A Dynamic Culture Method to Produce Ovarian Cancer Spheroids under Physiologically-Relevant Shear Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. COMSOL Multiphysics® Simulations
2.2. Cell Culture
2.3. Preparation of ULA Plates
2.4. Spheroid Culture
2.5. Microscopy
2.6. Spheroid Characterization
2.7. Scanning Electron Microscopy (SEM)
2.8. Live/Dead Staining of Spheroids
2.9. Spheroid Viability
2.10. Data Analysis
3. Results
3.1. Simulation of FSS in Well Plates on an Orbital Shaker
3.2. Optimization of Well Size and Rotation Speed
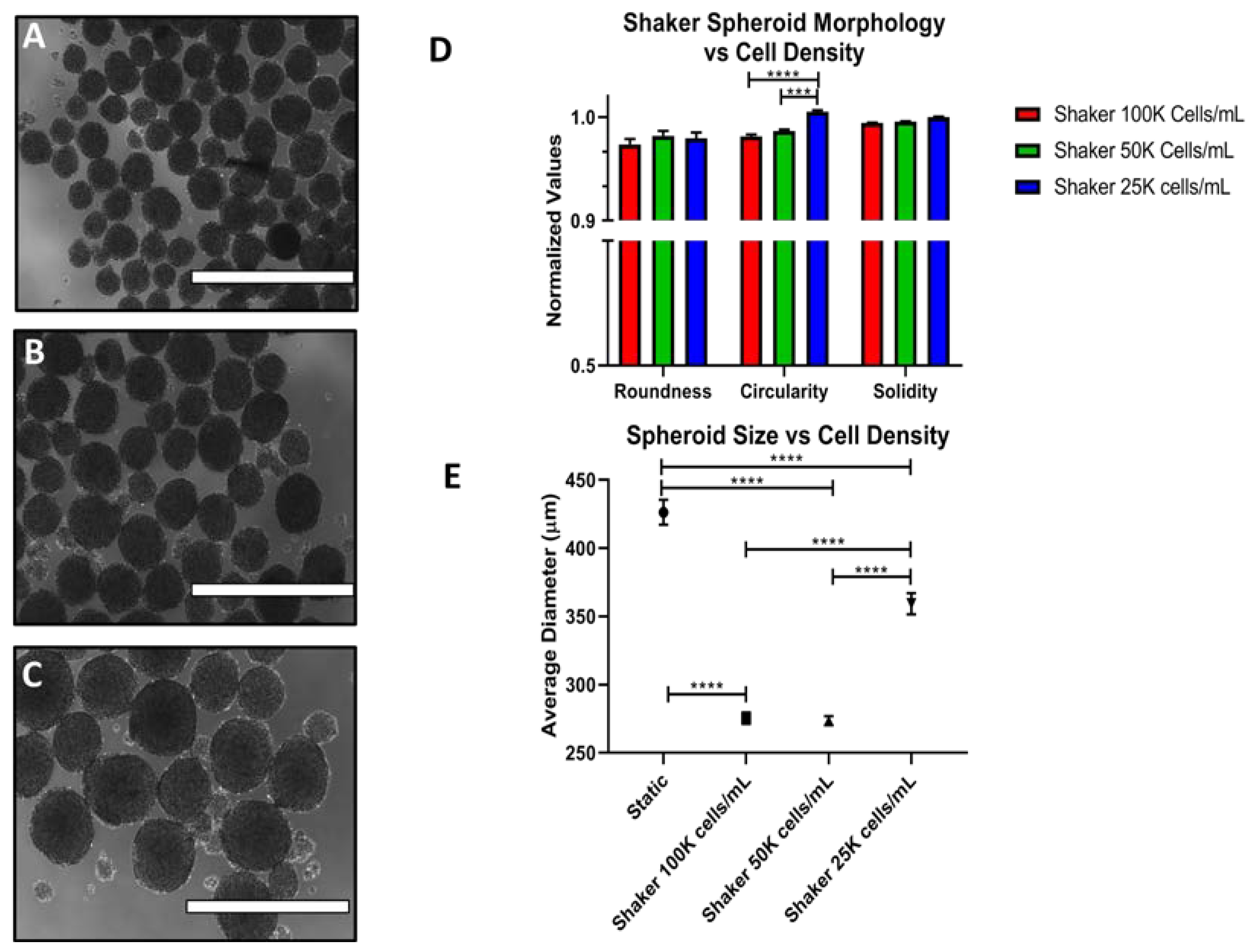
3.3. Effects of Cell Density on Spheroid Size and Morphology
3.4. Spheroid Viability
3.5. Long Term Culture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Gloss, B.S.; Samimi, G. Epigenetic biomarkers in epithelial ovarian cancer. Cancer Lett. 2014, 342, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Duska, L.R.; Kohn, E.C. The new classifications of ovarian, fallopian tube, and primary peritoneal cancer and their clinical implications. Ann. Oncol. 2017, 28, viii8–viii12. [Google Scholar] [CrossRef] [PubMed]
- Hemachandra, L.P.; Shin, D.H.; Dier, U.; Iuliano, J.N.; Engelberth, S.A.; Uusitalo, L.M.; Murphy, S.K.; Hempel, N. Mitochondrial superoxide dismutase has a protumorigenic role in ovarian clear cell carcinoma. Cancer Res. 2015, 75, 4973–4984. [Google Scholar] [CrossRef] [PubMed]
- Ku, F.C.; Wu, R.C.; Yang, L.Y.; Tang, Y.H.; Chang, W.Y.; Yang, J.E.; Wang, C.C.; Jung, S.M.; Lin, C.T.; Chang, T.C.; et al. Clear cell carcinomas of the ovary have poorer outcomes compared with serous carcinomas: Results from a single-center Taiwanese study. J. Formos Med. Assoc. 2018, 117, 117–125. [Google Scholar] [CrossRef]
- Argento, M.; Hoffman, P.; Gauchez, A.-S. Ovarian cancer detection and treatment: Current situation and future prospects. Anticancer Res. 2008, 28, 3135–3138. [Google Scholar]
- Gu, Z.; He, Y.; Zhang, Y.; Chen, M.; Song, K.; Huang, Y.; Li, Q.; Di, W. Postprandial increase in serum CA125 as a surrogate biomarker for early diagnosis of ovarian cancer. J. Transl. Med. 2018, 16, 114. [Google Scholar] [CrossRef] [Green Version]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef]
- Pradeep, S.; Kim, S.W.; Wu, S.Y.; Nishimura, M.; Chaluvally-Raghavan, P.; Miyake, T.; Pecot, C.V.; Kim, S.J.; Choi, H.J.; Bischoff, F.Z.; et al. Hematogenous metastasis of ovarian cancer: Rethinking mode of spread. Cancer Cell 2014, 26, 77–91. [Google Scholar] [CrossRef]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef]
- Kanda, M.; Kodera, Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J. Gastroenterol. 2016, 22, 6829–6840. [Google Scholar] [CrossRef] [PubMed]
- Kasagi, Y.; Harada, Y.; Morodomi, Y.; Iwai, T.; Saito, S.; Yoshida, K.; Oki, E.; Saeki, H.; Ohgaki, K.; Sugiyama, M.; et al. Peritoneal dissemination requires an Sp1-dependent CXCR4/CXCL12 signaling axis and extracellular matrix-directed spheroid formation. Cancer Res. 2016, 76, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.; Ackland, M.L.; Ahmed, N.; Rice, G.E. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol. Oncol. 2009, 113, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Davidowitz, R.A.; Iwanicki, M.P.; Brugge, J.S. In vitro mesothelial clearance assay that models the early steps of ovarian cancer metastasis. J. Vis. Exp. 2012, 10, 3791–3888. [Google Scholar] [CrossRef] [PubMed]
- Pettee, K.M.; Dvorak, K.M.; Nestor-Kalinoski, A.L.; Eisenmann, K.M. An mDia2/ROCK signaling axis regulates invasive egress from epithelial ovarian cancer spheroids. PLoS ONE 2014, 9, e90371. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.M.; Volpato, M.; Chi, H.Y.; Musiwaro, P.; Poterlowicz, K.; Peng, Y.; Scally, A.J.; Patterson, L.H.; Phillips, R.M.; Sutton, C.W. Characterization of changes in the proteome in different regions of 3D multicell tumor spheroids. J. Proteome Res. 2012, 11, 2863–2875. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Raghavan, S.; Ward, M.R.; Rowley, K.R.; Wold, R.M.; Takayama, S.; Buckanovich, R.J.; Mehta, G. Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays. Gynecol. Oncol. 2015, 138, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef] [Green Version]
- Cavnar, S.P.; Salomonsson, E.; Luker, K.E.; Luker, G.D.; Takayama, S. Transfer, imaging, and analysis plate for facile handling of 384 hanging drop 3D tissue spheroids. J. Lab. Autom. 2014, 19, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lal-Nag, M.; McGee, L.; Guha, R.; Lengyel, E.; Kenny, H.A.; Ferrer, M. A high-throughput screening model of the tumor microenvironment for ovarian cancer cell growth. SLAS Discov. 2017, 22, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.K.; Li, S.S.; Tang, M.Y.; Sy, S.K.; Ren, Y.; Shum, H.C.; Wong, A.S. Stemness and chemoresistance in epithelial ovarian carcinoma cells under shear stress. Sci. Rep. 2016, 6, 26788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.S.; Ip, C.K.; Tang, M.Y.; Sy, S.K.; Yung, S.; Chan, T.M.; Yang, M.; Shum, H.C.; Wong, A.S. Modeling ovarian cancer multicellular spheroid behavior in a dynamic 3D peritoneal microdevice. J. Vis. Exp. 2017, 10, 3791–55337. [Google Scholar] [CrossRef] [PubMed]
- Hyler, A.R.; Baudoin, N.C.; Brown, M.S.; Stremler, M.A.; Cimini, D.; Davalos, R.V.; Schmelz, E.M. Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability. PLoS ONE 2018, 13, e0194170. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.E.; van den End, E.J.; Streefland, M. Configuration of bioreactors. In Animal Cell Biotechnology; Springer: New York, NY, USA, 2014; Volume 4, pp. 285–311. [Google Scholar]
- Massai, D.; Isu, G.; Madeddu, D.; Cerino, G.; Falco, A.; Frati, C.; Gallo, D.; Deriu, M.A.; Falvo D’Urso Labate, G.; Quaini, F.; et al. A Versatile bioreactor for dynamic suspension cell culture. application to the culture of cancer cell spheroids. PLoS ONE 2016, 11, e0154610. [Google Scholar] [CrossRef]
- Kraiss, L.W.; Weyrich, A.S.; Alto, N.M.; Dixon, D.A.; Ennis, T.M.; Modur, V.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A. Fluid flow activates a regulator of translation, p70/p85 S6 kinase, in human endothelial cells. Am. J. Physiol. Heart Circulatory Physiol. 2000, 278, H1537–H1544. [Google Scholar] [CrossRef]
- Ziko, L.; Riad, S.; Amer, M.; Zdero, R.; Bougherara, H.; Amleh, A. Mechanical stress promotes cisplatin-induced hepatocellular carcinoma cell death. Biomed Res. Int. 2015, 2015, 430569. [Google Scholar] [CrossRef]
- dela Paz, N.G.; Walshe, T.E.; Leach, L.L.; Saint-Geniez, M.; D’Amore, P.A. Role of shear-stress-induced VEGF expression in endothelial cell survival. J. Cell Sci. 2012, 125, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Park, J.I.; Lee, J.; Kwon, J.L.; Park, H.B.; Lee, S.Y.; Kim, J.Y.; Sung, J.; Kim, J.M.; Song, K.S.; Kim, K.H. Scaffold-Free coculture spheroids of human colonic adenocarcinoma cells and normal colonic fibroblasts promote tumorigenicity in nude mice. Transl. Oncol. 2016, 9, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Gru, R.; Hohn, H.-P.; Mareel, M.; Denker, H.-W. Adhesion and invasion of three human choriocarcinoma cell lines into human endometrium in a three-dimensional organ culture system. Placenta 1994, 15, 411–429. [Google Scholar]
- Jeffrey, B.; Udaykumar, H.S.; Schulze, K.S. Flow fields generated by peristaltic reflex in isolated guinea pig ileum: Impact of contraction depth and shoulders. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G907–G918. [Google Scholar] [CrossRef] [PubMed]
- Avraham-Chakim, L.; Elad, D.; Zaretsky, U.; Kloog, Y.; Jaffa, A.; Grisaru, D. Fluid-flow induced wall shear stress and epithelial ovarian cancer peritoneal spreading. PLoS ONE 2013, 8, e60965. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Gurkan, U.A.; Tasoglu, S.; Alagic, N.; Celli, J.P.; Mensah, L.B.; Mai, Z.; Demirci, U.; Hasan, T. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc. Natl. Acad. Sci. USA 2013, 110, E1974–E1983. [Google Scholar] [CrossRef] [PubMed]
- Dier, U.; Shin, D.H.; Hemachandra, L.P.; Uusitalo, L.M.; Hempel, N. Bioenergetic analysis of ovarian cancer cell lines: Profiling of histological subtypes and identification of a mitochondria-defective cell line. PLoS ONE 2014, 9, e98479. [Google Scholar] [CrossRef] [PubMed]
- Kropp, C.; Massai, D.; Zweigerdt, R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process Biochem. 2017, 59, 244–254. [Google Scholar] [CrossRef]
- Novak, C.; Horst, E.; Mehta, G. Review: Mechanotransduction in ovarian cancer: Shearing into the unknown. APL Bioeng. 2018, 2, 031701. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Ruan, Y.; Jiang, H.; Xu, C. MicroRNA-424 inhibits cell migration, invasion, and epithelial mesenchymal transition by downregulating doublecortin-like kinase 1 in ovarian clear cell carcinoma. Int. J. Biochem. Cell Biol. 2017, 85, 66–74. [Google Scholar] [CrossRef]
- Puiffe, M.-L.; Le Page, C.; Filali-Mouhim, A.; Zietarska, M.; Ouellet, V.; Tonin, P.N.; Chevrette, M.; Provencher, D.M.; Mes-Masson, A.-M. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007, 9, 820-IN828. [Google Scholar] [CrossRef]
- Kenny, H.A.; Dogan, S.; Zillhardt, M.; K Mitra, A.; Yamada, S.D.; Krausz, T.; Lengyel, E. Organotypic models of metastasis: A three-dimensional culture mimicking the human peritoneum and omentum for the study of the early steps of ovarian cancer metastasis. Cancer Treat. Res. 2009, 149, 335–351. [Google Scholar] [CrossRef] [PubMed]










| Cell Line | Plate Type | Well Diameter (cm) | Rotation Speed (rpm) | * Spheroid Quantity | Morphology | Size Consistency | ** Size Relevance |
|---|---|---|---|---|---|---|---|
| ES-2 | 6 | 3.5 | 0 | High | Irregular | Poor | Mixed |
| 6 | 3.5 | 100 | High | Irregular | Good | Yes | |
| 6 | 3.5 | 120 | High | Round | Good | Yes | |
| 6 | 3.5 | 140 | High | Round | Good | Yes | |
| 12 | 2.5 | 0 | High | Irregular | Poor | Mixed | |
| 12 | 2.5 | 100 | Low | Irregular | Good | No | |
| 12 | 2.5 | 120 | High | Round | Good | Yes | |
| 12 | 2.5 | 140 | High | Round | Good | Yes | |
| 24 | 1.8 | 0 | High | Irregular | Poor | Mixed | |
| 24 | 1.8 | 100 | Low | Irregular | Good | No | |
| 24 | 1.8 | 120 | Low | Round | Good | No | |
| 24 | 1.8 | 140 | Low | Round | Good | No | |
| OVCA420 | 6 | 3.5 | 0 | High | Round | Poor | Yes |
| 6 | 3.5 | 120 | Low | Round | Poor | Yes | |
| 6 | 3.5 | 140 | Low | Round | Good | Yes | |
| 12 | 2.5 | 0 | High | Round | Poor | Yes | |
| 12 | 2.5 | 120 | Low | Round | Good | No | |
| 12 | 2.5 | 140 | High | Round | Good | Yes | |
| 24 | 1.8 | 0 | High | Round | Poor | Yes | |
| 24 | 1.8 | 120 | Low | Round | Good | No | |
| 24 | 1.8 | 140 | Low | Round | Good | No |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masiello, T.; Dhall, A.; Hemachandra, L.P.M.; Tokranova, N.; Melendez, J.A.; Castracane, J. A Dynamic Culture Method to Produce Ovarian Cancer Spheroids under Physiologically-Relevant Shear Stress. Cells 2018, 7, 277. https://doi.org/10.3390/cells7120277
Masiello T, Dhall A, Hemachandra LPM, Tokranova N, Melendez JA, Castracane J. A Dynamic Culture Method to Produce Ovarian Cancer Spheroids under Physiologically-Relevant Shear Stress. Cells. 2018; 7(12):277. https://doi.org/10.3390/cells7120277
Chicago/Turabian StyleMasiello, Timothy, Atul Dhall, L. P. Madhubhani Hemachandra, Natalya Tokranova, J. Andres Melendez, and James Castracane. 2018. "A Dynamic Culture Method to Produce Ovarian Cancer Spheroids under Physiologically-Relevant Shear Stress" Cells 7, no. 12: 277. https://doi.org/10.3390/cells7120277
APA StyleMasiello, T., Dhall, A., Hemachandra, L. P. M., Tokranova, N., Melendez, J. A., & Castracane, J. (2018). A Dynamic Culture Method to Produce Ovarian Cancer Spheroids under Physiologically-Relevant Shear Stress. Cells, 7(12), 277. https://doi.org/10.3390/cells7120277






