SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Serum Biochemical Analysis
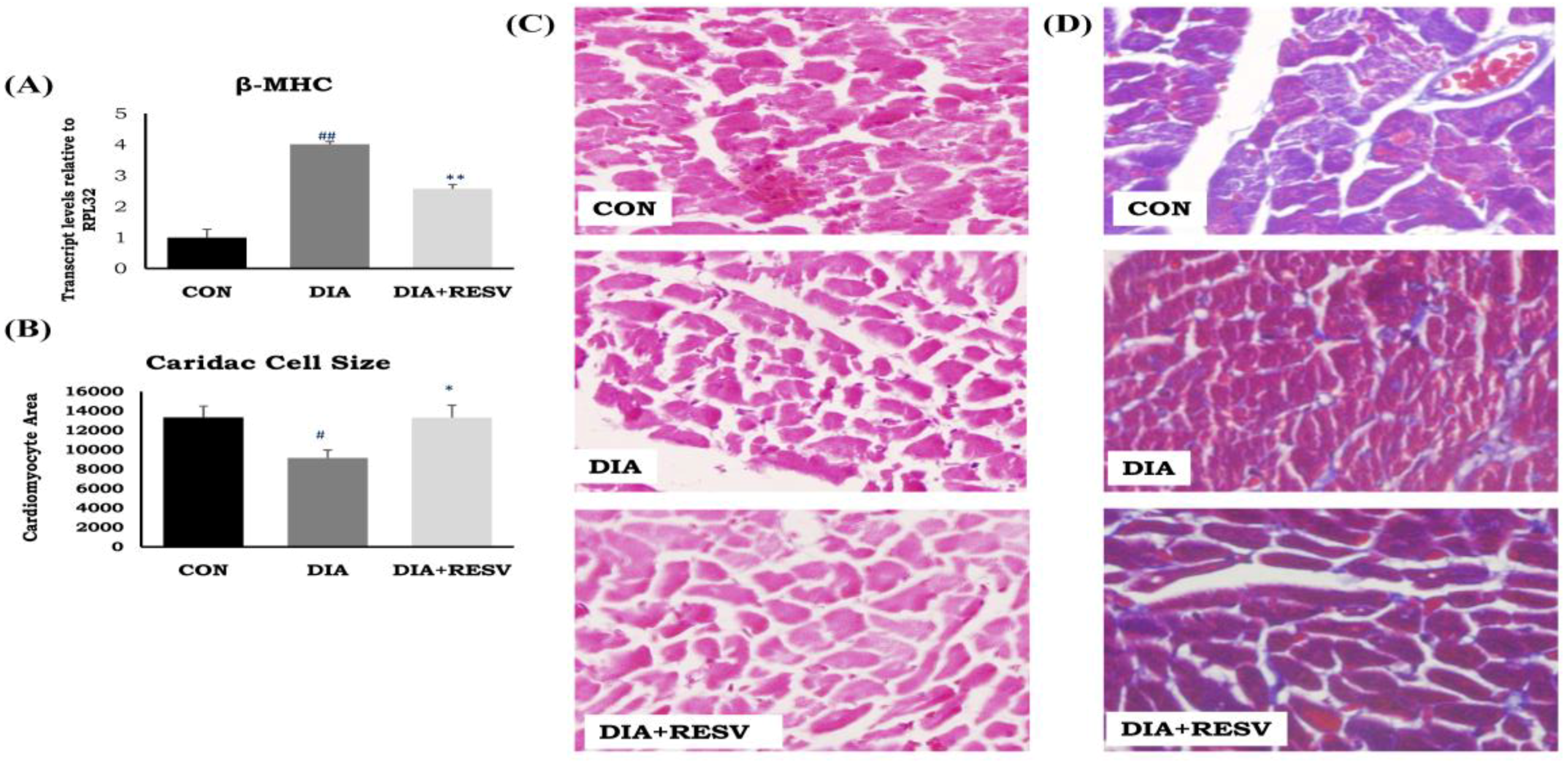
2.4. Measurement of Cardiac Cell Size and Histopathological Examination
2.5. Isolation of Mitochondria
2.6. Preparation of Heart Tissue Homogenate
2.7. Measurement of Cardiac ROS, Antioxidant Levels and Oxidative Stress Parameters
2.8. Electron Transport Chain Complex Assembly Activity
2.9. Gene Expression Analysis
2.10. Immunoblotting
2.11. Immunoprecipitation
2.12. Electrophoretic Mobility Shift Assay
2.13. Statistical Analysis
3. Results
3.1. Serum Biochemistry
3.2. Cardiac Atrophy and Fibrosis
3.3. Mitochondrial Citrate Synthase, β-Hydroxy Acyl Co-A Dehydrogenase Activity, and Mitochondrial Number
3.4. Activity of ETC Complex Assembly and ATP Generation
3.5. Reactive Oxygen Species Levels and Antioxidant Defense
3.6. mRNA Expression of Mitochondrial Encoded Genes
3.7. Expression and Activity of TFAM with Increased Acetylation Status
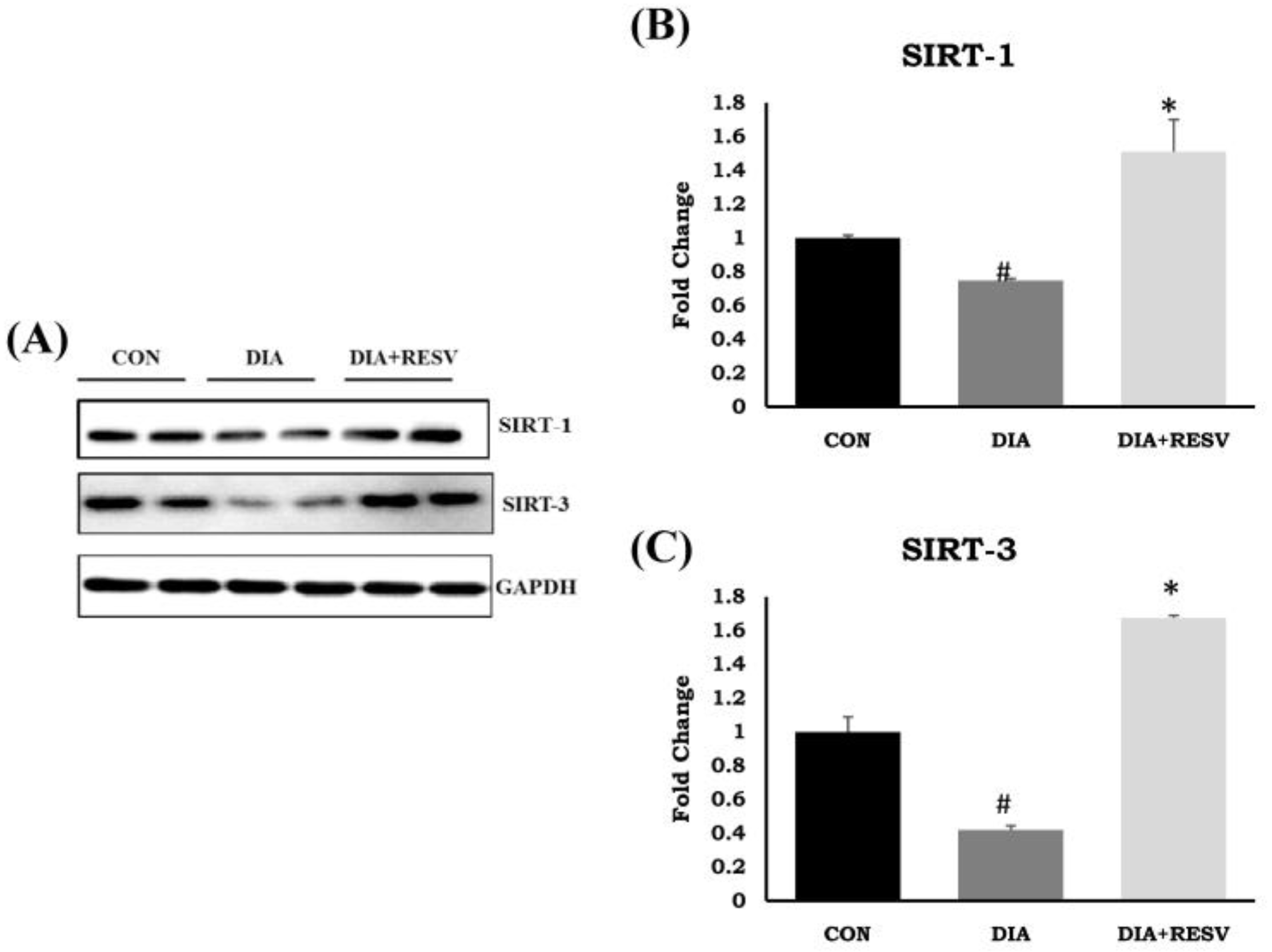
3.8. Expression and Activity of SIRT-1 and SIRT-3
3.9. TFAM Acetylation Is Regulated by SIRT-3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dittenhafer-Reed, K.E.; Richards, A.L.; Fan, J.; Smallegan, M.J.; Fotuhi Siahpirani, A.; Kemmerer, Z.A.; Prolla, T.A.; Roy, S.; Coon, J.J.; Denu, J.M. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 2015, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.H.; Kim, H.S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, L.L.; Wen, X.; Wang, X.Y.; Liu, J.; Cheng, Y.; Huang, J. Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer. Cell Death Dis. 2014, 5, e1047. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.A.; Zhang, H.J.; Hong, Z.; Pillai, V.B.; Sundaresan, N.R.; Wolfgeher, D.; Archer, S.L.; Chan, D.C.; Gupta, M.P. SIRT3 deacetylates and activates opa1 to regulate mitochondrial dynamics during stress. Mol. Cell. Biol. 2014, 34, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Chiang, W.F.; Liu, S.Y.; Chen, P.F.; Chiang, H.C. Role of SIRT3 in the regulation of redox balance during oral carcinogenesis. Mol. Cancer 2013, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Grillon, J.M.; Johnson, K.R.; Kotlo, K.; Danziger, R.S. Non-histone lysine acetylated proteins in heart failure. Biochim. Biophys. Acta 2012, 1822, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Milner, K.; Gupta, A.; Lopaschuk, G.D. Myocardial energy substrate metabolism in heart failure: From pathways to therapeutic targets. Curr. Pharm. Des. 2015, 21, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Banerjee, S.K. Insulin resistance, oxidative stress and cardiovascular complications: Role of sirtuins. Curr. Pharm. Des. 2013, 19, 5663–5677. [Google Scholar] [CrossRef] [PubMed]
- Hebert, A.S.; Dittenhafer-Reed, K.E.; Yu, W.; Bailey, D.J.; Selen, E.S.; Boersma, M.D.; Carson, J.J.; Tonelli, M.; Balloon, A.J.; Higbee, A.J. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 2013, 49, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Witt, C.N.; Bode, C. Mitochondrial sirtuins in the heart. Heart Fail. Rev. 2016, 21, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Koentges, C.; Bode, C.; Bugger, H. SIRT3 in cardiac physiology and disease. Front. Cardiovasc. Med. 2016, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Cheng, Z.; Xiong, Z.; Lv, J.; Yang, Z.; Li, T.; Jiang, S.; Gu, J.; Sun, D. Polydatin ameliorates diabetic cardiomyopathy via Sirt3 activation. Biochem. Biophys. Res. Commun. 2017, 493, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Dinda, A.K.; Banerjee, S.K. Effect of resveratrol on sirtuins expression and cardiac complications in diabetes. Biochem. Biophys. Res. Commun. 2015, 468, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, J.; Liu, J.; Li, N.; Wang, S.; Liu, H.; Zeng, M.; Zhang, Y.; Bu, P. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H424–H434. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Shahi, M.; Haidari, F.; Shiri, M.R. Comparison of effect of resveratrol and vanadium on diabetes related dyslipidemia and hyperglycemia in streptozotocin induced diabetic rats. Adv. Pharm. Bull. 2011, 1, 81–86. [Google Scholar] [PubMed]
- Helms, S.A.; Azhar, G.; Zuo, C.; Theus, S.A.; Bartke, A.; Wei, J.Y. Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of ames dwarf mice. Int. J. Biol. Sci. 2010, 6, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Cosyns, B.; Droogmans, S.; Hernot, S.; Degaillier, C.; Garbar, C.; Weytjens, C.; Roosens, B.; Schoors, D.; Lahoutte, T.; Franken, P.R.; et al. Effect of streptozotocin-induced diabetes on myocardial blood flow reserve assessed by myocardial contrast echocardiography in rats. Cardiovasc. Diabetol. 2008, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; Dinda, A.K.; Manchanda, S.C.; Maulik, S.K. Chronic garlic administration protects rat heart against oxidative stress induced by ischemic reperfusion injury. BMC Pharmacol. 2002, 2, 16. [Google Scholar] [CrossRef]
- Padiya, R.; Chowdhury, D.; Borkar, R.; Srinivas, R.; Pal Bhadra, M.; Banerjee, S.K. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS ONE 2014, 9, e94228. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Khatua, T.N.; Bagul, P.K.; Kuncha, M.; Banerjee, S.K. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr. Metab. 2011, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.; Garland, P.B. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem. J. 1969, 114, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Trounce, I.A.; Kim, Y.L.; Jun, A.S.; Wallace, D.C. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996, 264, 484–509. [Google Scholar] [PubMed]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Li, H.; Xu, J.; Liu, Y.; Gao, X.; Wang, J.; Ng, K.F.; Lau, W.B.; Ma, X.L.; Rodrigues, B.; et al. Hyperglycemia-induced protein kinase c β2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes 2013, 62, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.T.; Yau, S.K.; Mee, A.P.; Mawer, E.B.; Miller, C.A.; Garland, H.O.; Riccardi, D. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J. Am. Soc. Nephrol. 2001, 12, 779–790. [Google Scholar] [PubMed]
- Fuentes-Antras, J.; Picatoste, B.; Gomez-Hernandez, A.; Egido, J.; Tunon, J.; Lorenzo, O. Updating experimental models of diabetic cardiomyopathy. J. Diabetes Res. 2015, 2015, 656795. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hong, Y.; Lee, S.R.; Chang, K.T.; Hong, Y. Autophagy contributes to retardation of cardiac growth in diabetic rats. Lab. Anim. Res. 2012, 28, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tesiram, Y.A.; Towner, R.A.; Abbott, A.; Patterson, E.; Huang, S.; Garrett, M.W.; Chandrasekaran, S.; Matsuzaki, S.; Szweda, L.I.; et al. Early myocardial dysfunction in streptozotocin-induced diabetic mice: A study using in vivo magnetic resonance imaging (MRI). Cardiovasc. Diabetol. 2007, 6, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dobrzynski, E.; Montanari, D.; Agata, J.; Zhu, J.; Chao, J.; Chao, L. Adrenomedullin improves cardiac function and prevents renal damage in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1291–E1298. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, L.; Costa, A.L.; Le Bachelier, C.; Slama, A.; Lebre, A.-S.; Taylor, R.W.; Bastin, J.; Djouadi, F. Resveratrol attenuates oxidative stress in mitochondrial complex I deficiency: Involvement of SIRT3. Free Radic. Biol. Med. 2016, 96, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, M.; Zeng, X.; Yang, J.; Deng, H.; Yi, L.; Mi, M. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014, 5, e1576. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.-J.; Li, Y.-F.; Liu, F.-L.; Deng, J.; Wu, Y.-P.; Yuan, W.-L.; Tsoi, B.; Chen, J.-L.; Wang, Q.; Cai, S.-H. A SIRT3/AMPK/autophagy network orchestrates the protective effects of trans-resveratrol in stressed peritoneal macrophages and RAW 264.7 macrophages. Free Radic. Biol. Med. 2016, 95, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Nam, M.; Fan, W.; Akie, T.E.; Hoaglin, D.C.; Gao, G.; Keaney, J.F., Jr.; Cooper, M.P. Nutrient sensing by the mitochondrial transcription machinery dictates oxidative phosphorylation. J. Clin. Investig. 2014, 124, 768–784. [Google Scholar] [CrossRef] [PubMed]




| Parameters | CON | DIA | DIA + RESV |
|---|---|---|---|
| Blood glucose, mg/dL | 88.58 ± 2.90 | 429.00 ± 22.53 ## | 217.50 ± 37.83 ** |
| Glycated Hemoglobin, % | 5.84 ± 0.16 | 9.71 ± 0.13 ## | 7.46 ± 0.15 * |
| Insulin, pmol | 131.01 ± 15.74 | 44.87 ± 6.70 ## | 97.32 ± 8.26 * |
| Triglyceride, mg/dL | 116.87 ± 8.255 | 157.00 ± 15.38 # | 122.60 ± 6.47 * |
| Cholesterol, mg/dL | 72.77 ± 3.55 | 53.00 ± 2.58 # | 74.66 ± 5.40 ** |
| Uric acid, mg/dL | 0.85 ± 0.034 | 1.44 ± 0.15 # | 0.92 ± 0.05 * |
| Creatinine, mg/dL | 0.36 ± 0.01 | 0.52 ± 0.03 # | 0.26 ± 0.01 * |
| HDL, mg/dL | 62.71 ± 1.02 | 48.20 ± 2.09 # | 64.80 ± 3.69 * |
| HOMA IR | 4.00 ± 0.46 | 26.65 ± 8.25 # | 5.52 ± 1.18 ** |
| HOMA %B | 278.70 ± 19.15 | 13.61 ± 2.56 # | 58.20 ± 17.59 * |
| HOMA %S | 28.12 ± 3.07 | 15.46 ± 5.95 # | 20.60 ± 3.76 * |
| Parameters | CON | DIA | DIA + RESV |
|---|---|---|---|
| TBARS (uM/ gm weight of tissue) | 559.57 ± 26.44 | 707.53 ± 26.32 # | 576.29 ± 4.09 ** |
| ROS (%) | 100 | 160.18 ± 1.87 ## | 95.24 ± 1.07 ** |
| DPPH (% scavenging) | 26.68 ± 2.30 | 18.61 ± 1.43 # | 27.86 ± 5.01 * |
| GSH (ng/mg of protein) | 398.23 ± 6.00 | 378.67 ± 5.34 # | 417.50 ± 12.58 * |
| Catalase (mU/ug of protein) | 1.85 ± 0.11 | 1.04 ± 0.28 # | 3.26 ± 0.52 ** |
| SOD (% activity) | 100 | 68.84 ± 2.53 ## | 81.51 ± 3.62 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagul, P.K.; Katare, P.B.; Bugga, P.; Dinda, A.K.; Banerjee, S.K. SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM. Cells 2018, 7, 235. https://doi.org/10.3390/cells7120235
Bagul PK, Katare PB, Bugga P, Dinda AK, Banerjee SK. SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM. Cells. 2018; 7(12):235. https://doi.org/10.3390/cells7120235
Chicago/Turabian StyleBagul, Pankaj K., Parmeshwar B. Katare, Paramesha Bugga, Amit K. Dinda, and Sanjay K. Banerjee. 2018. "SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM" Cells 7, no. 12: 235. https://doi.org/10.3390/cells7120235
APA StyleBagul, P. K., Katare, P. B., Bugga, P., Dinda, A. K., & Banerjee, S. K. (2018). SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM. Cells, 7(12), 235. https://doi.org/10.3390/cells7120235





