, 89, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Reder, A.T.; Centonze, D.; Naylor, M.L.; Nagpal, A.; Rajbhandari, R.; Altincatal, A.; Kim, M.; Berdofe, A.; Radhakrishnan, M.; Jung, E.; et al. COVID-19 in Patients with Multiple Sclerosis: Associations with Disease-Modifying Therapies. CNS Drugs 2021, 35, 317–330. [Google Scholar] [CrossRef]
- Salter, A.; Fox, R.J.; Newsome, S.D.; Halper, J.; Li, D.K.B.; Kanellis, P.; Costello, K.; Bebo, B.; Rammohan, K.; Cutter, G.R.; et al. Outcomes and Risk Factors Associated with SARS-CoV-2 Infection in a North American Registry of Patients with Multiple Sclerosis. JAMA Neurol. 2021, 78, 699–708. [Google Scholar] [CrossRef]
- Cross, A.H.; Delgado, S.; Habek, M.; Davydovskaya, M.; Ward, B.J.; Cree, B.A.C.; Totolyan, N.; Pingili, R.; Mancione, L.; Hu, X.; et al. COVID-19 Outcomes and Vaccination in People with Relapsing Multiple Sclerosis Treated with Ofatumumab. Neurol. Ther. 2022, 11, 741–758. [Google Scholar] [CrossRef]
- Otani, I.M.; Lehman, H.K.; Jongco, A.M.; Tsao, L.R.; Azar, A.E.; Tarrant, T.K.; Engel, E.; Walter, J.E.; Truong, T.Q.; Khan, D.A.; et al. Practical guidance for the diagnosis and management of secondary hypogammaglobulinemia: A Work Group Report of the AAAAI Primary Immunodeficiency and Altered Immune Response Committees. J. Allergy Clin. Immunol. 2022, 149, 1525–1560. [Google Scholar] [CrossRef]
- Starvaggi Cucuzza, C.; Longinetti, E.; Ruffin, N.; Evertsson, B.; Kockum, I.; Jagodic, M.; Al Nimer, F.; Frisell, T.; Piehl, F. Sustained Low Relapse Rate with Highly Variable B-Cell Repopulation Dynamics with Extended Rituximab Dosing Intervals in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200056. [Google Scholar] [CrossRef]
- Kelly, H.; Vishnevetsky, A.; Chibnik, L.B.; Levy, M. Hypogammaglobulinemia secondary to B-cell depleting therapies in neuroimmunology: Comparing management strategies. Mult. Scler. J. Exp. Transl. Clin. 2023, 9, 20552173231182536. [Google Scholar] [CrossRef]
- Smets, I.; Giovannoni, G. Derisking CD20-therapies for long-term use. Mult. Scler. Relat. Disord. 2022, 57, 103418. [Google Scholar] [CrossRef]
- Wijetilleka, S.; Jayne, D.R.; Mukhtyar, C.; Ala, A.; Bright, P.D.; Chinoy, H.; Harper, L.; Kazmi, M.A.; Kiani-Alikhan, S.; Li, C.K.; et al. Recommendations for the management of secondary hypogammaglobulinaemia due to B cell targeted therapies in autoimmune rheumatic diseases. Rheumatology 2019, 58, 889–896. [Google Scholar] [CrossRef]
- Bar-Or, A.; Calkwood, J.C.; Chognot, C.; Evershed, J.; Fox, E.J.; Herman, A.; Manfrini, M.; McNamara, J.; Robertson, D.S.; Stokmaier, D.; et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology 2020, 95, e1999–e2008. [Google Scholar] [CrossRef] [PubMed]
- Vijenthira, A.; Gong, I.; Betschel, S.D.; Cheung, M.; Hicks, L.K. Vaccine response following anti-CD20 therapy: A systematic review and meta-analysis of 905 patients. Blood Adv. 2021, 5, 2624–2643. [Google Scholar] [CrossRef] [PubMed]
- Bingham, C.O.; Looney, R.J.; Deodhar, A.; Halsey, N.; Greenwald, M.; Codding, C.; Trzaskoma, B.; Martin, F.; Agarwal, S.; Kelman, A. Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial. Arthritis Rheum. 2010, 62, 64–74. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.-H.; Huh, S.-Y.; Kong, S.-Y.; Choi, Y.J.; Cheong, H.J.; Kim, H.J. Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur. J. Neurol. 2013, 20, 975–980. [Google Scholar] [CrossRef]
- van Assen, S.; Holvast, A.; Benne, C.A.; Posthumus, M.D.; van Leeuwen, M.A.; Voskuyl, A.E.; Blom, M.; Risselada, A.P.; de Haan, A.; Westra, J.; et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010, 62, 75–81. [Google Scholar] [CrossRef]
- Interim Results of Open-label Multicenter Phase 4 Study Assessing Immune Response to Influenza Vaccine in Patients with Relapsing Multiple Sclerosis Treated with Ofatumumab. Available online: https://mscare.sharefile.com/share/view/sdc1d528dd1704e7e90edae25e4631988 (accessed on 19 March 2025).
- Levit, E.; Longbrake, E.E.; Stoll, S.S. Seroconversion after COVID-19 vaccination for multiple sclerosis patients on high efficacy disease modifying medications. Mult. Scler. Relat. Disord. 2022, 60, 103719. [Google Scholar] [CrossRef]
- Bitoun, S.; Henry, J.; Desjardins, D.; Vauloup-Fellous, C.; Dib, N.; Belkhir, R.; Mouna, L.; Joly, C.; Bitu, M.; Ly, B.; et al. Rituximab Impairs B Cell Response But Not T Cell Response to COVID-19 Vaccine in Autoimmune Diseases. Arthritis Rheumatol. 2022, 74, 927–933. [Google Scholar] [CrossRef]
- Jaber, A.; Patel, M.; Sylvester, A.; Yarussi, M.; Kalina, J.T.; Mendoza, J.P.; Avila, R.L.; Tremblay, M.A. COVID-19 Vaccine Response in People with Multiple Sclerosis Treated with Dimethyl Fumarate, Diroximel Fumarate, Natalizumab, Ocrelizumab, or Interferon Beta Therapy. Neurol. Ther. 2023, 12, 687–700. [Google Scholar] [CrossRef]
- Räuber, S.; Willison, A.; Korsen, M.; Kölsche, T.; Golombeck, K.S.; Plaack, B.; Schüller, J.; Huntemann, N.; Rolfes, L.; Schroeter, C.B.; et al. Vaccine-based clinical protection against SARS-CoV-2 infection and the humoral immune response: A 1-year follow-up study of patients with multiple sclerosis receiving ocrelizumab. Front. Immunol. 2022, 13, 1037214. [Google Scholar] [CrossRef]
- Faissner, S.; Heitmann, N.; Plaza-Sirvent, C.; Trendelenburg, P.; Ceylan, U.; Motte, J.; Bessen, C.; Urlaub, D.; Watzl, C.; Overheu, O.; et al. Immune response in ofatumumab treated multiple sclerosis patients after SARS-CoV-2 vaccination. Front. Immunol. 2022, 13, 980526. [Google Scholar] [CrossRef]
- Ziemssen, T.; Groth, M.; Ettle, B.; Bopp, T. Immune Response to SARS-CoV-2 mRNA Vaccines in an Open-Label Multicenter Study in Participants with Relapsing Multiple Sclerosis Treated with Ofatumumab. Vaccines 2022, 10, 2167. [Google Scholar] [CrossRef] [PubMed]
- SARS-CoV-2 Vaccination Responses in Anti-CD20-Treated Progressive Multiple Sclerosis Patients Show Immunosenescence in Antigen-Specific B and T Cells—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/39204047/ (accessed on 13 February 2025).
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Centonze, D.; Rocca, M.A.; Gasperini, C.; Kappos, L.; Hartung, H.-P.; Magyari, M.; Oreja-Guevara, C.; Trojano, M.; Wiendl, H.; Filippi, M. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: An expert consensus. J. Neurol. 2021, 268, 3961–3968. [Google Scholar] [CrossRef]
- van der Kolk, L.E.; Grillo-López, A.J.; Baars, J.W.; Hack, C.E.; van Oers, M.H. Complement activation plays a key role in the side-effects of rituximab treatment. Br. J. Haematol. 2001, 115, 807–811. [Google Scholar] [CrossRef]
- Zecca, C.; Bovis, F.; Novi, G.; Capobianco, M.; Lanzillo, R.; Frau, J.; Repice, A.M.; Hakiki, B.; Realmuto, S.; Bonavita, S.; et al. Treatment of multiple sclerosis with rituximab: A multicentric Italian-Swiss experience. Mult. Scler. 2020, 26, 1519–1531. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Cree, B.A.C.; Hauser, S.L. Ocrelizumab and Other CD20+ B-Cell-Depleting Therapies in Multiple Sclerosis. Neurotherapeutics 2017, 14, 835–841. [Google Scholar] [CrossRef]
- Ciardi, M.R.; Iannetta, M.; Zingaropoli, M.A.; Salpini, R.; Aragri, M.; Annecca, R.; Pontecorvo, S.; Altieri, M.; Russo, G.; Svicher, V.; et al. Reactivation of Hepatitis B Virus with Immune-Escape Mutations After Ocrelizumab Treatment for Multiple Sclerosis. Open Forum Infect. Dis. 2019, 6, ofy356. [Google Scholar] [CrossRef]
- Ratzinger, G.; Reagan, J.L.; Heller, G.; Busam, K.J.; Young, J.W. Differential CD52 expression by distinct myeloid dendritic cell subsets: Implications for alemtuzumab activity at the level of antigen presentation in allogeneic graft-host interactions in transplantation. Blood 2003, 101, 1422–1429. [Google Scholar] [CrossRef]
- Hu, Y.; Turner, M.J.; Shields, J.; Gale, M.S.; Hutto, E.; Roberts, B.L.; Siders, W.M.; Kaplan, J.M. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 2009, 128, 260–270. [Google Scholar] [CrossRef]
- Hale, G. The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy 2001, 3, 137–143. [Google Scholar] [CrossRef]
- Lemtrada | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lemtrada (accessed on 5 October 2024).
- CAMMS223 Trial Investigators; Coles, A.J.; Compston, D.A.S.; Selmaj, K.W.; Lake, S.L.; Moran, S.; Margolin, D.H.; Norris, K.; Tandon, P.K. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N. Engl. J. Med. 2008, 359, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Coles, A.J.; Arnold, D.L.; Confavreux, C.; Fox, E.J.; Hartung, H.-P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; Fisher, E.; et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012, 380, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.J.; Twyman, C.L.; Arnold, D.L.; Cohen, J.A.; Confavreux, C.; Fox, E.J.; Hartung, H.-P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012, 380, 1829–1839. [Google Scholar] [CrossRef]
- Singer, B.A.; Alroughani, R.; Brassat, D.; Broadley, S.; Hartung, H.-P.; Havrdova, E.; Kim, H.J.; Oreja-Guevara, C.; Pozzilli, C.; Selmaj, K.W.; et al. Durable Clinical Outcomes with Alemtuzumab in Patients with Active RRMS in the Absence of Continuous Treatment: 7-Year Follow-up of CARE-MS II Patients (TOPAZ Study) (P6.369). Neurology 2018, 90, P6.369. [Google Scholar] [CrossRef]
- Havrdova, E.; Horakova, D.; Kovarova, I. Alemtuzumab in the treatment of multiple sclerosis: Key clinical trial results and considerations for use. Ther. Adv. Neurol. Disord. 2015, 8, 31–45. [Google Scholar] [CrossRef]
- Gross, C.C.; Ahmetspahic, D.; Ruck, T.; Schulte-Mecklenbeck, A.; Schwarte, K.; Jörgens, S.; Scheu, S.; Windhagen, S.; Graefe, B.; Melzer, N.; et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e289. [Google Scholar] [CrossRef]
- Rolla, S.; Maglione, A.; De Mercanti, S.F.; Clerico, M. The Meaning of Immune Reconstitution after Alemtuzumab Therapy in Multiple Sclerosis. Cells 2020, 9, 1396. [Google Scholar] [CrossRef]
- Baker, D.; Herrod, S.S.; Alvarez-Gonzalez, C.; Zalewski, L.; Albor, C.; Schmierer, K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e360. [Google Scholar] [CrossRef]
- Cossburn, M.D.; Harding, K.; Ingram, G.; El-Shanawany, T.; Heaps, A.; Pickersgill, T.P.; Jolles, S.; Robertson, N.P. Clinical relevance of differential lymphocyte recovery after alemtuzumab therapy for multiple sclerosis. Neurology 2013, 80, 55–61. [Google Scholar] [CrossRef]
- Gilmore: Leukocyte Repopulation Following Alemtuzumab…—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?journal=Mult+Scler&title=Leukocyte+repopulation+following+alemtuzumab+treatment+in+relapsing-remitting+MS+contains+multiple+regulatory+immune+cell+types&author=W+Gilmore&author=BT+Lund&author=A+Traboulsee&volume=23&issue=suppl+3&publication_year=2017&pages=P979& (accessed on 5 October 2024).
- Thompson, S.A.J.; Jones, J.L.; Cox, A.L.; Compston, D.A.S.; Coles, A.J. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J. Clin. Immunol. 2010, 30, 99–105. [Google Scholar] [CrossRef]
- Baker, D.; Herrod, S.S.; Alvarez-Gonzalez, C.; Giovannoni, G.; Schmierer, K. Interpreting Lymphocyte Reconstitution Data From the Pivotal Phase 3 Trials of Alemtuzumab. JAMA Neurol. 2017, 74, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Richards, S.; Surks, H.K.; Jacobs, A.; Panzara, M.A. Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis. Clin. Exp. Immunol. 2018, 194, 295–314. [Google Scholar] [CrossRef]
- Akgün, K.; Blankenburg, J.; Marggraf, M.; Haase, R.; Ziemssen, T. Event-Driven Immunoprofiling Predicts Return of Disease Activity in Alemtuzumab-Treated Multiple Sclerosis. Front. Immunol. 2020, 11, 56. [Google Scholar] [CrossRef]
- Kashani, N.; Kelland, E.E.; Vajdi, B.; Anderson, L.M.; Gilmore, W.; Lund, B.T. Immune Regulatory Cell Bias Following Alemtuzumab Treatment in Relapsing-Remitting Multiple Sclerosis. Front. Immunol. 2021, 12, 706278. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.; Shin, H.-J.; Hyun, J.-W.; Kim, S.-H.; Lee, E.; Kim, H.J. Restoration of regulatory B cell deficiency following alemtuzumab therapy in patients with relapsing multiple sclerosis. J. Neuroinflamm. 2018, 15, 300. [Google Scholar] [CrossRef]
- Lighaam, L.C.; Unger, P.-P.A.; Vredevoogd, D.W.; Verhoeven, D.; Vermeulen, E.; Turksma, A.W.; Ten Brinke, A.; Rispens, T.; van Ham, S.M. In vitro-Induced Human IL-10+ B Cells Do Not Show a Subset-Defining Marker Signature and Plastically Co-express IL-10 with Pro-Inflammatory Cytokines. Front. Immunol. 2018, 9, 1913. [Google Scholar] [CrossRef]
- Staun-Ram, E.; Miller, A. Effector and regulatory B cells in Multiple Sclerosis. Clin. Immunol. 2017, 184, 11–25. [Google Scholar] [CrossRef]
- Coles, A.J.; Wing, M.; Smith, S.; Coraddu, F.; Greer, S.; Taylor, C.; Weetman, A.; Hale, G.; Chatterjee, V.K.; Waldmann, H.; et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999, 354, 1691–1695. [Google Scholar] [CrossRef]
- Ziemssen, T.; Thomas, K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: An update on the clinical trial evidence and data from the real world. Ther. Adv. Neurol. Disord. 2017, 10, 343–359. [Google Scholar] [CrossRef]
- Meltzer, E.; Campbell, S.; Ehrenfeld, B.; Cruz, R.A.; Steinman, L.; Parsons, M.S.; Zamvil, S.S.; Frohman, E.M.; Frohman, T.C. Mitigating alemtuzumab-associated autoimmunity in MS: A “whack-a-mole” B-cell depletion strategy. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e868. [Google Scholar] [CrossRef]
- Coles, A.J.; Fox, E.; Vladic, A.; Gazda, S.K.; Brinar, V.; Selmaj, K.W.; Skoromets, A.; Stolyarov, I.; Bass, A.; Sullivan, H.; et al. Alemtuzumab more effective than interferon β-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology 2012, 78, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- LEMTRADA® (Alemtuzumab) Injection, for Intravenous Use Prescribing Information. Available online: https://products.sanofi.us/lemtrada/lemtrada.html (accessed on 19 October 2024).
- Pariani, N.; Willis, M.; Muller, I.; Healy, S.; Nasser, T.; McGowan, A.; Lyons, G.; Jones, J.; Chatterjee, K.; Dayan, C.; et al. Alemtuzumab-Induced Thyroid Dysfunction Exhibits Distinctive Clinical and Immunological Features. J. Clin. Endocrinol. Metab. 2018, 103, 3010–3018. [Google Scholar] [CrossRef] [PubMed]
- Sellner, J.; Rommer, P.S. Immunological consequences of “immune reconstitution therapy” in multiple sclerosis: A systematic review. Autoimmun. Rev. 2020, 19, 102492. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Phuah, C.-L.; Cox, A.L.; Thompson, S.A.; Ban, M.; Shawcross, J.; Walton, A.; Sawcer, S.J.; Compston, A.; Coles, A.J. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J. Clin. Investig. 2009, 119, 2052–2061. [Google Scholar] [CrossRef]
- Costelloe, L.; Jones, J.; Coles, A. Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev. Neurother. 2012, 12, 335–341. [Google Scholar] [CrossRef]
- Kuchen, S.; Robbins, R.; Sims, G.P.; Sheng, C.; Phillips, T.M.; Lipsky, P.E.; Ettinger, R. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 2007, 179, 5886–5896. [Google Scholar] [CrossRef]
- Sakuraba, K.; Oyamada, A.; Fujimura, K.; Spolski, R.; Iwamoto, Y.; Leonard, W.J.; Yoshikai, Y.; Yamada, H. Interleukin-21 signaling in B cells, but not in T cells, is indispensable for the development of collagen-induced arthritis in mice. Arthritis Res. Ther. 2016, 18, 188. [Google Scholar] [CrossRef]
- Wray, S.; Havrdova, E.; Snydman, D.R.; Arnold, D.L.; Cohen, J.A.; Coles, A.J.; Hartung, H.-P.; Selmaj, K.W.; Weiner, H.L.; Daizadeh, N.; et al. Infection risk with alemtuzumab decreases over time: Pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult. Scler. 2019, 25, 1605–1617. [Google Scholar] [CrossRef]
- Buonomo, A.R.; Zappulo, E.; Viceconte, G.; Scotto, R.; Borgia, G.; Gentile, I. Risk of opportunistic infections in patients treated with alemtuzumab for multiple sclerosis. Expert Opin. Drug Saf. 2018, 17, 709–717. [Google Scholar] [CrossRef]
- Rau, D.; Lang, M.; Harth, A.; Naumann, M.; Weber, F.; Tumani, H.; Bayas, A. Listeria Meningitis Complicating Alemtuzumab Treatment in Multiple Sclerosis--Report of Two Cases. Int. J. Mol. Sci. 2015, 16, 14669–14676. [Google Scholar] [CrossRef]
- Penkert, H.; Delbridge, C.; Wantia, N.; Wiestler, B.; Korn, T. Fulminant Central Nervous System Nocardiosis in a Patient Treated with Alemtuzumab for Relapsing-Remitting Multiple Sclerosis. JAMA Neurol. 2016, 73, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Hawkes, C.; Lechner-Scott, J.; Levy, M.; Waubant, E.; Gold, J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult. Scler. Relat. Disord. 2020, 39, 102073. [Google Scholar] [CrossRef]
- Iovino, A.; Olivieri, N.; Aruta, F.; Giaquinto, E.; Ruggiero, L.; Spina, E.; Tozza, S.; Manganelli, F.; Iodice, R. Alemtuzumab in Covid era. Mult. Scler. Relat. Disord. 2021, 51, 102908. [Google Scholar] [CrossRef]
- COVID-19 Severity and Vaccination Effect in Persons with MS Treated with Alemtuzumab. Available online: https://www.campus.sanofi/dam/jcr:9cb74030-9d2f-4321-8d8d-4a6e45c8cad5/ECTRIMS%202022%20Alem_Sormani_COVID-19%20Outcomes__Poster%20(1).pdf (accessed on 19 March 2025).
- Matías-Guiu, J.; Montero-Escribano, P.; Pytel, V.; Porta-Etessam, J.; Matias-Guiu, J.A. Potential COVID-19 infection in patients with severe multiple sclerosis treated with alemtuzumab. Mult. Scler. Relat. Disord. 2020, 44, 102297. [Google Scholar] [CrossRef]
- Ziemssen, T.; Bass, A.D.; Berkovich, R.; Comi, G.; Eichau, S.; Hobart, J.; Hunter, S.F.; LaGanke, C.; Limmroth, V.; Pelletier, D.; et al. Efficacy and Safety of Alemtuzumab Through 9 Years of Follow-up in Patients with Highly Active Disease: Post Hoc Analysis of CARE-MS I and II Patients in the TOPAZ Extension Study. CNS Drugs 2020, 34, 973–988. [Google Scholar] [CrossRef]
- Measures to Minimise Risk of Serious Side Effects of Multiple Sclerosis Medicine Lemtrada | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/news/measures-minimise-risk-serious-side-effects-multiple-sclerosis-medicine-lemtrada (accessed on 19 October 2024).
- Azevedo, C.J.; Kutz, C.; Dix, A.; Boster, A.; Sanossian, N.; Kaplan, J. Intracerebral haemorrhage during alemtuzumab administration. Lancet Neurol. 2019, 18, 329–331. [Google Scholar] [CrossRef]
- Syed, Y.Y. Alemtuzumab: A Review in Relapsing Remitting Multiple Sclerosis. Drugs 2021, 81, 157–168. [Google Scholar] [CrossRef]
- McCarthy, C.L.; Tuohy, O.; Compston, D.A.; Kumararatne, D.S.; Coles, A.J.; Jones, J.L. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology 2013, 81, 872–876. [Google Scholar] [CrossRef]
- Drulovic, J.; Ivanovic, J.; Martinovic, V.; Tamas, O.; Veselinovic, N.; Cujic, D.; Gnjatovic, M.; Mesaros, S.; Pekmezovic, T. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult. Scler. Relat. Disord. 2021, 54, 103150. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Magalashvili, D.; Menascu, S.; Warszawer, Y.; Dolev, M.; Didikin, M.; Harari, G.; Sonis, P.; et al. In-depth characterization of long-term humoral and cellular immune responses to COVID-19m-RNA vaccination in multiple sclerosis patients treated with teriflunomide or alemtuzumab. Mult. Scler. Relat. Disord. 2023, 72, 104616. [Google Scholar] [CrossRef]
- Empowering People Affected by MS to Live Their Best Lives. Available online: https://www.nationalmssociety.org (accessed on 12 January 2025).
- Leist, T.P.; Weissert, R. Cladribine: Mode of action and implications for treatment of multiple sclerosis. Clin. Neuropharmacol. 2011, 34, 28–35. [Google Scholar] [CrossRef] [PubMed]
- MAVENCLAD 10 mg Tablets—Summary of Product Characteristics (SmPC)—(emc). Available online: https://www.medicines.org.uk/emc/product/8435/smpc#gref (accessed on 5 October 2024).
- EMD Serono Inc. MAVENCLAD 10 mg Tablets Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022561s000lbl.pdf (accessed on 5 October 2024).
- Cook, S.; Vermersch, P.; Comi, G.; Giovannoni, G.; Rammohan, K.; Rieckmann, P.; Sørensen, P.S.; Hamlett, A.; Miret, M.; Weiner, J.; et al. Safety and tolerability of cladribine tablets in multiple sclerosis: The CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study. Mult. Scler. 2011, 17, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Leist, T.P.; Comi, G.; Cree, B.A.; Coyle, P.K.; Freedman, M.S.; Hartung, H.P.; Vermersch, P.; Casset-Semanaz, F.; Scaramozza, M.; on behalf of the oral cladribine for early MS (ORACLE MS) Study Group. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): A phase 3 randomised trial. Lancet Neurol. 2014, 13, 257–267. [Google Scholar] [CrossRef]
- Montalban, X.; Cohen, B.; Leist, T.; Moses, H.; Hicking, C.; Dangond, F. Efficacy of Cladribine Tablets as Add-On to IFN-beta Therapy in Patients with Active Relapsing MS: Final Results from the Phase II ONWARD Study (P3.029). Neurology 2016, 86, P3.029. [Google Scholar] [CrossRef]
- Beutler, E.; Sipe, J.C.; Romine, J.S.; Koziol, J.A.; McMillan, R.; Zyroff, J. The treatment of chronic progressive multiple sclerosis with cladribine. Proc. Natl. Acad. Sci. USA 1996, 93, 1716–1720. [Google Scholar] [CrossRef]
- Smal, C.; Vertommen, D.; Bertrand, L.; Ntamashimikiro, S.; Rider, M.H.; Van Den Neste, E.; Bontemps, F. Identification of in vivo phosphorylation sites on human deoxycytidine kinase. Role of Ser-74 in the control of enzyme activity. J. Biol. Chem. 2006, 281, 4887–4893. [Google Scholar] [CrossRef]
- Toy, G.; Austin, W.R.; Liao, H.-I.; Cheng, D.; Singh, A.; Campbell, D.O.; Ishikawa, T.; Lehmann, L.W.; Satyamurthy, N.; Phelps, M.E.; et al. Requirement for deoxycytidine kinase in T and B lymphocyte development. Proc. Natl. Acad. Sci. USA 2010, 107, 5551–5556. [Google Scholar] [CrossRef]
- Salvat: Cellular Expression Profiling of Genes Involved...—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?journal=Cellular+expression+profiling+of+genes+involved+in+the+cladribine+metabolic+pathway:+insights+into+mechanism+of+action+in+multiple+sclerosis&author=C+Salvat&author=ML+Curchod&author=E+Guedj& (accessed on 5 October 2024).
- Gil, M.; Ramil, F.; AgÍs, J.A. Hydroids (Cnidaria, Hydrozoa) from Mauritanian Coral Mounds. Zootaxa 2020, 4878, zootaxa.4878.3.2. [Google Scholar] [CrossRef]
- Giovannoni, G.; Mathews, J. Cladribine Tablets for Relapsing-Remitting Multiple Sclerosis: A Clinician’s Review. Neurol. Ther. 2022, 11, 571–595. [Google Scholar] [CrossRef]
- Moser, T.; Schwenker, K.; Seiberl, M.; Feige, J.; Akgün, K.; Haschke-Becher, E.; Ziemssen, T.; Sellner, J. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 2020, 7, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Ammoscato, F.; Wafa, M.; Skonieczna, J.; Bestwick, J.; Monero, R.; Andrews, M.; De Trane, S.; Holden, D.; Adams, A.; Bianchi, L.; et al. Cladribine tablets in relapsing-remitting multiple sclerosis preferentially target B-cells. Clin. Immunol. 2024, 269, 110380. [Google Scholar] [CrossRef] [PubMed]
- Wiendl, H.; Schmierer, K.; Hodgkinson, S.; Derfuss, T.; Chan, A.; Sellebjerg, F.; Achiron, A.; Montalban, X.; Prat, A.; De Stefano, N.; et al. Specific Patterns of Immune Cell Dynamics May Explain the Early Onset and Prolonged Efficacy of Cladribine Tablets: A MAGNIFY-MS Substudy. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200048. [Google Scholar] [CrossRef]
- Comi, G.; Cook, S.; Giovannoni, G.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Galazka, A.; Nolting, A.; Hicking, C.; Dangond, F. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 168–174. [Google Scholar] [CrossRef]
- Stuve, O.; Soelberg Soerensen, P.; Leist, T.; Giovannoni, G.; Hyvert, Y.; Damian, D.; Dangond, F.; Boschert, U. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: An extended analysis of surface markers. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419854986. [Google Scholar] [CrossRef]
- Holm Hansen, R.; von Essen, M.R.; Mahler, M.R.; Cobanovic, S.; Binko, T.S.; Sellebjerg, F. Cladribine Effects on T and B Cells and T Cell Reactivity in Multiple Sclerosis. Ann. Neurol. 2023, 94, 518–530. [Google Scholar] [CrossRef]
- Ruschil, C.; Gabernet, G.; Kemmerer, C.L.; Jarboui, M.A.; Klose, F.; Poli, S.; Ziemann, U.; Nahnsen, S.; Kowarik, M.C. Cladribine treatment specifically affects peripheral blood memory B cell clones and clonal expansion in multiple sclerosis patients. Front. Immunol. 2023, 14, 1133967. [Google Scholar] [CrossRef]
- Rolfes, L.; Pfeuffer, S.; Huntemann, N.; Schmidt, M.; Su, C.; Skuljec, J.; Aslan, D.; Hackert, J.; Kleinschnitz, K.; Hagenacker, T.; et al. Immunological consequences of cladribine treatment in multiple sclerosis: A real-world study. Mult. Scler. Relat. Disord. 2022, 64, 103931. [Google Scholar] [CrossRef]
- Spiezia, A.L.; Cerbone, V.; Molinari, E.A.; Capasso, N.; Lanzillo, R.; Carotenuto, A.; Petracca, M.; Novarella, F.; Covelli, B.; Scalia, G.; et al. Changes in lymphocytes, neutrophils and immunoglobulins in year-1 cladribine treatment in multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 57, 103431. [Google Scholar] [CrossRef]
- Holm Hansen, R.; von Essen, M.R.; Reith Mahler, M.; Cobanovic, S.; Sellebjerg, F. Sustained effects on immune cell subsets and autoreactivity in multiple sclerosis patients treated with oral cladribine. Front. Immunol. 2024, 15, 1327672. [Google Scholar] [CrossRef]
- Teschner, V.E.; Fleck, A.-K.; Walter, C.; Schwarze, A.-S.; Eschborn, M.; Wirth, T.; Steinberg, O.V.; Schulte-Mecklenbeck, A.; Lu, I.-N.; Herrera-Rivero, M.; et al. Single-cell profiling reveals preferential reduction of memory B cell subsets in cladribine patients that correlates with treatment response. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231211076. [Google Scholar] [CrossRef]
- Ceronie, B.; Jacobs, B.M.; Baker, D.; Dubuisson, N.; Mao, Z.; Ammoscato, F.; Lock, H.; Longhurst, H.J.; Giovannoni, G.; Schmierer, K. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J. Neurol. 2018, 265, 1199–1209. [Google Scholar] [CrossRef]
- Voo, V.T.F.; Butzkueven, H.; Stankovich, J.; O’Brien, T.; Monif, M. The development and impact of cladribine on lymphoid and myeloid cells in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 52, 102962. [Google Scholar] [CrossRef]
- Aruta, F.; Iovino, A.; Costa, C.; Manganelli, F.; Iodice, R. Lichenoid rash: A new side effect of oral Cladribine. Mult. Scler. Relat. Disord. 2020, 41, 102023. [Google Scholar] [CrossRef]
- Cook, S.; Leist, T.; Comi, G.; Montalban, X.; Giovannoni, G.; Nolting, A.; Hicking, C.; Galazka, A.; Sylvester, E. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis. Mult. Scler. Relat. Disord. 2019, 29, 157–167. [Google Scholar] [CrossRef]
- Oh, J.; Walker, B.; Giovannoni, G.; Jack, D.; Dangond, F.; Nolting, A.; Aldridge, J.; Lebson, L.A.; Leist, T.P. Treatment-emergent adverse events occurring early in the treatment course of cladribine tablets in two phase 3 trials in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 20552173211024296. [Google Scholar] [CrossRef]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Soelberg Sørensen, P.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef]
- Jack, D.; Damian, D.; Nolting, A.; Galazka, A. COVID-19 in patients with multiple sclerosis treated with cladribine tablets: An update. Mult. Scler. Relat. Disord. 2021, 51, 102929. [Google Scholar] [CrossRef]
- Rieckmann, P.; Centonze, D.; Giovannoni, G.; Hua, L.H.; Oreja-Guevara, C.; Selchen, D.; Sørensen, P.S.; Vermersch, P.; Wiendl, H.; Salloukh, H.; et al. Expert opinion on COVID-19 vaccination and the use of cladribine tablets in clinical practice. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211058298. [Google Scholar] [CrossRef]
- Leist, T.; Cook, S.; Comi, G.; Montalban, X.; Giovannoni, G.; Nolting, A.; Damian, D.; Syed, S.; Galazka, A. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 46, 102572. [Google Scholar] [CrossRef]
- Latest World Cancer Statistics—GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: https://www.iarc.who.int/news-events/latest-world-cancer-statistics-globocan-2012-estimated-cancer-incidence-mortality-and-prevalence-worldwide-in-2012 (accessed on 30 December 2024).
- Schmierer, K.; Wiendl, H.; Oreja-Guevara, C.; Centonze, D.; Chudecka, A.; Roy, S.; Boschert, U. Varicella zoster virus and influenza vaccine antibody titres in patients from MAGNIFY-MS who were treated with cladribine tablets for highly active relapsing multiple sclerosis. Mult. Scler. 2022, 28, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.F.; Boschert, U.; Hayward, B.; Lebson, L.A. Evalu ating the impact of cladribine tablets on the devel opment of antibody titres: Interim results from the CLOCK-MS influenza vaccine sub-study. Mult Scler. 2021, 27, P071. [Google Scholar]
- Moser, T.; O’Sullivan, C.; Puttinger, C.; Feige, J.; Pilz, G.; Haschke-Becher, E.; Cadamuro, J.; Oberkofler, H.; Hitzl, W.; Harrer, A.; et al. Pre-Existing Humoral Immunological Memory Is Retained in Patients with Multiple Sclerosis Receiving Cladribine Therapy. Biomedicines 2021, 9, 1584. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211012836. [Google Scholar] [CrossRef]
- Grothe, C.; Steffen, F.; Bittner, S. Humoral immune response and lymphocyte levels after complete vaccination against COVID-19 in a cohort of multiple sclerosis patients treated with cladribine tablets. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211060118. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Dolev, M.; Menascu, S.; Magalashvili, D.; Flechter, S.; Givon, U.; Guber, D.; et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross-sectional study. J. Neuroimmunol. 2021, 361, 577746. [Google Scholar] [CrossRef]
- Waring, R. A Randomized, Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar]
- Von Andrian, U.H.; Engelhardt, B. α4 Integrins as Therapeutic Targets in Autoimmune Disease. N. Engl. J. Med. 2003, 348, 68–72. [Google Scholar] [CrossRef]
- Morrow, S.A.; Clift, F.; Devonshire, V.; Lapointe, E.; Schneider, R.; Stefanelli, M.; Vosoughi, R. Use of natalizumab in persons with multiple sclerosis: 2022 update. Mult. Scler. Relat. Disord. 2022, 65, 103995. [Google Scholar] [CrossRef]
- Butzkueven, H.; Kappos, L.; Wiendl, H.; Trojano, M.; Spelman, T.; Chang, I.; Kasliwal, R.; Jaitly, S.; Campbell, N.; Ho, P.-R.; et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J. Neurol. Neurosurg. Psychiatry 2020, 91, 660–668. [Google Scholar] [CrossRef]
- Niino, M.; Bodner, C.; Simard, M.; Alatab, S.; Gano, D.; Kim, H.J.; Trigueiro, M.; Racicot, D.; Guérette, C.; Antel, J.P.; et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann. Neurol. 2006, 59, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, M.; Meinl, I.; Kümpfel, T.; Hohlfeld, R.; Meinl, E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology 2008, 71, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Harrer, A.; Pilz, G.; Einhaeupl, M.; Oppermann, K.; Hitzl, W.; Wipfler, P.; Sellner, J.; Golaszewski, S.; Afazel, S.; Haschke-Becher, E.; et al. Lymphocyte Subsets Show Different Response Patterns to In Vivo Bound Natalizumab—A Flow Cytometric Study on Patients with Multiple Sclerosis. PLoS ONE 2012, 7, e31784. [Google Scholar] [CrossRef]
- Mellergård, J.; Edström, M.; Jenmalm, M.C.; Dahle, C.; Vrethem, M.; Ernerudh, J. Increased B Cell and Cytotoxic NK Cell Proportions and Increased T Cell Responsiveness in Blood of Natalizumab-Treated Multiple Sclerosis Patients. PLoS ONE 2013, 8, e81685. [Google Scholar] [CrossRef]
- Koudriavtseva, T.; Sbardella, E.; Trento, E.; Bordignon, V.; D’Agosto, G.; Cordiali-Fei, P. Long-term follow-up of peripheral lymphocyte subsets in a cohort of multiple sclerosis patients treated with natalizumab. Clin. Exp. Immunol. 2014, 176, 320–326. [Google Scholar] [CrossRef]
- Saraste, M.; Penttilä, T.-L.; Airas, L. Natalizumab treatment leads to an increase in circulating CXCR3-expressing B cells. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e292. [Google Scholar] [CrossRef]
- Kaufmann, M.; Haase, R.; Proschmann, U.; Ziemssen, T.; Akgün, K. Real-World Lab Data in Natalizumab Treated Multiple Sclerosis Patients Up to 6 Years Long-Term Follow Up. Front. Neurol. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Traub, J.W.; Pellkofer, H.L.; Grondey, K.; Seeger, I.; Rowold, C.; Brück, W.; Husseini, L.; Häusser-Kinzel, S.; Weber, M.S. Natalizumab promotes activation and pro-inflammatory differentiation of peripheral B cells in multiple sclerosis patients. J. Neuroinflamm. 2019, 16, 228. [Google Scholar] [CrossRef]
- Canto-Gomes, J.; Boleixa, D.; Teixeira, C.; Martins Da Silva, A.; González-Suárez, I.; Cerqueira, J.; Correia-Neves, M.; Nobrega, C. Distinct disease-modifying therapies are associated with different blood immune cell profiles in people with relapsing-remitting multiple sclerosis. Int. Immunopharmacol. 2024, 131, 111826. [Google Scholar] [CrossRef]
- Camponeschi, A.; Gerasimcik, N.; Wang, Y.; Fredriksson, T.; Chen, D.; Farroni, C.; Thorarinsdottir, K.; Sjökvist Ottsjö, L.; Aranburu, A.; Cardell, S.; et al. Dissecting Integrin Expression and Function on Memory B Cells in Mice and Humans in Autoimmunity. Front. Immunol. 2019, 10, 534. [Google Scholar] [CrossRef]
- Skarica, M.; Eckstein, C.; Whartenby, K.A.; Calabresi, P.A. Novel mechanisms of immune modulation of natalizumab in multiple sclerosis patients. J. Neuroimmunol. 2011, 235, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Planas, R.; Jelčić, I.; Schippling, S.; Martin, R.; Sospedra, M. Natalizumab treatment perturbs memory- and marginal zone-like B-cell homing in secondary lymphoid organs in multiple sclerosis. Eur. J. Immunol. 2012, 42, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Bekeredjian-Ding, I.; Milkova, M.; Balint, B.; Schwarz, A.; Korporal, M.; Jarius, S.; Fritz, B.; Lorenz, H.-M.; Wildemann, B. B cells undergo unique compartmentalized redistribution in multiple sclerosis. J. Autoimmun. 2011, 37, 289–299. [Google Scholar] [CrossRef]
- Van Langelaar, J.; Rijvers, L.; Janssen, M.; Wierenga-Wolf, A.F.; Melief, M.; Siepman, T.A.; De Vries, H.E.; Unger, P.A.; Van Ham, S.M.; Hintzen, R.Q.; et al. Induction of brain-infiltrating T-bet–expressing B cells in multiple sclerosis. Ann. Neurol. 2019, 86, 264–278. [Google Scholar] [CrossRef]
- Stüve, O.; Marra, C.M.; Jerome, K.R.; Cook, L.; Cravens, P.D.; Cepok, S.; Frohman, E.M.; Phillips, J.T.; Arendt, G.; Hemmer, B.; et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann. Neurol. 2006, 59, 743–747. [Google Scholar] [CrossRef]
- Kowarik, M.C.; Pellkofer, H.L.; Cepok, S.; Korn, T.; Kümpfel, T.; Buck, D.; Hohlfeld, R.; Berthele, A.; Hemmer, B. Differential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology 2011, 76, 1214–1221. [Google Scholar] [CrossRef]
- Ganapathy Subramanian, R.; Horakova, D.; Vaneckova, M.; Lorincz, B.; Krasensky, J.; Kubala Havrdova, E.; Uher, T. Natalizumab Induces Changes of Cerebrospinal Fluid Measures in Multiple Sclerosis. Diagnostics 2021, 11, 2230. [Google Scholar] [CrossRef]
- Villar, L.M. Immunological Markers of Optimal Response to Natalizumab in Multiple Sclerosis. Arch. Neurol. 2012, 69, 191. [Google Scholar] [CrossRef]
- Warnke, C.; Stettner, M.; Lehmensiek, V.; Dehmel, T.; Mausberg, A.K.; Von Geldern, G.; Gold, R.; Kümpfel, T.; Hohlfeld, R.; Mäurer, M.; et al. Natalizumab exerts a suppressive effect on surrogates of B cell function in blood and CSF. Mult. Scler. 2015, 21, 1036–1044. [Google Scholar] [CrossRef]
- Schlüter, M.; Oswald, E.; Winklmeier, S.; Meinl, I.; Havla, J.; Eichhorn, P.; Meinl, E.; Kümpfel, T. Effects of Natalizumab Therapy on Intrathecal Immunoglobulin G Production Indicate Targeting of Plasmablasts. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1030. [Google Scholar] [CrossRef]
- Von Glehn, F.; Farias, A.S.; De Oliveira, A.C.P.; Damasceno, A.; Longhini, A.L.F.; Oliveira, E.C.; Damasceno, B.P.; Santos, L.M.; Brandão, C.O. Disappearance of cerebrospinal fluid oligoclonal bands after natalizumab treatment of multiple sclerosis patients. Mult. Scler. 2012, 18, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Harrer, A.; Tumani, H.; Niendorf, S.; Lauda, F.; Geis, C.; Weishaupt, A.; Kleinschnitz, C.; Rauer, S.; Kuhle, J.; Stangel, M.; et al. Cerebrospinal fluid parameters of B cell-related activity in patients with active disease during natalizumab therapy. Mult. Scler. 2013, 19, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Lereim, R.R.; Nytrova, P.; Guldbrandsen, A.; Havrdova, E.K.; Myhr, K.-M.; Barsnes, H.; Berven, F.S. Natalizumab promotes anti-inflammatory and repair effects in multiple sclerosis. PLoS ONE 2024, 19, e0300914. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.T.; Cyster, J.G. Integrin-Mediated Long-Term B Cell Retention in the Splenic Marginal Zone. Science 2002, 297, 409–412. [Google Scholar] [CrossRef]
- Zanotti, C.; Chiarini, M.; Serana, F.; Sottini, A.; Garrafa, E.; Torri, F.; Caimi, L.; Rasia, S.; Capra, R.; Imberti, L. Peripheral accumulation of newly produced T and B lymphocytes in natalizumab-treated multiple sclerosis patients. Clin. Immunol. 2012, 145, 19–26. [Google Scholar] [CrossRef]
- Teniente-Serra, A.; Grau-López, L.; Mansilla, M.J.; Fernández-Sanmartín, M.; Ester Condins, A.; Ramo-Tello, C.; Martínez-Cáceres, E. Multiparametric flow cytometric analysis of whole blood reveals changes in minor lymphocyte subpopulations of multiple sclerosis patients. Autoimmunity 2016, 49, 219–228. [Google Scholar] [CrossRef]
- Lesesve, J.-F.; Debouverie, M.; Decarvalho Bittencourt, M.; Béné, M.-C. CD49d blockade by natalizumab therapy in patients with multiple sclerosis increases immature B-lymphocytes. Bone Marrow Transplant. 2011, 46, 1489–1491. [Google Scholar] [CrossRef]
- Zohren, F.; Toutzaris, D.; Klärner, V.; Hartung, H.-P.; Kieseier, B.; Haas, R. The monoclonal anti–VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood 2008, 111, 3893–3895. [Google Scholar] [CrossRef]
- Benkert, T.F.; Dietz, L.; Hartmann, E.M.; Leich, E.; Rosenwald, A.; Serfling, E.; Buttmann, M.; Berberich-Siebelt, F. Natalizumab Exerts Direct Signaling Capacity and Supports a Pro-Inflammatory Phenotype in Some Patients with Multiple Sclerosis. PLoS ONE 2012, 7, e52208. [Google Scholar] [CrossRef]
- Mameli, G.; Arru, G.; Caggiu, E.; Niegowska, M.; Leoni, S.; Madeddu, G.; Babudieri, S.; Sechi, G.P.; Sechi, L.A. Natalizumab Therapy Modulates miR-155, miR-26a and Proinflammatory Cytokine Expression in MS Patients. PLoS ONE 2016, 11, e0157153. [Google Scholar] [CrossRef]
- Sievers, C.; Meira, M.; Hoffmann, F.; Fontoura, P.; Kappos, L.; Lindberg, R.L.P. Altered microRNA expression in B lymphocytes in multiple sclerosis. Clin. Immunol. 2012, 144, 70–79. [Google Scholar] [CrossRef]
- Stüve, O. The effects of natalizumab on the innate and adaptive immune system in the central nervous system. J. Neurol. Sci. 2008, 274, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.Y.; Perez, H.D.; Zollner, T.M. Fatalities in natalizumab treatment—A ‘no go’ for leukocyte recirculation approaches? Expert Opin. Ther. Targets 2006, 10, 489–499. [Google Scholar] [CrossRef]
- Perneczky, J.; Sellner, J. Natalizumab extended-interval dosing in multiple sclerosis to mitigate progressive multifocal leukoencephalopathy risk: Initial study evidence and real-world experience. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221135484. [Google Scholar] [CrossRef]
- Sabath, B.F.; Major, E.O. Traffic of JC Virus from Sites of Initial Infection to the Brain: The Path to Progressive Multifocal Leukoencephalopathy. J. Infect. Dis. 2002, 186, S180–S186. [Google Scholar] [CrossRef]
- Chapagain, M.L.; Nerurkar, V.R. Human Polyomavirus JC (JCV) Infection of Human B Lymphocytes: A Possible Mechanism for JCV Transmigration across the Blood-Brain Barrier. J. Infect. Dis. 2010, 202, 184–191. [Google Scholar] [CrossRef]
- Frohman, E.M.; Monaco, M.C.; Remington, G.; Ryschkewitsch, C.; Jensen, P.N.; Johnson, K.; Perkins, M.; Liebner, J.; Greenberg, B.; Monson, N.; et al. JC Virus in CD34 + and CD19 + Cells in Patients with Multiple Sclerosis Treated with Natalizumab. JAMA Neurol. 2014, 71, 596. [Google Scholar] [CrossRef]
- Warnke, C.; Smolianov, V.; Dehmel, T.; Andrée, M.; Hengel, H.; Zohren, F.; Arendt, G.; Wiendl, H.; Haas, R.; Hartung, H.-P.; et al. CD34+ progenitor cells mobilized by natalizumab are not a relevant reservoir for JC virus. Mult. Scler. 2011, 17, 151–156. [Google Scholar] [CrossRef]
- Marshall, L.J.; Ferenczy, M.W.; Daley, E.L.; Jensen, P.N.; Ryschkewitsch, C.F.; Major, E.O. Lymphocyte Gene Expression and JC Virus Noncoding Control Region Sequences Are Linked with the Risk of Progressive Multifocal Leukoencephalopathy. J. Virol. 2014, 88, 5177–5183. [Google Scholar] [CrossRef]
- Meira, M.; Sievers, C.; Hoffmann, F.; Derfuss, T.; Kuhle, J.; Kappos, L.; Lindberg, R.L. MiR-126: A novel route for natalizumab action? Mult. Scler. 2014, 20, 1363–1370. [Google Scholar] [CrossRef]
- Mancuso, R.; Agostini, S.; Hernis, A.; Caputo, D.; Galimberti, D.; Scarpini, E.; Clerici, M. Alterations of the miR-126-3p/POU2AF1/Spi-B Axis and JCPyV Reactivation in Multiple Sclerosis Patients Receiving Natalizumab. Front. Neurol. 2022, 13, 819911. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Houshi, S.; Sadeghi, E.; Hashemi, M.S.; Pishgahi, G.; Bagherieh, S.; Afshari-Safavi, A.; Mirmosayyeb, O.; Shaygannejad, V.; Zabeti, A. Association of Disease-Modifying Therapies with COVID-19 Susceptibility and Severity in Patients with Multiple Sclerosis: A Systematic Review and Network Meta-Analysis. Mult. Scler. Int. 2022, 2022, 9388813. [Google Scholar] [CrossRef] [PubMed]
- Cuculiza Henriksen, A.; Ammitzbøll, C.; Petersen, E.R.; McWilliam, O.; Sellebjerg, F.; Von Essen, M.R.; Romme Christensen, J. Natalizumab differentially affects plasmablasts and B cells in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 52, 102987. [Google Scholar] [CrossRef]
- Rovituso, D.; Heller, S.; Schroeter, M.; Kleinschnitz, C.; Kuerten, S. B1 cells are unaffected by immune modulatory treatment in remitting–relapsing multiple sclerosis patients. J. Neuroimmunol. 2014, 272, 86–90. [Google Scholar] [CrossRef]
- Lindquist, R.L.; Niesner, R.A.; Hauser, A.E. In the Right Place, at the Right Time: Spatiotemporal Conditions Determining Plasma Cell Survival and Function. Front. Immunol. 2019, 10, 788. [Google Scholar] [CrossRef]
- Wang, X.; Rodda, L.B.; Bannard, O.; Cyster, J.G. Integrin-Mediated Interactions between B Cells and Follicular Dendritic Cells Influence Germinal Center B Cell Fitness. J. Immunol. 2014, 192, 4601–4609. [Google Scholar] [CrossRef]
- Carrasco, Y.R.; Batista, F.D. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 2006, 25, 889–899. [Google Scholar] [CrossRef]
- Klein, A.; Flaskamp, M.; Berthele, A.; Held, F.; Muratovic, H.; Hemmer, B. The impact of disease-modifying therapies on immunoglobulin blood levels in patients with multiple sclerosis: A retrospective cross-sectional study. Ther. Adv. Neurol. Disord. 2023, 16, 175628642311626. [Google Scholar] [CrossRef]
- Selter, R.C.; Biberacher, V.; Grummel, V.; Buck, D.; Eienbröker, C.; Oertel, W.H.; Berthele, A.; Tackenberg, B.; Hemmer, B. Natalizumab treatment decreases serum IgM and IgG levels in multiple sclerosis patients. Mult. Scler. 2013, 19, 1454–1461. [Google Scholar] [CrossRef]
- Zoehner, G.; Miclea, A.; Salmen, A.; Kamber, N.; Diem, L.; Friedli, C.; Bagnoud, M.; Ahmadi, F.; Briner, M.; Sédille-Mostafaie, N.; et al. Reduced serum immunoglobulin G concentrations in multiple sclerosis: Prevalence and association with disease-modifying therapy and disease course. Ther. Adv. Neurol. Disord. 2019, 12, 175628641987834. [Google Scholar] [CrossRef]
- De Biasi, S.; Lo Tartaro, D.; Neroni, A.; Rau, M.; Paschalidis, N.; Borella, R.; Santacroce, E.; Paolini, A.; Gibellini, L.; Ciobanu, A.L.; et al. Immunosenescence and vaccine efficacy revealed by immunometabolic analysis of SARS-CoV-2-specific cells in multiple sclerosis patients. Nat. Commun. 2024, 15, 2752. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, J.R.; Valtcheva, M.V.; Cross, A.H. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult. Scler. Relat. Disord. 2020, 45, 102439. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, K.B.; Healy, B.C.; Conway, S.; Houtchens, M.; Bakshi, R.; Bhattacharyya, S.; Bose, G.; Galetta, K.; Kaplan, T.; Severson, C.; et al. Humoral response to COVID-19 vaccination in MS patients on disease modifying therapy: Immune profiles and clinical outcomes. Mult. Scler. Relat. Disord. 2022, 67, 104079. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.; Pardo, G.; Rossman, H.; Sweetser, M.T.; Forrestal, F.; Duda, P. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2014, 341, 22–27. [Google Scholar] [CrossRef]
- Gombolay, G.Y.; Dutt, M.; Tyor, W. Immune responses to SARS-COV-2 vaccination in multiple sclerosis: A systematic review/meta-analysis. Ann. Clin. Transl. Neurol. 2022, 9, 1321–1331. [Google Scholar] [CrossRef]
- Meyer-Arndt, L.; Braun, J.; Fauchere, F.; Vanshylla, K.; Loyal, L.; Henze, L.; Kruse, B.; Dingeldey, M.; Jürchott, K.; Mangold, M.; et al. SARS-CoV-2 mRNA vaccinations fail to elicit humoral and cellular immune responses in patients with multiple sclerosis receiving fingolimod. J. Neurol. Neurosurg. Psychiatry 2022, 93, 960–971. [Google Scholar] [CrossRef]
- Coyle, P.K.; Freedman, M.S.; Cohen, B.A.; Cree, B.A.C.; Markowitz, C.E. Sphingosine 1-phosphate receptor modulators in multiple sclerosis treatment: A practical review. Ann. Clin. Transl. Neurol. 2024, 11, 842–855. [Google Scholar] [CrossRef]
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 2011, 76, S3–S8. [Google Scholar] [CrossRef]
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-Phosphate and Lymphocyte Egress from Lymphoid Organs. Annu. Rev. Immunol. 2012, 30, 69–94. [Google Scholar] [CrossRef]
- Sinha, R.K.; Park, C.; Hwang, I.-Y.; Davis, M.D.; Kehrl, J.H. B Lymphocytes Exit Lymph Nodes through Cortical Lymphatic Sinusoids by a Mechanism Independent of Sphingosine-1-Phosphate-Mediated Chemotaxis. Immunity 2009, 30, 434–446. [Google Scholar] [CrossRef]
- Chiba, K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol. Ther. 2005, 108, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H.; Shimizu, F.; Sano, Y.; Takeshita, Y.; Maeda, T.; Abe, M.; Koga, M.; Kanda, T. Fingolimod Prevents Blood-Brain Barrier Disruption Induced by the Sera from Patients with Multiple Sclerosis. PLoS ONE 2015, 10, e0121488. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Kappos, L.; O’Connor, P.; Collins, W.; Tang, D.; Mercier, F.; Cohen, J. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult. Scler. 2014, 20, 471–480. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.-W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A Placebo-Controlled Trial of Oral Fingolimod in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Gergely, P.; Nuesslein-Hildesheim, B.; Guerini, D.; Brinkmann, V.; Traebert, M.; Bruns, C.; Pan, S.; Gray, N.; Hinterding, K.; Cooke, N.; et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br. J. Pharmacol. 2012, 167, 1035–1047. [Google Scholar] [CrossRef]
- Scott, F.L.; Clemons, B.; Brooks, J.; Brahmachary, E.; Powell, R.; Dedman, H.; Desale, H.G.; Timony, G.A.; Martinborough, E.; Rosen, H.; et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br. J. Pharmacol. 2016, 173, 1778–1792. [Google Scholar] [CrossRef]
- Harris, S.; Tran, J.Q.; Southworth, H.; Spencer, C.M.; Cree, B.A.C.; Zamvil, S.S. Effect of the sphingosine-1-phosphate receptor modulator ozanimod on leukocyte subtypes in relapsing MS. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e839. [Google Scholar] [CrossRef]
- Kappos, L.; Bar-Or, A.; Cree, B.A.C.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef]
- Krumbholz, M.; Derfuss, T.; Hohlfeld, R.; Meinl, E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat. Rev. Neurol. 2012, 8, 613–623. [Google Scholar] [CrossRef]
- Galicia-Rosas, G.; Pikor, N.; Schwartz, J.A.; Rojas, O.; Jian, A.; Summers-Deluca, L.; Ostrowski, M.; Nuesslein-Hildesheim, B.; Gommerman, J.L. A Sphingosine-1-Phosphate Receptor 1-Directed Agonist Reduces Central Nervous System Inflammation in a Plasmacytoid Dendritic Cell-Dependent Manner. J. Immunol. 2012, 189, 3700–3706. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Niino, M.; Fukazawa, T.; Takahashi, E.; Nonaka, T.; Amino, I.; Tashiro, J.; Minami, N.; Fujiki, N.; Doi, S.; et al. Suppressed pro-inflammatory properties of circulating B cells in patients with multiple sclerosis treated with fingolimod, based on altered proportions of B-cell subpopulations. Clin. Immunol. 2014, 151, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Matsuoka, T.; Chihara, N.; Miyake, S.; Sato, W.; Araki, M.; Okamoto, T.; Lin, Y.; Ogawa, M.; Murata, M.; et al. Differential effects of fingolimod on B-cell populations in multiple sclerosis. Mult. Scler. 2014, 20, 1371–1380. [Google Scholar] [CrossRef]
- Claes, N.; Dhaeze, T.; Fraussen, J.; Broux, B.; Van Wijmeersch, B.; Stinissen, P.; Hupperts, R.; Hellings, N.; Somers, V. Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study. PLoS ONE 2014, 9, e111115. [Google Scholar] [CrossRef]
- Grützke, B.; Hucke, S.; Gross, C.C.; Herold, M.V.B.; Posevitz-Fejfar, A.; Wildemann, B.T.; Kieseier, B.C.; Dehmel, T.; Wiendl, H.; Klotz, L. Fingolimod treatment promotes regulatory phenotype and function of B cells. Ann. Clin. Transl. Neurol. 2015, 2, 119–130. [Google Scholar] [CrossRef]
- Lohmann, L.; Janoschka, C.; Schulte-Mecklenbeck, A.; Klinsing, S.; Kirstein, L.; Hanning, U.; Wirth, T.; Schneider-Hohendorf, T.; Schwab, N.; Gross, C.C.; et al. Immune Cell Profiling During Switching from Natalizumab to Fingolimod Reveals Differential Effects on Systemic Immune-Regulatory Networks and on Trafficking of Non-T Cell Populations into the Cerebrospinal Fluid—Results from the ToFingo Successor Study. Front. Immunol. 2018, 9, 1560. [Google Scholar] [CrossRef]
- Moreno-Torres, I.; González-García, C.; Marconi, M.; García-Grande, A.; Rodríguez-Esparragoza, L.; Elvira, V.; Ramil, E.; Campos-Ruíz, L.; García-Hernández, R.; Al-Shahrour, F.; et al. Immunophenotype and Transcriptome Profile of Patients with Multiple Sclerosis Treated with Fingolimod: Setting Up a Model for Prediction of Response in a 2-Year Translational Study. Front. Immunol. 2018, 9, 1693. [Google Scholar] [CrossRef]
- Cohen, J.A.; Bar-Or, A.; Cree, B.A.C.; Mao-Draayer, Y.; Han, M.H.; Singer, B.; Jannu, A.; Kolodny, S.; Meng, X.; Winger, R.C. The FLUENT study design: Investigating immune cell subset and neurofilament changes in patients with relapsing multiple sclerosis treated with fingolimod. Mult. Scler. J.—Exp. Transl. Clin. 2019, 5, 205521731881924. [Google Scholar] [CrossRef]
- Cellerino, M.; Ivaldi, F.; Pardini, M.; Rotta, G.; Vila, G.; Bäcker-Koduah, P.; Berge, T.; Laroni, A.; Lapucci, C.; Novi, G.; et al. Impact of treatment on cellular immunophenotype in MS: A cross-sectional study. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e693. [Google Scholar] [CrossRef]
- Chiarini, M.; Sottini, A.; Bertoli, D.; Serana, F.; Caimi, L.; Rasia, S.; Capra, R.; Imberti, L. Newly produced T and B lymphocytes and T-cell receptor repertoire diversity are reduced in peripheral blood of fingolimod-treated multiple sclerosis patients. Mult. Scler. 2015, 21, 726–734. [Google Scholar] [CrossRef]
- Mao-Draayer, Y.; Cohen, J.A.; Bar-Or, A.; Han, M.H.; Singer, B.; Williams, I.M.; Meng, X.; Elam, C.; Weiss, J.L.; Cox, G.M.; et al. Immune cell subset profiling in multiple sclerosis after fingolimod initiation and continued treatment: The FLUENT study. Mult. Scler. J.—Exp. Transl. Clin. 2022, 8, 205521732211150. [Google Scholar] [CrossRef]
- Hawke, S.; Zinger, A.; Juillard, P.-G.; Holdaway, K.; Byrne, S.N.; Grau, G.E. Selective modulation of trans-endothelial migration of lymphocyte subsets in multiple sclerosis patients under fingolimod treatment. J. Neuroimmunol. 2020, 349, 577392. [Google Scholar] [CrossRef] [PubMed]
- Smets, I.; Prezzemolo, T.; Imbrechts, M.; Mallants, K.; Mitera, T.; Humblet-Baron, S.; Dubois, B.; Matthys, P.; Liston, A.; Goris, A. Treatment-Induced BAFF Expression and B Cell Biology in Multiple Sclerosis. Front. Immunol. 2021, 12, 676619. [Google Scholar] [CrossRef] [PubMed]
- Angerer, I.C.; Hecker, M.; Koczan, D.; Roch, L.; Friess, J.; Rüge, A.; Fitzner, B.; Boxberger, N.; Schröder, I.; Flechtner, K.; et al. Transcriptome profiling of peripheral blood immune cell populations in multiple sclerosis patients before and during treatment with a sphingosine-1-phosphate receptor modulator. CNS Neurosci. Ther. 2018, 24, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Piancone, F.; Saresella, M.; Marventano, I.; La Rosa, F.; Zoppis, M.; Agostini, S.; Longhi, R.; Caputo, D.; Mendozzi, L.; Rovaris, M.; et al. B Lymphocytes in Multiple Sclerosis: Bregs and BTLA/CD272 Expressing-CD19+ Lymphocytes Modulate Disease Severity. Sci. Rep. 2016, 6, 29699. [Google Scholar] [CrossRef]
- Blumenfeld-Kan, S.; Staun-Ram, E.; Miller, A. Fingolimod reduces CXCR4-mediated B cell migration and induces regulatory B cells-mediated anti-inflammatory immune repertoire. Mult. Scler. Relat. Disord. 2019, 34, 29–37. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Niino, M.; Takahashi, E.; Suzuki, M.; Mizuno, M.; Hisahara, S.; Fukazawa, T.; Amino, I.; Nakano, F.; Nakamura, M.; et al. Fingolimod induces BAFF and expands circulating transitional B cells without activating memory B cells and plasma cells in multiple sclerosis. Clin. Immunol. 2018, 187, 95–101. [Google Scholar] [CrossRef]
- Sánchez-Sanz, A.; Muñoz-Viana, R.; Sabín-Muñoz, J.; Moreno-Torres, I.; Brea-Álvarez, B.; Rodríguez-De La Fuente, O.; García-Merino, A.; Sánchez-López, A.J. Response to Fingolimod in Multiple Sclerosis Patients Is Associated with a Differential Transcriptomic Regulation. Int. J. Mol. Sci. 2024, 25, 1372. [Google Scholar] [CrossRef]
- Wu, Q.; Mills, E.A.; Wang, Q.; Dowling, C.A.; Fisher, C.; Kirch, B.; Lundy, S.K.; Fox, D.A.; Mao-Draayer, Y. Siponimod enriches regulatory T and B lymphocytes in secondary progressive multiple sclerosis. JCI Insight 2020, 5, e134251. [Google Scholar] [CrossRef]
- Spiezia, A.L.; Scalia, G.; Petracca, M.; Caliendo, D.; Moccia, M.; Fiore, A.; Cerbone, V.; Lanzillo, R.; Brescia Morra, V.; Carotenuto, A. Effect of siponimod on lymphocyte subsets in active secondary progressive multiple sclerosis and clinical implications. J. Neurol. 2024, 271, 4281–4291. [Google Scholar] [CrossRef]
- Friedli, C.; Wagner, F.; Hammer, H.N.; Kamber, N.; Wiest, R.; Diem, L.; Chan, A.; Salmen, A.; Hoepner, R. Leptomeningeal enhancement under different MS immunotherapies: A monocentric retrospective cohort study of 214 patients. Mult. Scler. 2023, 29, 63–73. [Google Scholar] [CrossRef]
- Bail, K.; Notz, Q.; Rovituso, D.M.; Schampel, A.; Wunsch, M.; Koeniger, T.; Schropp, V.; Bharti, R.; Scholz, C.-J.; Foerstner, K.U.; et al. Differential effects of FTY720 on the B cell compartment in a mouse model of multiple sclerosis. J. Neuroinflamm. 2017, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Diddens, J.; Friedrich, V.; Pfaller, M.; Radbruch, H.; Hemmer, B.; Steiger, K.; Lehmann-Horn, K. Siponimod Inhibits the Formation of Meningeal Ectopic Lymphoid Tissue in Experimental Autoimmune Encephalomyelitis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1117. [Google Scholar] [CrossRef]
- Kaufmann, M.; Haase, R.; Proschmann, U.; Ziemssen, T.; Akgün, K. Real World Lab Data: Patterns of Lymphocyte Counts in Fingolimod Treated Patients. Front. Immunol. 2018, 9, 2669. [Google Scholar] [CrossRef]
- Epstein, D.J.; Dunn, J.; Deresinski, S. Infectious Complications of Multiple Sclerosis Therapies: Implications for Screening, Prophylaxis, and Management. Open Forum Infect. Dis. 2018, 5, ofy174. [Google Scholar] [CrossRef]
- Fox, E.J.; Lublin, F.D.; Wolinsky, J.S.; Cohen, J.A.; Williams, I.M.; Meng, X.; Ziehn, M.; Kolodny, S.; Cree, B.A.C. Lymphocyte counts and infection rates: Long-term fingolimod treatment in primary progressive MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e614. [Google Scholar] [CrossRef]
- Ziemssen, T.; Lang, M.; Schmidt, S.; Albrecht, H.; Klotz, L.; Haas, J.; Lassek, C.; Lang, S.; Winkelmann, V.E.; Ettle, B.; et al. Long-term real-world effectiveness and safety of fingolimod over 5 years in Germany. J. Neurol. 2022, 269, 3276–3285. [Google Scholar] [CrossRef]
- Langer-Gould, A.M.; Smith, J.B.; Gonzales, E.G.; Piehl, F.; Li, B.H. Multiple Sclerosis, Disease-Modifying Therapies, and Infections. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200164. [Google Scholar] [CrossRef]
- Carpenter, K.; Etemady-Deylamy, A.; Costello, V.; Khasawneh, M.; Chamberland, R.; Tian, K.; Donlin, M.; Moreira-Walsh, B.; Reisenbichler, E.; Abate, G. Cryptococcal chest wall mass and rib osteomyelitis associated with the use of fingolimod: A case report and literature review. Front. Med. 2022, 9, 942751. [Google Scholar] [CrossRef]
- Kammeyer, J.A.; Lehmann, N.M. Cerebral Venous Thrombosis due to Cryptococcus in a Multiple Sclerosis Patient on Fingolimod. Case Rep. Neurol. 2022, 14, 286–290. [Google Scholar] [CrossRef]
- Harirchian, M.H.; Poursadeghfard, M.; Sadeghipour, A.; Kamali, H.; Sarraf, P. Necrotizing fungal osteomyelitis and fingolimod, 4 years after treatment with fingolimod. Mult. Scler. Relat. Disord. 2020, 41, 102021. [Google Scholar] [CrossRef]
- Berger, J.R.; Cree, B.A.; Greenberg, B.; Hemmer, B.; Ward, B.J.; Dong, V.M.; Merschhemke, M. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology 2018, 90, e1815–e1821. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Papathanasiou, A.; Coclitu, C.; Garjani, A.; Evangelou, N.; Constantinescu, C.S.; Popescu, B.O.; Tanasescu, R. An update on the use of sphingosine 1-phosphate receptor modulators for the treatment of relapsing multiple sclerosis. Expert Opin. Pharmacother. 2023, 24, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Sharifian-Dorche, M.; Sahraian, M.A.; Fadda, G.; Osherov, M.; Sharifian-Dorche, A.; Karaminia, M.; Saveriano, A.W.; La Piana, R.; Antel, J.P.; Giacomini, P.S. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: A systematic review. Mult. Scler. Relat. Disord. 2021, 50, 102800. [Google Scholar] [CrossRef]
- Schiavetti, I.; Cordioli, C.; Stromillo, M.L.; Ferrò, M.T.; Laroni, A.; Cocco, E.; Cola, G.; Pasquali, L.; Rilla, M.T.; Signoriello, E.; et al. Breakthrough SARS-CoV-2 infections in MS patients on disease-modifying therapies. Mult. Scler. J. 2022, 28, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Recurrent Varicella Following Steroids and Fingolimod in a Multiple Sclerosis Patient—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24163092/ (accessed on 13 February 2025).
- Cohen, J.A.; Comi, G.; Arnold, D.L.; Bar-Or, A.; Selmaj, K.W.; Steinman, L.; Havrdová, E.K.; Cree, B.A.; Montalbán, X.; Hartung, H.-P.; et al. Efficacy and safety of ozanimod in multiple sclerosis: Dose-blinded extension of a randomized phase II study. Mult. Scler. 2019, 25, 1255–1262. [Google Scholar] [CrossRef]
- Swallow, E.; Patterson-Lomba, O.; Yin, L.; Mehta, R.; Pelletier, C.; Kao, D.; Sheffield, J.K.; Stonehouse, T.; Signorovitch, J. Comparative safety and efficacy of ozanimod versus fingolimod for relapsing multiple sclerosis. J. Comp. Eff. Res. 2020, 9, 275–285. [Google Scholar] [CrossRef]
- Kappos, L.; Mehling, M.; Arroyo, R.; Izquierdo, G.; Selmaj, K.; Curovic-Perisic, V.; Keil, A.; Bijarnia, M.; Singh, A.; Von Rosenstiel, P. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology 2015, 84, 872–879. [Google Scholar] [CrossRef]
- Sabatino, J.J.; Mittl, K.; Rowles, W.M.; McPolin, K.; Rajan, J.V.; Laurie, M.T.; Zamecnik, C.R.; Dandekar, R.; Alvarenga, B.D.; Loudermilk, R.P.; et al. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine–induced antibody and T cell immunity and function. JCI Insight 2022, 7, e156978. [Google Scholar] [CrossRef]
- Disanto, G.; Galante, A.; Cantu’, M.; Sacco, R.; Mele, F.; Eisler, J.J.; Keller, F.; Bernasconi, E.; Sallusto, F.; Zecca, C.; et al. Longitudinal Postvaccine SARS-CoV-2 Immunoglobulin G Titers, Memory B-Cell Responses, and Risk of COVID-19 in Multiple Sclerosis Over 1 Year. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200043. [Google Scholar] [CrossRef]
- Schraad, M.; Runkel, S.; Hitzler, W.; Protopapa, M.; Bittner, S.; Uphaus, T.; Zipp, F. Long-Term Observation of SARS-CoV-2 Vaccination Response upon High Efficacy Treatment in Multiple Sclerosis—A Real-World Scenario. Vaccines 2024, 12, 296. [Google Scholar] [CrossRef]
- Han, S.; Zhang, X.; Wang, G.; Guan, H.; Garcia, G.; Li, P.; Feng, L.; Zheng, B. FTY720 suppresses humoral immunity by inhibiting germinal center reaction. Blood 2004, 104, 4129–4133. [Google Scholar] [CrossRef] [PubMed]
- Cinamon, G.; Zachariah, M.A.; Lam, O.M.; Foss, F.W.; Cyster, J.G. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 2008, 9, 54–62. [Google Scholar] [CrossRef]
- Arnon, T.I.; Horton, R.M.; Grigorova, I.L.; Cyster, J.G. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature 2013, 493, 684–688. [Google Scholar] [CrossRef]
- Proschmann, U.; Mueller-Enz, M.; Woopen, C.; Katoul Al Rahbani, G.; Haase, R.; Dillenseger, A.; Dunsche, M.; Atta, Y.; Ziemssen, T.; Akgün, K. Differential effects of selective versus unselective sphingosine 1-phosphate receptor modulators on T- and B-cell response to SARS-CoV-2 vaccination. Mult. Scler. 2023, 29, 1849–1859. [Google Scholar] [CrossRef]
- Torke, S.; Weber, M.S. Inhibition of Bruton’s tyrosine kinase as a novel therapeutic approach in multiple sclerosis. Expert Opin. Investig. Drugs 2020, 29, 1143–1150. [Google Scholar] [CrossRef]
- Li, R.; Tang, H.; Burns, J.C.; Hopkins, B.T.; Le Coz, C.; Zhang, B.; De Barcelos, I.P.; Romberg, N.; Goldstein, A.C.; Banwell, B.L.; et al. BTK inhibition limits B-cell–T-cell interaction through modulation of B-cell metabolism: Implications for multiple sclerosis therapy. Acta Neuropathol. 2022, 143, 505–521. [Google Scholar] [CrossRef]
- Nyhoff, L.E.; Clark, E.S.; Barron, B.L.; Bonami, R.H.; Khan, W.N.; Kendall, P.L. Bruton’s Tyrosine Kinase Is Not Essential for B Cell Survival beyond Early Developmental Stages. J. Immunol. 2018, 200, 2352–2361. [Google Scholar] [CrossRef]
- Estupiñán, H.Y.; Berglöf, A.; Zain, R.; Smith, C.I.E. Comparative Analysis of BTK Inhibitors and Mechanisms Underlying Adverse Effects. Front. Cell Dev. Biol. 2021, 9, 630942. [Google Scholar] [CrossRef]
- Airas, L.; Bermel, R.A.; Chitnis, T.; Hartung, H.-P.; Nakahara, J.; Stuve, O.; Williams, M.J.; Kieseier, B.C.; Wiendl, H. A review of Bruton’s tyrosine kinase inhibitors in multiple sclerosis. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241233040. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.D.; Qiu, H.; Askew, B.C.; Bender, A.T.; Brugger, N.; Camps, M.; Dhanabal, M.; Dutt, V.; Eichhorn, T.; Gardberg, A.S.; et al. Discovery of Evobrutinib: An Oral, Potent, and Highly Selective, Covalent Bruton’s Tyrosine Kinase (BTK) Inhibitor for the Treatment of Immunological Diseases. J. Med. Chem. 2019, 62, 7643–7655. [Google Scholar] [CrossRef]
- Montalban, X.; Arnold, D.L.; Weber, M.S.; Staikov, I.; Piasecka-Stryczynska, K.; Willmer, J.; Martin, E.C.; Dangond, F.; Syed, S.; Wolinsky, J.S. Placebo-Controlled Trial of an Oral BTK Inhibitor in Multiple Sclerosis. N. Engl. J. Med. 2019, 380, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Rijvers, L.; Van Langelaar, J.; Bogers, L.; Melief, M.-J.; Koetzier, S.C.; Blok, K.M.; Wierenga-Wolf, A.F.; De Vries, H.E.; Rip, J.; Corneth, O.B.J.; et al. Human T-bet+ B cell development is associated with BTK activity and suppressed by evobrutinib. JCI Insight 2022, 7, e160909. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.L.; Waede, M.R.; Kingo, C.; Damsbo, K.; Illes, Z. Expression of Bruton’s tyrosine kinase in different type of brain lesions of multiple sclerosis patients and during experimental demyelination. Front. Immunol. 2023, 14, 1264128. [Google Scholar] [CrossRef]
- Torke, S.; Pretzsch, R.; Häusler, D.; Haselmayer, P.; Grenningloh, R.; Boschert, U.; Brück, W.; Weber, M.S. Inhibition of Bruton’s tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease. Acta Neuropathol. 2020, 140, 535–548. [Google Scholar] [CrossRef]
- Bhargava, P.; Kim, S.; Reyes, A.A.; Grenningloh, R.; Boschert, U.; Absinta, M.; Pardo, C.; Van Zijl, P.; Zhang, J.; Calabresi, P.A. Imaging meningeal inflammation in CNS autoimmunity identifies a therapeutic role for BTK inhibition. Brain 2021, 144, 1396–1408. [Google Scholar] [CrossRef]
- Nuesslein-Hildesheim, B.; Ferrero, E.; Schmid, C.; Huck, C.; Smith, P.; Tisserand, S.; Rubert, J.; Bornancin, F.; Eichlisberger, D.; Cenni, B. Remibrutinib (LOU064) inhibits neuroinflammation driven by B cells and myeloid cells in preclinical models of multiple sclerosis. J. Neuroinflamm. 2023, 20, 194. [Google Scholar] [CrossRef]
- Evonuk, K.S.; Wang, S.; Mattie, J.; Cracchiolo, C.J.; Mager, R.; Ferenčić, Ž.; Sprague, E.; Carrier, B.; Schofield, K.; Martinez, E.; et al. Bruton’s tyrosine kinase inhibition reduces disease severity in a model of secondary progressive autoimmune demyelination. Acta Neuropathol. Commun. 2023, 11, 115. [Google Scholar] [CrossRef]
- Arnold, D.L.; Elliott, C.; Martin, E.C.; Hyvert, Y.; Tomic, D.; Montalban, X. Effect of Evobrutinib on Slowly Expanding Lesion Volume in Relapsing Multiple Sclerosis: A Post Hoc Analysis of a Phase 2 Trial. Neurology 2024, 102, e208058. [Google Scholar] [CrossRef]
- Barboza, A. It is time to rethink clinical trials on Bruton’s tyrosine kinase inhibitors in multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 82, 105395. [Google Scholar] [CrossRef]
- Kappos, L.; Wolinsky, J.S.; Giovannoni, G.; Arnold, D.L.; Wang, Q.; Bernasconi, C.; Model, F.; Koendgen, H.; Manfrini, M.; Belachew, S.; et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020, 77, 1132. [Google Scholar] [CrossRef]
- Papathanasiou, A.; Hibbert, A.; Tallantyre, E.; Harding, K.; Selvam, A.P.; Morgan, M.; Quainton, C.; Talaei, M.; Arun, T.; Ingram, G.; et al. Real-world annualized relapse rates from contemporary multiple sclerosis clinics in the UK: A retrospective multicentre cohort study. Neurol. Sci. 2023, 44, 3629–3635. [Google Scholar] [CrossRef] [PubMed]
- Chataway, J.; Williams, T.; Li, V.; Marrie, R.A.; Ontaneda, D.; Fox, R.J. Clinical trials for progressive multiple sclerosis: Progress, new lessons learned, and remaining challenges. Lancet Neurol. 2024, 23, 277–301. [Google Scholar] [CrossRef]
- Reich, D.S.; Arnold, D.L.; Vermersch, P.; Bar-Or, A.; Fox, R.J.; Matta, A.; Turner, T.; Wallström, E.; Zhang, X.; Mareš, M.; et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: A phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021, 20, 729–738. [Google Scholar] [CrossRef]
- Tolebrutinib Demonstrated a 31% Delay in Time to Onset of Confirmed Disability Progression in Non-Relapsing Secondary Progressive Multiple Sclerosis Phase 3 Study. Available online: https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-20-09-30-00-2949552 (accessed on 24 September 2024).
- ECTRIMS 2024—Late Breaking Oral Presentations. Mult. Scler. J. 2024, 30 (Suppl. 3), 1138–1147. [CrossRef]
- Zurmati, B.M.; Khan, J. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: A phase 2b, randomized, double-blind, placebo-controlled trial by Daniel S Reich et Al. Mult. Scler. Relat. Disord. 2023, 77, 104850. [Google Scholar] [CrossRef]
- Bar-Or, A.; Cross, A.H.; Cunningham, A.L.; Hyvert, Y.; Seitzinger, A.; Gühring, H.; Drouin, E.E.; Alexandri, N.; Tomic, D.; Montalban, X. Antibody response to SARS-CoV-2 vaccines in patients with relapsing multiple sclerosis treated with evobrutinib: A Bruton’s tyrosine kinase inhibitor. Mult. Scler. 2023, 29, 1471–1481. [Google Scholar] [CrossRef]
- Carnero Contentti, E.; Correale, J. Current Perspectives: Evidence to Date on BTK Inhibitors in the Management of Multiple Sclerosis. DDDT 2022, 16, 3473–3490. [Google Scholar] [CrossRef]
- Correale, J.; Halfon, M.J.; Jack, D.; Rubstein, A.; Villa, A. Acting centrally or peripherally: A renewed interest in the central nervous system penetration of disease-modifying drugs in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 56, 103264. [Google Scholar] [CrossRef]
- Lock, C. A Study to Investigate the Safety, Tolerability, and Processing by the Body of Intravenous and Subcutaneous RO7121932 in Participants with Multiple Sclerosis. Available online: https://clinicaltrials.stanford.edu/trials/a/NCT05704361.html (accessed on 22 October 2024).
- Hauser, S.L.; Bar-Or, A.; Weber, M.S.; Kletzl, H.; Günther, A.; Manfrini, M.; Model, F.; Mercier, F.; Petry, C.; Wing, Q.; et al. Association of Higher Ocrelizumab Exposure with Reduced Disability Progression in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200094. [Google Scholar] [CrossRef]
- A Study to Evaluate the Efficacy, Safety and Pharmacokinetics of a Higher Dose of Ocrelizumab in Adults with Primary Progressive Multiple Sclerosis (PPMS). Available online: https://clinicaltrials.gov/study/NCT04548999?cond=multiple%20sclerosis&term=ocrelizumab&rank=8#more-information (accessed on 1 October 2024).
- A Study to Evaluate the Efficacy, Safety and Pharmacokinetics of a Higher Dose of Ocrelizumab in Adults with Relapsing Multiple Sclerosis (RMS). Available online: https://clinicaltrials.gov/study/NCT04544436?cond=multiple%20sclerosis&term=ocrelizumab&rank=7 (accessed on 1 October 2024).
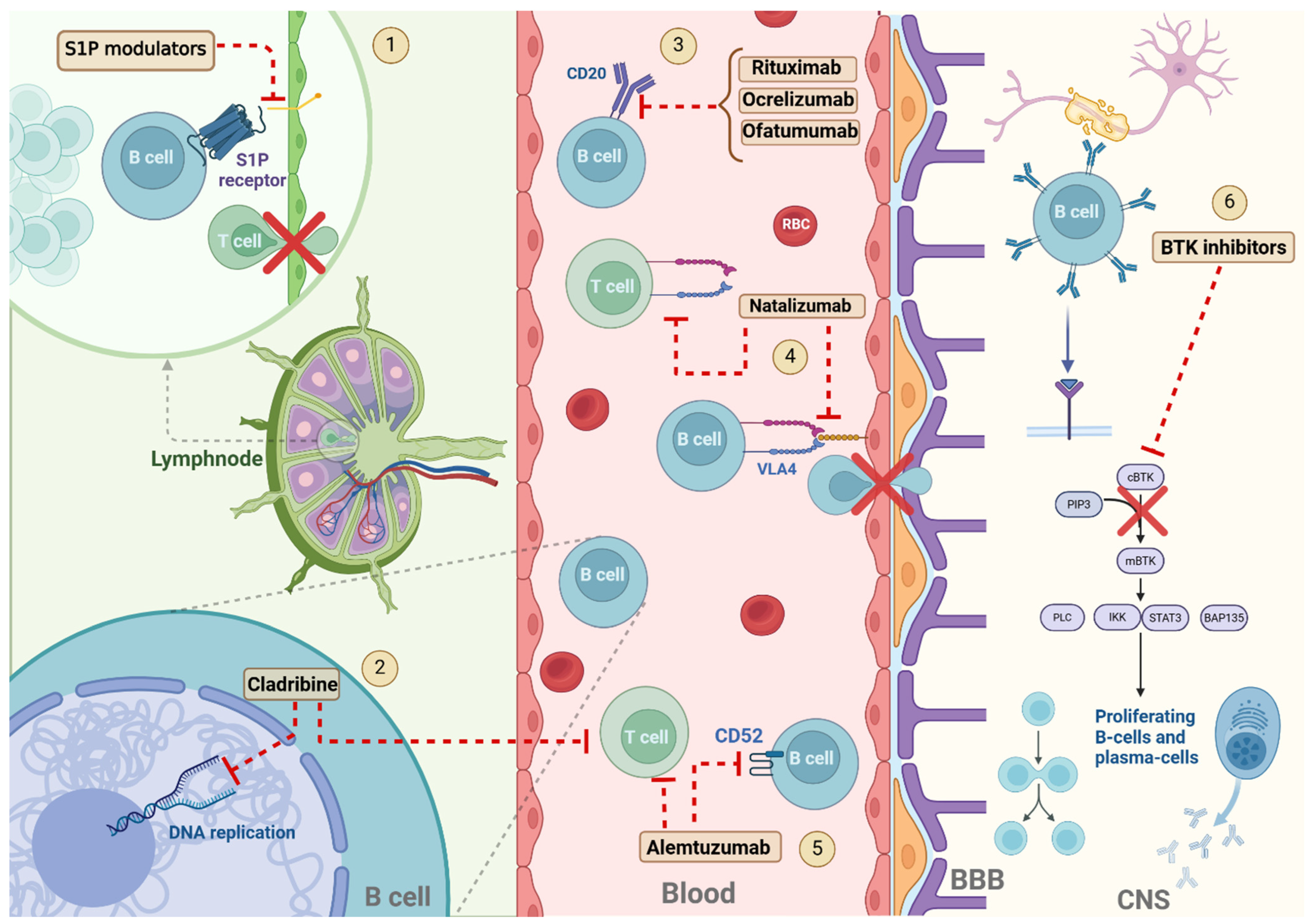
| Drugs | |||||
|---|---|---|---|---|---|
| Adverse Events | Anti-CD20 | Alemtuzumab | Cladribine | Natalizumab | S1P-Modulators |
| Lymphopenia | + | + | + | NA | + |
| Hypogammaglobulinemia | ++ | NA | NA | ++ | NA |
| Reduced vaccine effectiveness | ++ | +/− | +/− | +/− | + |
| Infections other than PML | ++ | + | + | +/− | + |
| PML | + | + | NA | + | +/− |
| Neoplasms | NA | NA | +/− | NA | +/− |
| Cardiovascular effects | +/− | + | NA | NA | − |
| Macular oedema | NA | NA | NA | NA | − |
| Hepatic toxicity | − | − | − | − | − |
| Infusion reactions | + | + | NA | + | NA |
| Autoimmunity | + | ++ | NA | NA | NA |
| Blineage Cell Types Drugs | Pre-B | Immature | Transitional | Naïve | Memory | Regulatory | Plasmablasts | Plasma Cells |
|---|---|---|---|---|---|---|---|---|
| Anti-CD20 mAbs | ↓ | ↓ | ↓ | ↓↓ | ↓↓ | -↓ | ↔ | ↔ |
| Alemtuzumab | ↓ | ↓ | ↓ | ↓↓ | ↓↓ | ↓ | ↓ | ↓ |
| Cladribine | - | - | ↓ | ↓ | ↓↓ | ↓ | ↓↓ | ↓ |
| Natalizumab | ↑ | ↑ | ↑ | ↑↓ | ↑↑ | - | ↓ | - |
| S1P-modulators | - | ↑ | ↑ | ↑↓ | ↓↓ | ↑↑ | ↔ | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galota, F.; Marcheselli, S.; De Biasi, S.; Gibellini, L.; Vitetta, F.; Fiore, A.; Smolik, K.; De Napoli, G.; Cardi, M.; Cossarizza, A.; et al. Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells. Cells 2025, 14, 606. https://doi.org/10.3390/cells14080606
Galota F, Marcheselli S, De Biasi S, Gibellini L, Vitetta F, Fiore A, Smolik K, De Napoli G, Cardi M, Cossarizza A, et al. Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells. Cells. 2025; 14(8):606. https://doi.org/10.3390/cells14080606
Chicago/Turabian StyleGalota, Federica, Simone Marcheselli, Sara De Biasi, Lara Gibellini, Francesca Vitetta, Alessia Fiore, Krzysztof Smolik, Giulia De Napoli, Martina Cardi, Andrea Cossarizza, and et al. 2025. "Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells" Cells 14, no. 8: 606. https://doi.org/10.3390/cells14080606
APA StyleGalota, F., Marcheselli, S., De Biasi, S., Gibellini, L., Vitetta, F., Fiore, A., Smolik, K., De Napoli, G., Cardi, M., Cossarizza, A., & Ferraro, D. (2025). Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells. Cells, 14(8), 606. https://doi.org/10.3390/cells14080606






