Tendon Remodeling in Response to Resistance Training, Anabolic Androgenic Steroids and Aging
Abstract
:1. Introduction
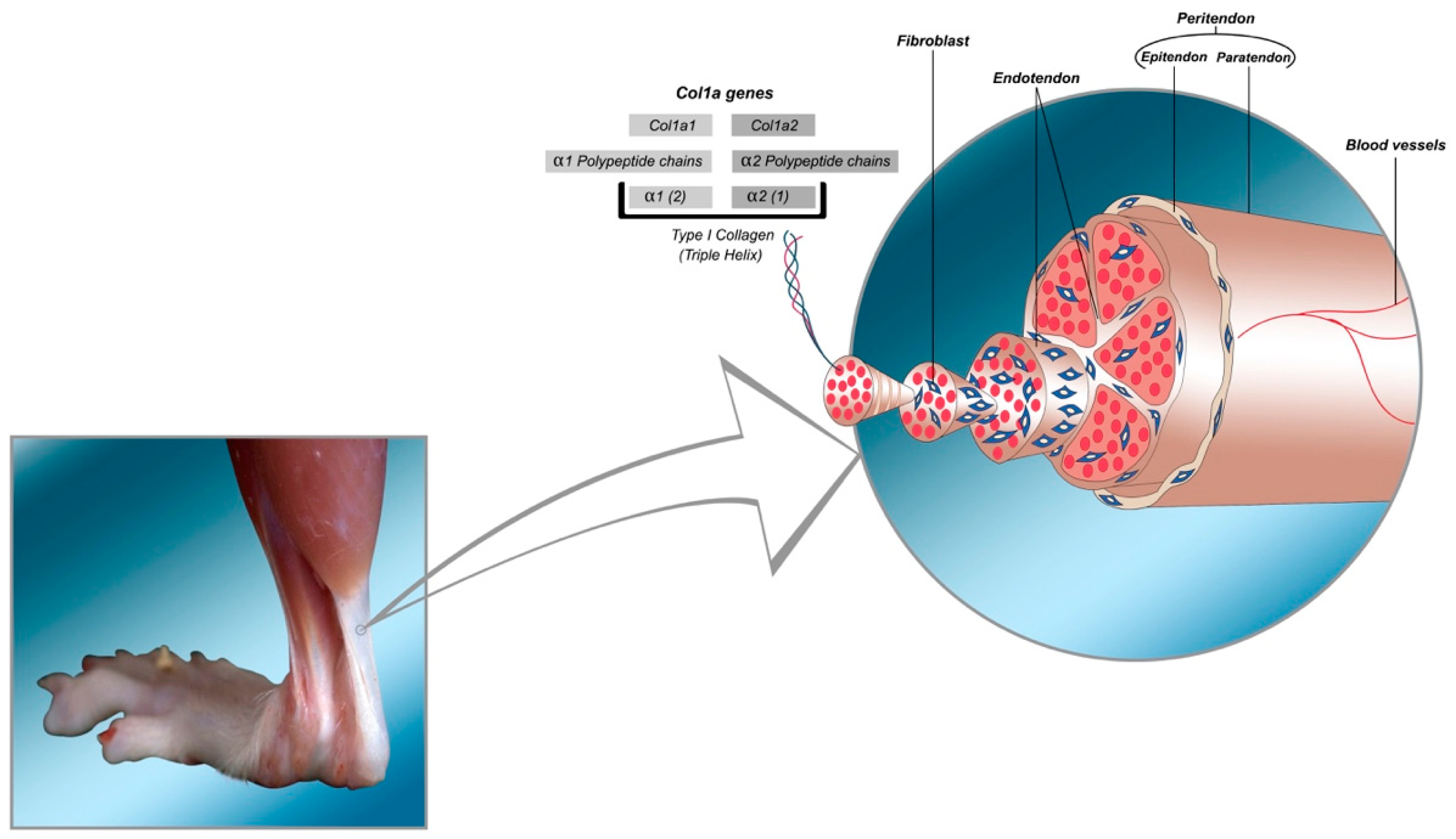
2. Structure and Function of Tendons
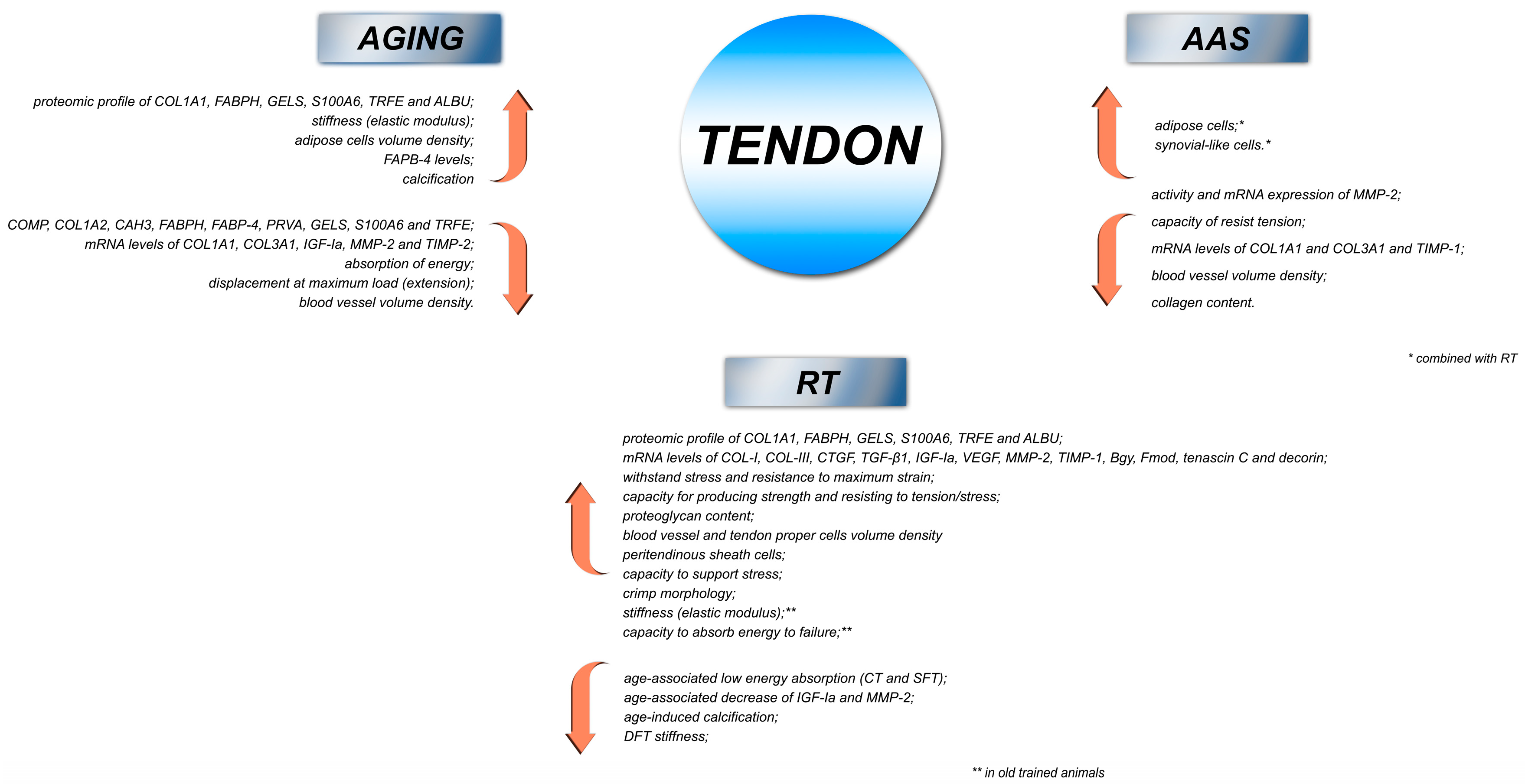
3. Effect of Training Modalities on Tendon Remodeling
4. Effect of AAS on Tendon Remodeling
5. Effect of Aging on Tendon Remodeling
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | anabolic androgenic steroids |
| ALBU | serum albumin |
| Bgn | biglycan |
| CAH3 | carbonic anhydrase |
| COL1A1 | type I collagen-α1 |
| COL3A1 | type III collagen-α1 |
| COL-I | type I collagen |
| COL-III | type III collagen |
| COMP | cartilage oligomeric matrix protein |
| CSA | cross-sectional area |
| CT | calcaneal tendon |
| CTGF | connective tissue growth factor |
| DFT | deep flexor tendon |
| ECM | extracellular matrix |
| FABP4 | acid-binding protein-4 |
| FABPH | acid-binding protein heart |
| Fmod | fibromodulin |
| GAGS | glycosaminoglycans |
| GAPDH | glyceraldehyde-3-Phosphatase Dehydrogenase |
| GELS | gelsolin |
| IGF-IEa | insulin-like grow factor I-Ea |
| MMP-2 | metalloproteinase 2 |
| PRVA | parvalbumin alpha |
| RT | resistance training |
| S100A6 | protein S-100A6 |
| SFT | superficial flexor tendon |
| TGFβ-1 | transforming growth factor beta 1 |
| TIMP-1 | tissue inhibitor of metalloproteinase 1 |
| TIMP-2 | tissue inhibitor of metalloproteinase 2 |
| TRFE | serotransferrin |
| TSPC | tendon-derived stem/progenitor cells |
| VEGF | vascular endothelial growth factor |
References
- Khan, K.M.; Cook, J.L.; Bonar, F.; Harcourt, P.; Astrom, M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999, 27, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, E.; Natali, P.G.; Postacchini, F.; Accinni, L.; De Martino, C. Morphological, immunochemical, and biochemical study of rabbit Achilles tendon at various ages. J. Bone Jt. Surg. Am. 1980, 62, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Kader, D.; Mosconi, M.; Benazzo, F.; Maffulli, N. Achilles tendon rupture. In Tendon Injuries; Springer: London, UK, 2005. [Google Scholar]
- Thampatty, B.P.; Wang, J.H.C. Mechanobiology of young and aging tendons: In vivo studies with treadmill running. J. Orthop. Res. 2018, 36, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.B.; Heinemeier, K.M.; Couppé, C.; Kjaer, M.; Magnusson, S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. 2016, 121, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Pope, H.G.; Kanayama, G.; Athey, A.; Ryan, E.; Hudson, J.I.; Baggish, A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am. J. Addict. 2014, 23, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Schwingel, P.A.; Cotrim, H.P.; dos Santos, C.R.; dos Santos, A.O.; de Andrade, A.R.C.F.; Carruego, M.V.V.B.; Zoppi, C.C. Recreational anabolic-androgenic steroid use associated with liver injuries among Brazilian young men. Subst. Use Misuse 2015, 50, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.; Peters, R.; Dillon, P. Anabolic-androgenic steroid use disorders among a sample of Australian competitive and recreational users. Drug Alcohol Depend. 2000, 60, 91–96. [Google Scholar] [CrossRef]
- Graham, M.R.; Davies, B.; Grace, F.M.; Kicman, A.; Baker, J.S. Anabolic Steroid Use. Sports Med. 2008, 38, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Guzzoni, V.; Cunha, T.S.; das Neves, V.J.; Briet, L.; Costa, R.; Moura, M.J.C.S.; Oliveira, V.; do Carmo Pinho Franco, M.; Novaes, P.D.; Marcondes, F.K. Nandrolone combined with strenuous resistance training reduces vascular nitric oxide bioavailability and impairs endothelium-dependent vasodilation. Steroids 2018, 131, 7–13. [Google Scholar] [CrossRef]
- Haupt, H.A.; Rovere, G.D. Anabolic steroids: A review of the literature. Am. J. Sports Med. 1984, 12, 469–484. [Google Scholar] [CrossRef]
- Yesalis, C.E.; Wright, J.E.; Bahrke, M.S. Epidemiological and policy issues in the measurement of the long-term health effects of anabolic-androgenic steroids. Sports Med. 1989, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kutscher, E.C.; Lund, B.C.; Perry, P.J. Anabolic steroids: A review for the clinician. Sports Med. 2002, 32, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, G.; Hudson, J.I.; Pope, H.G. Illicit anabolic-androgenic steroid use. Horm. Behav. 2010, 58, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.F.; Felisbino, S.L.; Keene, D.R.; Vogel, K.G. Identification, content, and distribution of type VI collagen in bovine tendons. Cell Tissue Res. 2006, 325, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Marqueti, R.C.; Prestes, J.; Paschoal, M.; Ramos, O.H.; Perez, S.E.; Carvalho, H.F.; Selistre-de-Araujo, H.S. Matrix metallopeptidase 2 activity in tendon regions: Effects of mechanical loading exercise associated to anabolic-androgenic steroids. Eur. J. Appl. Physiol. 2008, 104, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.P.; Narici, M.V.; Maganaris, C.N.; Kjaer, M. Human tendon behaviour and adaptation, in vivo. J. Physiol. 2008, 586, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannukainen, J.; Kalliokoski, K.K.; Nuutila, P.; Fujimoto, T.; Kemppainen, J.; Viljanen, T.; Laaksonen, M.S.; Parkkola, R.; Knuuti, J.; Kjær, M. In vivo measurements of glucose uptake in human Achilles tendon during different exercise intensities. Int. J. Sports Med. 2005, 26, 727–731. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Peffers, M.J.; Simpson, D.; Halliwell, E.; Screen, H.R.C.; Clegg, P.D. Anatomical heterogeneity of tendon: Fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci. Rep. 2016, 6, 20455. [Google Scholar] [CrossRef] [Green Version]
- Birch, H.L. Tendon matrix composition and turnover in relation to functional requirements. Int. J. Exp. Pathol. 2007, 88, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Vanderby, R. Collagen fibril morphology and organization: Implications for force transmission in ligament and tendon. Matrix Biol. 2006, 25, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, E.; Blitz, E.; Killian, M.L.; Thomopoulos, S. Tendon-to-bone attachment: From development to maturity. Birth Defects Res. Part C-Embryo Today Rev. 2014, 102, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-function relationships in tendons: A review. J. Anat. 2008, 212, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Jozsa, L.; Kannus, P.; Balint, J.B.; Reffy, A. Three-dimensional ultrastructure of human tendons. Acta Anat. (Basel) 1991, 142, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.W.D. The structure of rat tail tendon fascicles. Connect. Tissue Res. 1985, 14, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D. Structure and function of mammalian tendon. Biol. Rev. 1965, 40, 392–421. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.P.; Cappiello, W.L.; Poole, R.M.; Hunter, S.C. Prevention and Treatment of Overuse Tendon Injuries. Sports Med. 1989, 8, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon injury: From biology to tendon repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014, 802, 5–29. [Google Scholar]
- Angel, G.; Gheorghe, V. Interferometric evaluation of collagen concentration in tendon fibers. Connect. Tissue Res. 1985, 13, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; De Beaux, A. A quantitative ultrastructural study of rat tendon from birth to maturity. J. Anat. 1987, 153, 163–169. [Google Scholar] [PubMed]
- Viidik, A.; Ekholm, R. Light and electron microscopic studies of collagen fibers under strain. Anat. Embryol. (Berl) 1968, 127, 154–164. [Google Scholar] [CrossRef]
- Vidal, C.B. Crimp as part of a helical structure. C. R. Acad. Sci. III 1995, 318, 173–178. [Google Scholar]
- Hansen, K.A.; Weiss, J.A.; Barton, J.K. Recruitment of tendon crimp with applied tensile strain. J. Biomech. Eng. 2002, 124, 72. [Google Scholar] [CrossRef]
- Franchi, M.; Fini, M.; Quaranta, M.; De Pasquale, V.; Raspanti, M.; Giavaresi, G.; Ottani, V.; Ruggeri, A. Crimp morphology in relaxed and stretched rat Achilles tendon. J. Anat. 2007, 210, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Raspanti, M.; Manelli, A.; Franchi, M.; Ruggeri, A. The 3D structure of crimps in the rat Achilles tendon. Matrix Biol. 2005, 24, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, D.T.; Garrett, W.E. Function and biomechanics of tendons. Scand. J. Med. Sci. Sports 2007, 7, 62–66. [Google Scholar] [CrossRef]
- Pang, X.; Wu, J.P.; Allison, G.T.; Xu, J.; Rubenson, J.; Zheng, M.H.; Lloyd, D.G.; Gardiner, B.; Wang, A.; Kirk, T.B. Three-dimensional microstructural network of elastin, collagen, and cells in Achilles tendons. J. Orthop. Res. 2017, 35, 1203–1214. [Google Scholar] [CrossRef]
- Giusti, B.; Pepe, G. Fibrillins in tendon. Front. Aging Neurosci. 2016, 8, 237. [Google Scholar] [CrossRef]
- Mithieux, S.M.; Weiss, A.S. Elastin. In Advances in Protein Chemistry; Academic Press: New York, NY, USA, 2005; Volume 70, pp. 437–461. [Google Scholar]
- Green, E.M.; Mansfield, J.C.; Bell, J.S.; Winlove, C.P. The structure and micromechanics of elastic tissue. Interface Focus 2014, 4, 20130058. [Google Scholar] [CrossRef] [Green Version]
- Kielty, C.M. Elastic fibres in health and disease. Expert Rev. Mol. Med. 2006, 8, 1–23. [Google Scholar] [CrossRef]
- Yoon, J.H.; Halper, J. Tendon proteoglycans: Biochemistry and function. J. Musculoskelet. Neuronal Interact. 2005, 5, 22–34. [Google Scholar]
- Samiric, T.; Ilic, M.Z.; Handley, C.J. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004, 23, 127–140. [Google Scholar] [CrossRef]
- Screen, H.R.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef]
- Järvinen, M.; Kannus, P.; Kvist, M.; Isola, J.; Lehto, M.; Jozsa, L. Macromolecular composition of the myotendinous junction. Exp. Mol. Pathol. 1991, 55, 230–237. [Google Scholar] [CrossRef]
- Minor, R.R. Collagen metabolism: A comparison of diseases of collagen and diseases affecting collagen. Am. J. Pathol. 1980, 98, 225–280. [Google Scholar] [CrossRef]
- O’Brien, M. Structure and metabolism of tendons. Scand. J. Med. Sci. Sports 1997, 7, 55–61. [Google Scholar] [CrossRef]
- Bojsen-Møller, J.; Kalliokoski, K.K.; Seppänen, M.; Kjaer, M.; Magnusson, S.P. Low-intensity tensile loading increases intratendinous glucose uptake in the Achilles tendon. J. Appl. Physiol. 2006, 101, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Kalliokoski, K.K.; Langberg, H.; Ryberg, A.K.; Scheede-Bergdahl, C.; Doessing, S.; Kjaer, A.; Boushel, R.; Kjaer, M. The effect of dynamic knee-extension exercise on patellar tendon and quadriceps femoris muscle glucose uptake in humans studied by positron emission tomography. J. Appl. Physiol. 2005, 99, 1189–1192. [Google Scholar] [CrossRef]
- Langberg, H.; Rosendal, L.; Kjær, M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J. Physiol. 2001, 534, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Salingcarnboriboon, R.; Yoshitake, H.; Tsuji, K.; Obinata, M.; Amagasa, T.; Nifuji, A.; Noda, M. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp. Cell Res. 2003, 287, 289–300. [Google Scholar] [CrossRef]
- De Mos, M.; Koevoet, W.J.L.M.; Jahr, H.; Verstegen, M.M.A.; Heijboer, M.P.; Kops, N.; Van Leeuwen, J.P.T.M.; Weinans, H.; Verhaar, J.A.N.; Van Osch, G.J.V.M. Intrinsic differentiation potential of adolescent human tendon tissue: An in-vitro cell differentiation study. BMC Musculoskelet. Disord. 2007, 8, 16. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Lake, S.P.; Ansorge, H.L.; Soslowsky, L.J. Animal models of tendinopathy. Disabil. Rehabil. 2008, 30, 1530–1541. [Google Scholar] [CrossRef]
- Dudhia, J.; Scott, C.M.; Draper, E.R.C.; Heinegård, D.; Pitsillides, A.A.; Smith, R.K. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell 2007, 6, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.M.; Scott, A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br. J. Sports Med. 2009, 43, 247–252. [Google Scholar] [CrossRef]
- Freedman, B.R.; Bade, N.D.; Riggin, C.N.; Zhang, S.; Haines, P.G.; Ong, K.L.; Janmey, P.A. The (dys) functional extracellular matrix. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 3153–3164. [Google Scholar] [CrossRef]
- Gauthier, N.C.; Roca-Cusachs, P. Mechanosensing at integrin-mediated cell–matrix adhesions: From molecular to integrated mechanisms. Curr. Opin. Cell Biol. 2018, 50, 20–26. [Google Scholar] [CrossRef]
- Arampatzis, A.; Karamanidis, K.; Albracht, K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J. Exp. Biol. 2007, 210, 2743–2753. [Google Scholar] [CrossRef] [Green Version]
- Heinemeier, K.M.; Kjaer, M. In vivo investigation of tendon responses to mechanical loading. J. Musculoskelet. Neuronal Interact. 2011, 11, 115–123. [Google Scholar]
- Wang, J.H.C.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology-A minireview of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133–141. [Google Scholar] [CrossRef]
- Shwartz, Y.; Blitz, E.; Zelzer, E. One load to rule them all: Mechanical control of the musculoskeletal system in development and aging. Differentiation 2013, 86, 104–111. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Olesen, J.L.; Haddad, F.; Schjerling, P.; Baldwin, K.M.; Kjaer, M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J. Appl. Physiol. 2008, 106, 178–186. [Google Scholar] [CrossRef]
- Langberg, H.; Skovgaard, D.; Petersen, L.J.; Bülow, J.; Kjær, M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J. Physiol. 1999, 521, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.F.; Olesen, J.L.; Hansen, M.; Døssing, S.; Crameri, R.M.; Welling, R.J.; Langberg, H.; Flyvbjerg, A.; Kjaer, M.; Babraj, J.A.; et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J. Physiol. 2005, 567, 1021–1033. [Google Scholar] [CrossRef] [Green Version]
- Barkhausen, T.; van Griensven, M.; Zeichen, J.; Bosch, U. Modulation of cell functions of human tendon fibroblasts by different repetitive cyclic mechanical stress patterns. Exp. Toxicol. Pathol. 2003, 55, 153–158. [Google Scholar] [CrossRef]
- Kilts, T.; Ameye, L.; Syed-Picard, F.; Ono, M.; Berendsen, A.D.; Oldberg, A.; Heegaard, A.M.; Bi, Y.; Young, M.F. Potential roles for the small leucine-rich proteoglycans biglycan and fibromodulin in ectopic ossification of tendon induced by exercise and in modulating rotarod performance. Scand. J. Med. Sci. Sports 2009, 19, 536–546. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.L.; Ritter, M.A.; Amiel, D.; Sanders, T.M.; Gomez, M.A.; Kuei, S.C.; Garfin, S.R.; Akeson, W.H. The biomechanical and biochemical properties of swine tendons-long term effects of exercise on the digital extensors. Connect. Tissue Res. 1980, 7, 177–183. [Google Scholar] [CrossRef]
- Wiesinger, H.P.; Kösters, A.; Müller, E.; Seynnes, O.R. Effects of increased loading on in vivo tendon properties: A systematic review. Med. Sci. Sports Exerc. 2015, 47, 1885. [Google Scholar] [CrossRef]
- Kongsgaard, M.; Reitelseder, S.; Pedersen, T.G.; Holm, L.; Aagaard, P.; Kjaer, M.; Magnusson, S.P. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol. 2007, 191, 111–121. [Google Scholar] [CrossRef]
- Couppe, C.; Kongsgaard, M.; Aagaard, P.; Hansen, P.; Bojsen-Moller, J.; Kjaer, M.; Magnusson, S.P. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J. Appl. Physiol. 2008, 105, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Houghton, L.A.; Dawson, B.T.; Rubenson, J. Effects of plyometric training on Achilles tendon properties and shuttle running during a simulated cricket batting innings. J. Strength Cond. Res. 2013, 27, 1036–1046. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H.-C. The effects of mechanical loading on tendons—An in vivo and in vitro model study. PLoS ONE 2013, 8, e71740. [Google Scholar] [CrossRef]
- Thornton, G.M.; Hart, D.A. The interface of mechanical loading and biological variables as they pertain to the development of tendinosis. J. Musculoskelet. Neuronal Interact. 2011, 11, 94–105. [Google Scholar]
- Davis, M.E.; Gumucio, J.P.; Sugg, K.B.; Bedi, A.; Mendias, C.L. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J. Appl. Physiol. 2013, 115, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Kjaer, M.; Kjær, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Koskinen, S.O.A.; Heinemeier, K.M.; Olesen, J.L.; Langberg, H.; Kjaer, M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J. Appl. Physiol. 2004, 96, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Arnoczky, S.P.; Tian, T.; Lavagnino, M.; Gardner, K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J. Orthop. Res. 2004, 22, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Lavagnino, M.; Arnoczky, S.P.; Tian, T.; Vaupel, Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: An in vitro study. Connect. Tissue Res. 2003, 44, 181–187. [Google Scholar] [CrossRef]
- Barin, F.R.; Durigan, J.L.Q.; de S. Oliveira, K.; Migliolo, L.; Almeida, J.A.; Carvalho, M.; Petriz, B.; Selistre-de-Araujo, H.S.; Fontes, W.; Franco, O.L.; et al. Beneficial effects of resistance training on the protein profile of the calcaneal tendon during aging. Exp. Gerontol. 2017, 100, 54–62. [Google Scholar] [CrossRef]
- Kongsgaard, M.; Qvortrup, K.; Larsen, J.; Aagaard, P.; Doessing, S.; Hansen, P.; Kjaer, M.; Magnusson, S.P. Fibril morphology and tendon mechanical properties in patellar tendinopathy. Am. J. Sports Med. 2010, 38, 749–756. [Google Scholar] [CrossRef]
- American College of Sports Medicine. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med. Sci. Sports Exerc. 1998, 30, 975–991. [Google Scholar]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American heart association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Olesen, J.L.; Haddad, F.; Langberg, H.; Kjaer, M.; Baldwin, K.M.; Schjerling, P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiol. 2007, 582, 1303–1316. [Google Scholar] [CrossRef] [Green Version]
- Kjær, M.; Langberg, H.; Heinemeier, K.; Bayer, M.L.; Hansen, M.; Holm, L.; Doessing, S.; Kongsgaard, M.; Krogsgaard, M.R.; Magnusson, S.P. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports 2009, 19, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Borst, S.E.; de Hoyos, D.V.; Garzarella, L.; Vincent, K.; Pollock, B.H.; Lowenthal, D.T.; Pollock, M.L. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med. Sci. Sports Exerc. 2001, 33, 648–653. [Google Scholar] [CrossRef]
- Guzzoni, V.; Ribeiro, M.B.T.; Lopes, G.N.; de Cássia Marqueti, R.; de Andrade, R.V.; Selistre-de-Araujo, H.S.; Durigan, J.L.Q. Effect of resistance training on extracellular matrix adaptations in skeletal muscle of older rats. Front. Physiol. 2018, 9, 374. [Google Scholar] [CrossRef]
- Evanko, S.P.; Vogel, K.G. Ultrastructure and proteoglycan composition in the developing fibrocartilaginous region of bovine tendon. Matrix 1990, 10, 420–436. [Google Scholar] [CrossRef]
- De Cassia Marqueti, R.; Almeida, J.A.; Nakagaki, W.R.; Guzzoni, V.; Boghi, F.; Renner, A.; Silva, P.E.; Durigan, J.L.Q.; Selistre-de-Araujo, H.S. Resistance training minimizes the biomechanical effects of aging in three different rat tendons. J. Biomech. 2017, 53, 29–35. [Google Scholar] [CrossRef]
- Killian, M.L.; Cavinatto, L.; Galatz, L.M.; Thomopoulos, S. The role of mechanobiology in tendon healing. J. Shoulder Elb. Surg. 2012, 21, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Marqueti, R.C.; Durigan, J.L.Q.; Oliveira, A.J.S.; Mekaro, M.S.; Guzzoni, V.; Aro, A.A.; Pimentel, E.R.; Selistre-De-Araujo, H.S. Effects of aging and resistance training in rat tendon remodeling. FASEB J. 2018, 32, 353–368. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, C.S.; Henkel, C.; Svensson, R.B.; Agergaard, A.-S.; Couppe, C.; Kjaer, M.; Magnusson, S.P. Lower tendon stiffness in very old compared to old individuals is unaffected by short term resistance training of skeletal muscle. J. Appl. Physiol. 2018, 125, 205–214. [Google Scholar] [CrossRef]
- Wood, L.K.; Brooks, S.V. Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice. J. Orthop. Res. 2016, 34, 346–353. [Google Scholar] [CrossRef]
- Bhasin, S.; Storer, T.W.; Berman, N.; Callegari, C.; Clevenger, B.; Phillips, J.; Bunnell, T.J.; Tricker, R.; Shirazi, A.; Casaburi, R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N. Engl. J. Med. 1996, 335, 1–7. [Google Scholar] [CrossRef]
- Evans, N.A. Current concepts in anabolic-androgenic steroids. Am. J. Sports Med. 2004, 32, 534–542. [Google Scholar] [CrossRef]
- Parssinen, M.; Karila, T.; Kovanen, V.; Seppälä, T. The effect of supraphysiological doses of anabolic androgenic steroids on collagen metabolism. Int. J. Sports Med. 2000, 21, 406–411. [Google Scholar] [CrossRef]
- Michna, H. Tendon injuries induced by exercise and anabolic steroids in experimental mice. Int. Orthop. 1987, 11, 157–162. [Google Scholar] [CrossRef]
- Michna, H. Organisation of collagen fibrils in tendon: changes induced by an anabolic steroid-I. Functional and ultrastructural studies. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1986, 52, 75–86. [Google Scholar] [CrossRef]
- Inhofe, P.D.; Grana, W.A.; Egle, D.; Min, K.W.; Tomasek, J. The effects of anabolic steroids on rat tendon: An ultrastructural, biomechanical, and biochemical analysis. Am. J. Sports Med. 1995, 23, 227–232. [Google Scholar] [CrossRef]
- Hill, J.A.; Suker, J.R.; Sachs, K.; Brigham, C. The athletic polydrug abuse phenomenon. A case report. Am. J. Sports Med. 1983, 11, 269–271. [Google Scholar] [CrossRef]
- Kramhøft, M.; Solgaard, S. Spontaneous rupture of the extensor pollicis longus tendon after anabolic steroids. J. Hand Surg. Am. 1986, 11, 87. [Google Scholar] [CrossRef]
- Wood, T.O.; Cooke, P.H.; Goodship, A.E. The effect of exercise and anabolic steroids on the mechanical properties and crimp morphology of the rat tendon. Am. J. Sports Med. 1988, 16, 153–158. [Google Scholar] [CrossRef]
- Laseter, J.T.; Russell, J.A. Anabolic steroid-induced tendon pathology: A review of the literature. Med. Sci. Sports Exerc. 1991, 23, 1–3. [Google Scholar] [CrossRef]
- Evans, N.A.; Bowrey, D.J.; Newman, G.R. Ultrastructural analysis of ruptured tendon from anabolic steroid users. Injury 1998, 29, 769–773. [Google Scholar] [CrossRef]
- Kanayama, G.; Deluca, J.; Meehan, W.P.; Hudson, J.I.; Isaacs, S.; Baggish, A.; Weiner, R.; Micheli, L.; Pope, H.G. Ruptured tendons in anabolic-androgenic steroid users. Am. J. Sports Med. 2015, 43, 2638–2644. [Google Scholar] [CrossRef]
- Miles, J.W.; Grana, W.A.; Egle, D.; Min, K.W.; Chitwood, J. The effect of anabolic steroids on the biomechanical and histological properties of rat tendon. J. Bone Jt. Surg. Am. 1992, 74, 411–422. [Google Scholar] [CrossRef]
- Marqueti, R.C.; Parizotto, N.A.; Chriguer, R.S.; Perez, S.E.A.; Selistre-de-Araujo, H.S. Androgenic-anabolic steroids associated with mechanical loading inhibit matrix metallopeptidase activity and affect the remodeling of the Achilles tendon in rats. Am. J. Sports Med. 2006, 34, 1274–1280. [Google Scholar] [CrossRef]
- Urso, M.L.; Pierce, J.R.; Alemany, J.A.; Harman, E.A.; Nindl, B.C. Effects of exercise training on the matrix metalloprotease response to acute exercise. Eur. J. Appl. Physiol. 2009, 106, 655–663. [Google Scholar] [CrossRef]
- De Sousa Neto, I.V.; Durigan, J.L.Q.; Guzzoni, V.; Tibana, R.A.; Prestes, J.; de Araujo, H.S.S.; Marqueti, R.C. Effects of resistance training on matrix metalloproteinase activity in skeletal muscles and blood circulation during aging. Front. Physiol 2018, 9. [Google Scholar] [CrossRef]
- Carmeli, E.; Moas, M.; Lennon, S.; Powers, S.K. High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibres. Exp. Physiol. 2005, 90, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Rullman, E.; Norrbom, J.; Strömberg, A.; Wågsäter, D.; Rundqvist, H.; Haas, T.; Gustafsson, T. Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J. Appl. Physiol. 2009, 106, 804–812. [Google Scholar] [CrossRef] [Green Version]
- Huisman, E.; Lu, A.; Jamil, S.; Mousavizadeh, R.; McCormack, R.; Roberts, C.; Scott, A. Influence of repetitive mechanical loading on MMP2 activity in tendon fibroblasts. J. Orthop. Res. 2016, 34, 1991–2000. [Google Scholar] [CrossRef]
- Peviani, S.M.; Guzzoni, V.; Pinheiro-Dardis, C.M.; Da Silva, Y.P.; Fioravante, A.C.R.; Sagawa, A.H.; Delfino, G.B.; Durigan, J.L.Q.; Salvini, T.F. Regulation of extracellular matrix elements and sarcomerogenesis in response to different periods of passive stretching in the soleus muscle of rats. Sci. Rep. 2018, 8, 9010. [Google Scholar] [CrossRef]
- Yang, G.; Im, H.J.; Wang, J.H.C. Repetitive mechanical stretching modulates IL-1β induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 2005, 363, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Asundi, K.R.; Rempel, D.M. Cyclic loading inhibits expression of MMP-3 but not MMP-1 in an in vitro rabbit flexor tendon model. Clin. Biomech. 2008, 23, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Waggett, A.D.; Ralphs, J.R.; Kwan, A.P.L.; Woodnutt, D.; Benjamin, M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 1998, 16, 457–470. [Google Scholar] [CrossRef]
- Marqueti, R.C.; Prestes, J.; Wang, C.C.; Ramos, O.H.P.; Perez, S.E.A.; Nakagaki, W.R.; Carvalho, H.F.; Selistre-de-Araujo, H.S. Biomechanical responses of different rat tendons to nandrolone decanoate and load exercise. Scand. J. Med. Sci. Sports 2011, 21, e91–e99. [Google Scholar] [CrossRef]
- Seynnes, O.R.; Kamandulis, S.; Kairaitis, R.; Helland, C.; Campbell, E.-L.; Brazaitis, M.; Skurvydas, A.; Narici, M.V. Effect of androgenic-anabolic steroids and heavy strength training on patellar tendon morphological and mechanical properties. J. Appl. Physiol. 2013, 115, 84–89. [Google Scholar] [CrossRef]
- Marqueti, R.D.C.; Heinemeier, K.M.; Durigan, J.L.Q.; de Andrade Perez, S.E.; Schjerling, P.; Kjaer, M.; Carvalho, H.F.; Selistre-De-Araujo, H.S. Gene expression in distinct regions of rat tendons in response to jump training combined with anabolic androgenic steroid administration. Eur. J. Appl. Physiol. 2012, 112, 1505–1515. [Google Scholar] [CrossRef]
- Legerlotz, K.; Schjerling, P.; Langberg, H.; Brüggemann, G.-P.; Niehoff, A. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats. J. Appl. Physiol. 2007, 102, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Minkwitz, S.; Schmock, A.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B.; Klatte-Schulz, F. Time-dependent alterations of MMPs, TIMPs and tendon structure in human Achilles tendons after acute rupture. Int. J. Mol. Sci. 2017, 18, 2199. [Google Scholar] [CrossRef]
- Marqueti, R.C.; Paulino, M.G.; Fernandes, M.N.; de Oliveira, E.M.; Selistre-de-Araujo, H.S. Tendon structural adaptations to load exercise are inhibited by anabolic androgenic steroids. Scand. J. Med. Sci. Sports 2014, 24, e39–e51. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Hatch, G.F.R.; Weber, A.E.; Vangsness, C.T. Anabolic steroids and tendons: A review of their mechanical, structural, and biologic effects. J. Orthop. Res. 2018. [Google Scholar] [CrossRef]
- Clayton, R.A.E.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344. [Google Scholar] [CrossRef]
- Peffers, M.J.; Thorpe, C.T.; Collins, J.A.; Eong, R.; Wei, T.K.J.; Screen, H.R.C.; Clegg, P.D. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J. Biol. Chem. 2014, 289, 25867–25878. [Google Scholar] [CrossRef]
- Gagliano, N.; Menon, A.; Cabitza, F.; Compagnoni, R.; Randelli, P. Morphological and molecular characterization of human hamstrings shows that tendon features are not influenced by donor age. Knee Surgery Sports Traumatol. Arthrosc. 2018, 26, 343–352. [Google Scholar] [CrossRef]
- Wood, L.K.; Arruda, E.M.; Brooks, S.V. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J. Appl. Physiol. 2011, 111, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Couppé, C.; Svensson, R.B.; Grosset, J.F.; Kovanen, V.; Nielsen, R.H.; Olsen, M.R.; Larsen, J.O.; Praet, S.F.E.; Skovgaard, D.; Hansen, M.; et al. Life-long endurance running is associated with reduced glycation and mechanical stress in connective tissue. Age (Omaha) 2014, 36, 9665. [Google Scholar] [CrossRef]
- Kubo, K.; Kanehisa, H.; Kawakami, Y.; Fukanaga, T. Growth changes in the elastic properties of human tendon structures. Int. J. Sports Med. 2001, 22, 138–143. [Google Scholar] [CrossRef]
- Shadwick, R.E. Elastic energy storage in tendons: Mechanical differences related to function and age. J. Appl. Physiol. 1990, 68, 1033–1040. [Google Scholar] [CrossRef]
- Noyes, F.R.; Grood, E.S. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J. Bone Jt. Surg. Am. 1976, 58, 1074–1082. [Google Scholar] [CrossRef]
- Nachemson, A.L.; Evans, J.H. Some mechanical properties of the third human lumbar interlaminar ligament (ligamentum flavum). J. Biomech. 1968, 1, 211–220. [Google Scholar] [CrossRef]
- Reeves, N.D. Adaptation of the tendon to mechanical usage. J. Musculoskelet. Neuronal Interact. 2006, 6, 174–180. [Google Scholar]
- Vogel, H.G. Species differences of elastic and collagenous tissue-Influence of maturation and age. Mech. Ageing Dev. 1991, 57, 15–24. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Streeter, I.; Pinchbeck, G.L.; Goodship, A.E.; Clegg, P.D.; Birch, H.L. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J. Biol. Chem. 2010, 285, 15674–15681. [Google Scholar] [CrossRef]
- Avery, N.C.; Bailey, A.J. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: Relevance to aging and exercise. Scand. J. Med. Sci. Sports 2005, 15, 231–240. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Birch, H.L.; Goodman, S.; Heinegård, D.; Goodship, A.E. The influence of ageing and exercise on tendon growth and degeneration-Hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 1039–1050. [Google Scholar] [CrossRef]
- Dedkov, E.I.; Kostrominova, T.Y.; Borisov, A.B.; Carlson, B.M. MyoD and myogenin protein expression in skeletal muscles of senile rats. Cell Tissue Res. 2003, 311, 401–416. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Cai, J.; Gu, B.; Lv, Y.; Zhao, L. The frequency-dependent aerobic exercise effects of hypothalamic GABAergic expression and cardiovascular functions in aged rats. Front. Aging Neurosci. 2017, 9, 212. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Pang, J.-H.S.; Wu, K.P.-H.; Chen, M.J.-L.; Chen, C.-H.; Tsai, W.-C. Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskelet. Disord. 2013, 14, 2. [Google Scholar] [CrossRef]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef]
- Bank, R.A.; Tekoppele, J.M.; Oostingh, G.; Hazleman, B.L.; Riley, G.P. Lysylhydroxylation and non-reducible crosslinking of human supraspinatus tendon collagen: Changes with age and in chronic rotator cuff tendinitis. Ann. Rheum. Dis. 1999, 58, 35–41. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Magnusson, S.P.; Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J. 2013, 27, 2074–2079. [Google Scholar] [CrossRef]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 47306, 2068–2076. [Google Scholar] [CrossRef]
- James, V.J.; Delbridge, L.; McLennan, S.V.; Yue, D.K. Use of X-ray diffraction in study of human diabetic and aging collagen. Diabetes 1991, 40, 391–394. [Google Scholar] [CrossRef]
- Miles, C.A.; Avery, N.C.; Rodin, V.V.; Bailey, A.J. The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J. Mol. Biol. 2005, 346, 551–556. [Google Scholar] [CrossRef]
- Bailey, A.J. Molecular mechanisms of ageing in connective tissues. Mech. Ageing Dev. 2001, 122, 735–755. [Google Scholar] [CrossRef]
- Couppé, C.; Hansen, P.; Kongsgaard, M.; Kovanen, V.; Suetta, C.; Aagaard, P.; Kjaer, M.; Magnusson, S.P. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J. Appl. Physiol. 2009, 107, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Investig. 1993, 91, 2463–2469. [Google Scholar] [CrossRef]
- Reddy, G.K.; Stehno-Bittel, L.; Enwemeka, C.S. Glycation-induced matrix stability in the rabbit Achilles tendon. Arch. Biochem. Biophys. 2002, 399, 174–180. [Google Scholar] [CrossRef]
- Reddy, G.K. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp. Diabesity Res. 2004, 5, 143–153. [Google Scholar] [CrossRef]
- Karamanidis, K.; Arampatzis, A. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J. Biomech. 2006, 39, 406–417. [Google Scholar] [CrossRef]
- Mian, O.S.; Thom, J.M.; Ardigò, L.P.; Minetti, A.E.; Narici, M.V. Gastrocnemius muscle-tendon behaviour during walking in young and older adults. Acta Physiol. 2007, 189, 57–65. [Google Scholar] [CrossRef]
- Onambele, G.L. Calf muscle-tendon properties and postural balance in old age. J. Appl. Physiol. 2006, 100, 2048–2056. [Google Scholar] [CrossRef] [Green Version]
- Bai, P.; Phua, K.; Hardt, T.; Cernadas, M.; Brodsky, B. Glycation alters collagen fibril organization. Connect. Tissue Res. 1992, 28, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Fessel, G.; Georgiadis, M.; Snedeker, J.G. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013, 32, 169–177. [Google Scholar] [CrossRef]
- Nielsen, H.M.; Skalicky, M.; Viidik, A. Influence of physical exercise on aging rats. III. Life-long exercise modifies the aging changes of the mechanical properties of limb muscle tendons. Mech. Ageing Dev. 1998, 100, 243–260. [Google Scholar] [CrossRef]
- Viidik, A.; Nielsen, H.M.; Skalicky, M. Influence of physical exercise on aging rats: II. Life-long exercise delays aging of tail tendon collagen. Mech. Ageing Dev. 1996, 88, 139–148. [Google Scholar] [CrossRef]
- Vogel, H.G. Influence of maturation and age on mechanical and biochemical parameters of connective tissue of various organs in the rat. Connect. Tissue Res. 1978, 6, 161–166. [Google Scholar] [CrossRef]
- Dressler, M.R.; Butler, D.L.; Wenstrup, R.; Awad, H.A.; Smith, F.; Boivin, G.P. A potential mechanism for age-related declines in patellar tendon biomechanics. J. Orthop. Res. 2002, 20, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, A.S.; Duenwald-Kuehl, S.E.; Brickson, S.; Akins, T.L.; Diffee, G.; Aiken, J.; Vanderby, R.; Lakes, R.S. Effect of age and exercise on the viscoelastic properties of rat tail Tendon. Ann. Biomed. Eng. 2013, 41, 1120–1128. [Google Scholar] [CrossRef]
- Simonsen, E.B.; Klitgaard, H.; Bojsen-Møller, F. The influence of strength training, swim training and ageing on the Achilles tendon and m. Soleus of the rat. J. Sports Sci. 1995, 13, 291–295. [Google Scholar] [CrossRef]
- Hubbard, R.P.; Soutas-Little, R.W. Mechanical properties of human tendon and their age dependence. J. Biomech. Eng 1984, 106, 144–150. [Google Scholar] [CrossRef]
- Flahiff, C.M.; Brooks, A.T.; Hollis, J.M.; Vander Schilden, J.L.; Nicholas, R.W. Biomechanical Analysis of Patellar Tendon Allografts as a Function of Donor Age. Am. J. Sports Med. 1995, 23, 354–358. [Google Scholar] [CrossRef]
- Bojsen-Moller, J. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J. Appl. Physiol. 2005, 99, 986–994. [Google Scholar] [CrossRef] [Green Version]
- Nordez, A.; Gallot, T.; Catheline, S.; Guével, A.; Cornu, C.; Hug, F. Electromechanical delay revisited using very high frame rate ultrasound. J. Appl. Physiol. 2009, 106, 1970–1975. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.S.; Wong, W.K.; Lam, K.S.L. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Torricelli, P.; Veronesi, F.; Pagani, S.; Maffulli, N.; Masiero, S.; Frizziero, A.; Fini, M. In vitro tenocyte metabolism in aging and oestrogen deficiency. Age (Omaha) 2013, 35, 2125–2136. [Google Scholar] [CrossRef]
- Kohler, J.; Popov, C.; Klotz, B.; Alberton, P.; Prall, W.C.; Haasters, F.; Müller-Deubert, S.; Ebert, R.; Klein-Hitpass, L.; Jakob, F.; et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 2013, 12, 988–999. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Akinbiyi, T.; Xu, L.; Ramcharan, M.; Leong, D.J.; Ros, S.J.; Colvin, A.C.; Schaffler, M.B.; Majeska, R.J.; Flatow, E.L.; et al. Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell 2010, 9, 911–915. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H.C. Moderate exercise mitigates the detrimental effects of aging on tendon stem cells. PLoS ONE 2015, 10, e0130454. [Google Scholar] [CrossRef] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzzoni, V.; Selistre-de-Araújo, H.S.; De Cássia Marqueti, R. Tendon Remodeling in Response to Resistance Training, Anabolic Androgenic Steroids and Aging. Cells 2018, 7, 251. https://doi.org/10.3390/cells7120251
Guzzoni V, Selistre-de-Araújo HS, De Cássia Marqueti R. Tendon Remodeling in Response to Resistance Training, Anabolic Androgenic Steroids and Aging. Cells. 2018; 7(12):251. https://doi.org/10.3390/cells7120251
Chicago/Turabian StyleGuzzoni, Vinicius, Heloisa Sobreiro Selistre-de-Araújo, and Rita De Cássia Marqueti. 2018. "Tendon Remodeling in Response to Resistance Training, Anabolic Androgenic Steroids and Aging" Cells 7, no. 12: 251. https://doi.org/10.3390/cells7120251
APA StyleGuzzoni, V., Selistre-de-Araújo, H. S., & De Cássia Marqueti, R. (2018). Tendon Remodeling in Response to Resistance Training, Anabolic Androgenic Steroids and Aging. Cells, 7(12), 251. https://doi.org/10.3390/cells7120251





