Can We Design a Nogo Receptor-Dependent Cellular Therapy to Target MS?
Abstract
1. Introduction
2. Clinical Features of MS
3. Pathological Features of MS
3.1. Inflammation Driven by Peripheral Immune Infiltrates
3.2. Demyelinating Plaques
3.3. Mechanisms of Axonal Pathology
4. Nogo-A/NgR-Dependent Mechanisms Governing Neuroinflammation and MS Pathology
4.1. Myelin Associated Inhibitory Factors (MAIFs)
4.2. Nogo Receptor 1 (NgR1)-Dependent Neurobiological Events
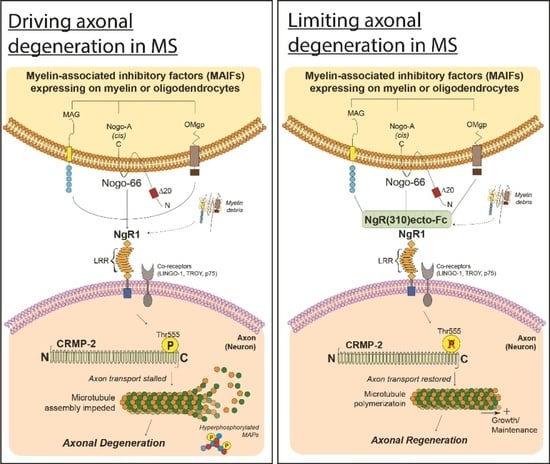
4.3. Axonal Transport Deficits
5. Current Therapy Options for Progressive MS
5.1. Immunomodulatory Drugs
5.2. Autologous Hematopoietic Stem Cell Transplantation
6. Potential Therapeutic Interventions for Remyelination
6.1. Utilizing Biological Peptide—NgR(310)ecto-Fc to Drive Neural Repair in the CNS
6.2. Hematopoietic Stem Cell Delivery of the NgR(310)ecto-Fc Therapeutic Fusion Protein
7. Conclusions
Funding
Conflicts of Interest
References
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Lublin, D.M. Blood group antigens: So many jobs to do. Transfusion 1996, 36, 293–295. [Google Scholar] [CrossRef]
- Klineova, S.; Lublin, F.D. Clinical course of multiple sclerosis. Cold Spring Harbor Perspect. Med. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Okuda, D.T.; Mowry, E.M.; Beheshtian, A.; Waubant, E.; Baranzini, S.E.; Goodin, D.S.; Hauser, S.L.; Pelletier, D. Incidental MRI anomalies suggestive of multiple sclerosis: The radiologically isolated syndrome. Neurology 2009, 72, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Taghian, K.; Petratos, S. Axonal degeneration in multiple sclerosis: Can we predict and prevent permanent disability? Acta Neuropathol. Commun. 2014, 2, 97. [Google Scholar] [CrossRef] [PubMed]
- Hagemeier, K.; Bruck, W.; Kuhlmann, T. Multiple sclerosis-remyelination failure as a cause of disease progression. Histol. Histopathol. 2012, 27, 277–287. [Google Scholar] [CrossRef]
- Bjartmar, C.; Kidd, G.; Mork, S.; Rudick, R.; Trapp, B.D. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann. Neurol. 2000, 48, 893–901. [Google Scholar] [CrossRef]
- Lovas, G.; Szilagyi, N.; Majtenyi, K.; Palkovits, M.; Komoly, S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain 2000, 123, 308–317. [Google Scholar] [CrossRef]
- Medana, I.M.; Esiri, M.M. Axonal damage: A key predictor of outcome in human CNS diseases. Brain 2003, 126, 515–530. [Google Scholar] [CrossRef]
- Friese, M.A.; Jakobsen, K.B.; Friis, L.; Etzensperger, R.; Craner, M.J.; McMahon, R.M.; Jensen, L.T.; Huygelen, V.; Jones, E.Y.; Bell, J.I.; et al. Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis. Nat. Med. 2008, 14, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Babbe, H.; Roers, A.; Waisman, A.; Lassmann, H.; Goebels, N.; Hohlfeld, R.; Friese, M.; Schroder, R.; Deckert, M.; Schmidt, S.; et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 2000, 192, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Skulina, C.; Schmidt, S.; Dornmair, K.; Babbe, H.; Roers, A.; Rajewsky, K.; Wekerle, H.; Hohlfeld, R.; Goebels, N. Multiple sclerosis: Brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc. Natl. Acad. Sci. USA 2004, 101, 2428–2433. [Google Scholar] [CrossRef] [PubMed]
- Saikali, P.; Antel, J.P.; Newcombe, J.; Chen, Z.; Freedman, M.; Blain, M.; Cayrol, R.; Prat, A.; Hall, J.A.; Arbour, N. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J. Neurosci. 2007, 27, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Rawji, K.S.; Kappen, J.; Tang, W.; Teo, W.; Plemel, J.R.; Stys, P.K.; Yong, V.W. Deficient surveillance and phagocytic activity of myeloid cells within demyelinated lesions in aging mice visualized by ex vivo live multiphoton imaging. J. Neurosci. 2018, 38, 1973–1988. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Vereyken, E.J.; Glim, J.E.; Heijnen, P.D.; Moeton, M.; van der Valk, P.; Amor, S.; Teunissen, C.E.; van Horssen, J.; Dijkstra, C.D. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 2013, 10, 35. [Google Scholar] [CrossRef]
- Kim, C.C.; Nakamura, M.C.; Hsieh, C.L. Brain trauma elicits non-canonical macrophage activation states. J. Neuroinflamm. 2016, 13, 117. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Raine, C.S. Multiple sclerosis: The resolving lesion revealed. J. Neuroimmunol. 2017, 304, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Ludwin, S.; Prat, A.; Antel, J.; Bruck, W.; Lassmann, H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017, 133, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Stigliano, E.; Aloisi, F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004, 14, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007, 130, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.; Abdul-Majid, K.B.; Bauer, J.; Lassmann, H.; Harris, R.A.; Holmdahl, R. A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. Eur. J. Immunol. 2002, 32, 1939–1946. [Google Scholar] [CrossRef]
- Qin, Y.; Duquette, P.; Zhang, Y.; Talbot, P.; Poole, R.; Antel, J. Clonal expansion and somatic hypermutation of V(H) genes of B cells from cerebrospinal fluid in multiple sclerosis. J. Clin. Investig. 1998, 102, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Cepok, S.; Rosche, B.; Grummel, V.; Vogel, F.; Zhou, D.; Sayn, J.; Sommer, N.; Hartung, H.P.; Hemmer, B. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain 2005, 128, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Duquette, P.; Zhang, Y.; Olek, M.; Da, R.R.; Richardson, J.; Antel, J.P.; Talbot, P.; Cashman, N.R.; Tourtellotte, W.W.; et al. Intrathecal B-cell clonal expansion, an early sign of humoral immunity, in the cerebrospinal fluid of patients with clinically isolated syndrome suggestive of multiple sclerosis. Lab. Investig. 2003, 83, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Kannel, K.; Alnek, K.; Vahter, L.; Gross-Paju, K.; Uibo, R.; Kisand, K.V. Changes in blood b cell-activating factor (baff) levels in multiple sclerosis: A sign of treatment outcome. PLoS ONE 2015, 10, e0143393. [Google Scholar] [CrossRef]
- Krumbholz, M.; Theil, D.; Derfuss, T.; Rosenwald, A.; Schrader, F.; Monoranu, C.M.; Kalled, S.L.; Hess, D.M.; Serafini, B.; Aloisi, F.; et al. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J. Exp. Med. 2005, 201, 195–200. [Google Scholar] [CrossRef]
- Popescu, B.F.; Lucchinetti, C.F. Pathology of demyelinating diseases. Annu. Rev. Pathol. 2012, 7, 185–217. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Rocca, M.A.; Barkhof, F.; Bruck, W.; Chen, J.T.; Comi, G.; DeLuca, G.; De Stefano, N.; Erickson, B.J.; Evangelou, N.; et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012, 11, 349–360. [Google Scholar] [CrossRef]
- Lucchinetti, C.; Bruck, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Bramow, S.; Frischer, J.M.; Lassmann, H.; Koch-Henriksen, N.; Lucchinetti, C.F.; Sorensen, P.S.; Laursen, H. Demyelination versus remyelination in progressive multiple sclerosis. Brain 2010, 133, 2983–2998. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.H.; Chambers, C.; Franklin, R.J. Remyelination: The true regeneration of the central nervous system. J. Comp. Pathol. 2013, 149, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Petratos, S. Multiple sclerosis: Does Nogo play a role? Neuroscientist 2013, 19, 394–408. [Google Scholar] [CrossRef]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- Bjartmar, C.; Kinkel, R.P.; Kidd, G.; Rudick, R.A.; Trapp, B.D. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology 2001, 57, 1248–1252. [Google Scholar] [CrossRef]
- Nikic, I.; Merkler, D.; Sorbara, C.; Brinkoetter, M.; Kreutzfeldt, M.; Bareyre, F.M.; Bruck, W.; Bishop, D.; Misgeld, T.; Kerschensteiner, M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 2011, 17, 495–499. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Tang, X.; Lan, M.; Zhang, M.; Yao, Z. Effect of nitric oxide to axonal degeneration in multiple sclerosis via downregulating monocarboxylate transporter 1 in oligodendrocytes. Nitric Oxide 2017, 67, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Nijland, P.G.; Michailidou, I.; Witte, M.E.; Mizee, M.R.; van der Pol, S.M.; van Het Hof, B.; Reijerkerk, A.; Pellerin, L.; van der Valk, P.; de Vries, H.E.; et al. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 2014, 62, 1125–1141. [Google Scholar] [CrossRef] [PubMed]
- Libby, R.T.; Li, Y.; Savinova, O.V.; Barter, J.; Smith, R.S.; Nickells, R.W.; John, S.W. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005, 1, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Soto, I.; Libby, R.T.; John, S.W. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp. Neurol. 2013, 246, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Terayama, R.; Bando, Y.; Murakami, K.; Kato, K.; Kishibe, M.; Yoshida, S. Neuropsin promotes oligodendrocyte death, demyelination and axonal degeneration after spinal cord injury. Neuroscience 2007, 148, 175–187. [Google Scholar] [CrossRef]
- Filbin, M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003, 4, 703–713. [Google Scholar] [CrossRef]
- Petratos, S.; Ozturk, E.; Azari, M.F.; Kenny, R.; Lee, J.Y.; Magee, K.A.; Harvey, A.R.; McDonald, C.; Taghian, K.; Moussa, L.; et al. Limiting multiple sclerosis related axonopathy by blocking Nogo receptor and CRMP-2 phosphorylation. Brain 2012, 135, 1794–1818. [Google Scholar] [CrossRef]
- Rinner, W.A.; Bauer, J.; Schmidts, M.; Lassmann, H.; Hickey, W.F. Resident microglia and hematogenous macrophages as phagocytes in adoptively transferred experimental autoimmune encephalomyelitis: An investigation using rat radiation bone marrow chimeras. Glia 1995, 14, 257–266. [Google Scholar] [CrossRef]
- Baer, A.S.; Syed, Y.A.; Kang, S.U.; Mitteregger, D.; Vig, R.; Ffrench-Constant, C.; Franklin, R.J.; Altmann, F.; Lubec, G.; Kotter, M.R. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 2009, 132, 465–481. [Google Scholar] [CrossRef]
- Robinson, S.; Miller, R.H. Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Dev. Biol. 1999, 216, 359–368. [Google Scholar] [CrossRef]
- Niederost, B.; Oertle, T.; Fritsche, J.; McKinney, R.A.; Bandtlow, C.E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002, 22, 10368–10376. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.E.; GrandPre, T.; Strittmatter, S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001, 409, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Lee, X.; Shao, Z.; Thill, G.; Ji, B.; Relton, J.; Levesque, M.; Allaire, N.; Perrin, S.; Sands, B.; et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Kim, J.A.; Sivasankaran, R.; Segal, R.; He, Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 2002, 420, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Prinjha, R.; Moore, S.E.; Vinson, M.; Blake, S.; Morrow, R.; Christie, G.; Michalovich, D.; Simmons, D.L.; Walsh, F.S. Inhibitor of neurite outgrowth in humans. Nature 2000, 403, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.E.; Gould, G.C.; Liu, B.P.; Strittmatter, S.M. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J. Neurosci. 2002, 22, 8876–8883. [Google Scholar] [CrossRef] [PubMed]
- GrandPre, T.; Nakamura, F.; Vartanian, T.; Strittmatter, S.M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 2000, 403, 439–444. [Google Scholar] [CrossRef]
- Park, J.B.; Yiu, G.; Kaneko, S.; Wang, J.; Chang, J.; He, X.L.; Garcia, K.C.; He, Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron 2005, 45, 345–351. [Google Scholar] [CrossRef]
- Ineichen, B.V.; Kapitza, S.; Bleul, C.; Good, N.; Plattner, P.S.; Seyedsadr, M.S.; Kaiser, J.; Schneider, M.P.; Zorner, B.; Martin, R.; et al. Nogo-A antibodies enhance axonal repair and remyelination in neuro-inflammatory and demyelinating pathology. Acta Neuropathol. 2017, 134, 423–440. [Google Scholar] [CrossRef]
- Domeniconi, M.; Cao, Z.; Spencer, T.; Sivasankaran, R.; Wang, K.; Nikulina, E.; Kimura, N.; Cai, H.; Deng, K.; Gao, Y.; et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron 2002, 35, 283–290. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; He, J.; Zhu, Z.; Rusckowski, M.; Hnatowich, D.J. Improving the labeling of S-acetyl NHS-MAG(3)-conjugated morpholino oligomers. Bioconjugate Chem. 2002, 13, 893–897. [Google Scholar] [CrossRef]
- Lauren, J.; Hu, F.; Chin, J.; Liao, J.; Airaksinen, M.S.; Strittmatter, S.M. Characterization of myelin ligand complexes with neuronal Nogo-66 receptor family members. J. Biol. Chem. 2007, 282, 5715–5725. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Raiker, S.J.; Venkatesh, K.; Geary, R.; Robak, L.A.; Zhang, Y.; Yeh, H.H.; Shrager, P.; Giger, R.J. Synaptic function for the Nogo-66 receptor NgR1: Regulation of dendritic spine morphology and activity-dependent synaptic strength. J. Neurosci. 2008, 28, 2753–2765. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, M.J.; Li, L.; Velumian, A.A.; Aui, P.M.; Fehlings, M.G.; Petratos, S. Nogo receptor 1 regulates Caspr distribution at axo-glial units in the central nervous system. Sci. Rep. 2017, 7, 8958. [Google Scholar] [CrossRef] [PubMed]
- Theotokis, P.; Touloumi, O.; Lagoudaki, R.; Nousiopoulou, E.; Kesidou, E.; Siafis, S.; Tselios, T.; Lourbopoulos, A.; Karacostas, D.; Grigoriadis, N.; et al. Nogo receptor complex expression dynamics in the inflammatory foci of central nervous system experimental autoimmune demyelination. J. Neuroinflamm. 2016, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Alrehaili, A.A.; Lee, J.Y.; Bakhuraysah, M.M.; Kim, M.J.; Aui, P.M.; Magee, K.A.; Petratos, S. Nogo receptor expression in microglia/macrophages during experimental autoimmune encephalomyelitis progression. Neural. Regen. Res. 2018, 13, 896–907. [Google Scholar] [CrossRef]
- Iobbi, C.; Korte, M.; Zagrebelsky, M. Nogo-66 Restricts Synaptic Strengthening via Lingo1 and the ROCK2-Cofilin Pathway to Control Actin Dynamics. Cereb. Cortex 2017, 27, 2779–2792. [Google Scholar] [CrossRef]
- Jitsuki, S.; Nakajima, W.; Takemoto, K.; Sano, A.; Tada, H.; Takahashi-Jitsuki, A.; Takahashi, T. Nogo receptor signaling restricts adult neural plasticity by limiting synaptic AMPA receptor delivery. Cereb. Cortex 2016, 26, 427–439. [Google Scholar] [CrossRef]
- Pernet, V.; Schwab, M.E. The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res. 2012, 349, 97–104. [Google Scholar] [CrossRef]
- Teng, F.Y.; Tang, B.L. Nogo signaling and non-physical injury-induced nervous system pathology. J. Neurosci. Res. 2005, 79, 273–278. [Google Scholar] [CrossRef]
- Karnezis, T.; Mandemakers, W.; McQualter, J.L.; Zheng, B.; Ho, P.P.; Jordan, K.A.; Murray, B.M.; Barres, B.; Tessier-Lavigne, M.; Bernard, C.C. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat. Neurosci. 2004, 7, 736–744. [Google Scholar] [CrossRef]
- Hu, F.; Liu, B.P.; Budel, S.; Liao, J.; Chin, J.; Fournier, A.; Strittmatter, S.M. Nogo-A interacts with the Nogo-66 receptor through multiple sites to create an isoform-selective subnanomolar agonist. J. Neurosci. 2005, 25, 5298–5304. [Google Scholar] [CrossRef] [PubMed]
- Petratos, S.; Azari, M.F.; Ozturk, E.; Papadopoulos, R.; Bernard, C.C. Novel therapeutic targets for axonal degeneration in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2010, 69, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, J.; Arzeno, A.; Choi, J.Y.; Boccio, J.; Frieden, E.; Bhargava, A.; Maynard, G.; Tsai, J.C.; Strittmatter, S.M. Intravitreal delivery of human NgR-Fc decoy protein regenerates axons after optic nerve crush and protects ganglion cells in glaucoma models. Invest. Ophthalmol. Visual Sci. 2015, 56, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yigitkanli, K.; Kim, C.Y.; Sekine-Komo, T.; Wirak, D.; Frieden, E.; Bhargava, A.; Maynard, G.; Cafferty, W.B.; Strittmatter, S.M. Human NgR-Fc decoy protein via lumbar intrathecal bolus administration enhances recovery from rat spinal cord contusion. J. Neurotrauma 2014, 31, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Iketani, M.; Kurihara, Y.; Yamaguchi, M.; Yamashita, N.; Nakamura, F.; Arie, Y.; Kawasaki, T.; Hirata, T.; Abe, T.; et al. Cartilage acidic protein-1B (LOTUS), an endogenous Nogo receptor antagonist for axon tract formation. Science 2011, 333, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Takeuchi, H.; Kurihara, Y.; Doi, H.; Kunii, M.; Tanaka, K.; Nakamura, H.; Fukai, R.; Tomita-Katsumoto, A.; Tada, M.; et al. Cerebrospinal fluid level of Nogo receptor 1 antagonist lateral olfactory tract usher substance (LOTUS) correlates inversely with the extent of neuroinflammation. J. Neuroinflamm. 2018, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Kurihara, Y.; Saito, Y.; Fujita, Y.; Yamashita, T.; Takei, K. The soluble form of LOTUS inhibits Nogo receptor-mediated signaling by interfering with the interaction between Nogo receptor type 1 and p75 neurotrophin receptor. J. Neurosci. 2018, 38, 2589–2604. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, Y.; Arie, Y.; Iketani, M.; Ito, H.; Nishiyama, K.; Sato, Y.; Nakamura, F.; Mizuki, N.; Goshima, Y.; Takei, K. The carboxyl-terminal region of Crtac1B/LOTUS acts as a functional domain in endogenous antagonism to Nogo receptor-1. Biochem. Biophys. Res. Commun. 2012, 418, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanaka, F.; Takei, K. LOTUS, a possible endogenous inhibitor of axonal degeneration, as a new biomarker for multiple sclerosis. Neurodegener. Dis. Manag. 2015, 5, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Millecamps, S.; Julien, J.P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Chihara, K.; Arimura, N.; Menager, C.; Kawano, Y.; Matsuo, N.; Nishimura, T.; Amano, M.; Kaibuchi, K. CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci. 2001, 4, 781–782. [Google Scholar] [CrossRef]
- Mokhtar, S.H.; Kim, M.J.; Magee, K.A.; Aui, P.M.; Thomas, S.; Bakhuraysah, M.M.; Alrehaili, A.A.; Lee, J.Y.; Steer, D.L.; Kenny, R.; et al. Amyloid-beta-dependent phosphorylation of collapsin response mediator protein-2 dissociates kinesin in Alzheimer’s disease. Neural. Regen. Res. 2018, 13, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Aulchenko, Y.S.; Hoppenbrouwers, I.A.; Ramagopalan, S.V.; Broer, L.; Jafari, N.; Hillert, J.; Link, J.; Lundstrom, W.; Greiner, E.; Dessa Sadovnick, A.; et al. Genetic variation in the KIF1B locus influences susceptibility to multiple sclerosis. Nat. Genet. 2008, 40, 1402–1403. [Google Scholar] [CrossRef]
- Van den Berg, R.; Hoogenraad, C.C.; Hintzen, R.Q. Axonal transport deficits in multiple sclerosis: Spiraling into the abyss. Acta Neuropathol. 2017, 134, 1–14. [Google Scholar] [CrossRef]
- Hares, K.; Redondo, J.; Kemp, K.; Rice, C.; Scolding, N.; Wilkins, A. Axonal motor protein KIF5A and associated cargo deficits in multiple sclerosis lesional and normal-appearing white matter. Neuropathol. Appl. Neurobiol. 2017, 43, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, C.D.; Wagner, N.E.; Ladwig, A.; Nikic, I.; Merkler, D.; Kleele, T.; Marinkovic, P.; Naumann, R.; Godinho, L.; Bareyre, F.M.; et al. Pervasive axonal transport deficits in multiple sclerosis models. Neuron 2014, 84, 1183–1190. [Google Scholar] [CrossRef]
- Lin, T.H.; Kim, J.H.; Perez-Torres, C.; Chiang, C.W.; Trinkaus, K.; Cross, A.H.; Song, S.K. Axonal transport rate decreased at the onset of optic neuritis in EAE mice. Neuroimage 2014, 100, 244–253. [Google Scholar] [CrossRef]
- Lyons, D.A.; Naylor, S.G.; Scholze, A.; Talbot, W.S. Kif1b is essential for mRNA localization in oligodendrocytes and development of myelinated axons. Nat. Genet. 2009, 41, 854–858. [Google Scholar] [CrossRef]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef]
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D.R. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar]
- Hawker, K.; O’Connor, P.; Freedman, M.S.; Calabresi, P.A.; Antel, J.; Simon, J.; Hauser, S.; Waubant, E.; Vollmer, T.; Panitch, H.; et al. Rituximab in patients with primary progressive multiple sclerosis: Results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009, 66, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Antel, J.; Comi, G.; Montalban, X.; O’Connor, P.; Polman, C.H.; Haas, T.; Korn, A.A.; Karlsson, G.; Radue, E.W.; et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 355, 1124–1140. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.; Comi, G.; Montalban, X.; Antel, J.; Radue, E.W.; de Vera, A.; Pohlmann, H.; Kappos, L.; Group, F.D.S. Oral fingolimod (FTY720) in multiple sclerosis: Two-year results of a phase II extension study. Neurology 2009, 72, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.; Miller, D.H.; Freedman, M.S.; Cree, B.A.C.; Wolinsky, J.S.; Weiner, H.; Lubetzki, C.; Hartung, H.P.; Montalban, X.; Uitdehaag, B.M.J.; et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016, 387, 1075–1084. [Google Scholar] [CrossRef]
- Pan, S.; Gray, N.S.; Gao, W.; Mi, Y.; Fan, Y.; Wang, X.; Tuntland, T.; Che, J.; Lefebvre, S.; Chen, Y.; et al. Discovery of BAF312 (Siponimod), a Potent and Selective S1P Receptor Modulator. ACS Med. Chem. Lett. 2013, 4, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Bar-Or, A.; Cree, B.A.C.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef]
- Mancardi, G.; Saccardi, R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008, 7, 626–636. [Google Scholar] [CrossRef]
- Burt, R.K.; Cohen, B.A.; Russell, E.; Spero, K.; Joshi, A.; Oyama, Y.; Karpus, W.J.; Luo, K.; Jovanovic, B.; Traynor, A.; et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: Failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood 2003, 102, 2373–2378. [Google Scholar] [CrossRef]
- Farge, D.; Labopin, M.; Tyndall, A.; Fassas, A.; Mancardi, G.L.; Van Laar, J.; Ouyang, J.; Kozak, T.; Moore, J.; Kotter, I.; et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: An observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica 2010, 95, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.A.; Hutton, G.J.; Racke, M.K.; Popat, U.; Devine, S.M.; Griffith, L.M.; Muraro, P.A.; Openshaw, H.; Sayre, P.H.; Stuve, O.; et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): A 3-year interim report. JAMA Neurol. 2015, 72, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Atkins, H.L.; Bowman, M.; Allan, D.; Anstee, G.; Arnold, D.L.; Bar-Or, A.; Bence-Bruckler, I.; Birch, P.; Bredeson, C.; Chen, J.; et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: A multicentre single-group phase 2 trial. Lancet 2016, 388, 576–585. [Google Scholar] [CrossRef]
- Muraro, P.A.; Pasquini, M.; Atkins, H.L.; Bowen, J.D.; Farge, D.; Fassas, A.; Freedman, M.S.; Georges, G.E.; Gualandi, F.; Hamerschlak, N.; et al. Long-term Outcomes After Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis. JAMA Neurol. 2017, 74, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.A.; Liu, B.P.; Tzvetkova, D.; Jeffrey, P.D.; Fournier, A.E.; Sah, D.; Cate, R.; Strittmatter, S.M.; Nikolov, D.B. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 2003, 22, 3291–3302. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Bazan, J.F.; McDermott, G.; Park, J.B.; Wang, K.; Tessier-Lavigne, M.; He, Z.; Garcia, K.C. Structure of the Nogo receptor ectodomain: A recognition module implicated in myelin inhibition. Neuron 2003, 38, 177–185. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Bruck, W. Immunoglobulins induce increased myelin debris clearance by mouse macrophages. Neurosci. Lett. 1999, 275, 191–194. [Google Scholar] [CrossRef]
- Li, S.; Liu, B.P.; Budel, S.; Li, M.; Ji, B.; Walus, L.; Li, W.; Jirik, A.; Rabacchi, S.; Choi, E.; et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 2004, 24, 10511–10520. [Google Scholar] [CrossRef]
- Wang, X.; Baughman, K.W.; Basso, D.M.; Strittmatter, S.M. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann. Neurol. 2006, 60, 540–549. [Google Scholar] [CrossRef]
- Ji, B.; Li, M.; Budel, S.; Pepinsky, R.B.; Walus, L.; Engber, T.M.; Strittmatter, S.M.; Relton, J.K. Effect of combined treatment with methylprednisolone and soluble Nogo-66 receptor after rat spinal cord injury. Eur. J. Neurosci. 2005, 22, 587–594. [Google Scholar] [CrossRef]
- Wang, X.; Duffy, P.; McGee, A.W.; Hasan, O.; Gould, G.; Tu, N.; Harel, N.Y.; Huang, Y.; Carson, R.E.; Weinzimmer, D.; et al. Recovery from chronic spinal cord contusion after Nogo receptor intervention. Ann. Neurol. 2011, 70, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, J.E.; Sivula, M.; Strittmatter, S.M. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004, 24, 6209–6217. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.A.; Lee, D.H.; Qian, F.; Weinreb, P.H.; Frank, E. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J. Neurosci. 2009, 29, 6285–6295. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, W.B.; McGee, A.W.; Strittmatter, S.M. Axonal growth therapeutics: Regeneration or sprouting or plasticity? Trends Neurosci. 2008, 31, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; De Palma, M.; Quattrini, A.; Del Carro, U.; Amadio, S.; Visigalli, I.; Sessa, M.; Fasano, S.; Brambilla, R.; Marchesini, S.; et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J. Clin. Investig. 2004, 113, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Akbik, F.V.; Bhagat, S.M.; Patel, P.R.; Cafferty, W.B.; Strittmatter, S.M. Anatomical plasticity of adult brain is titrated by Nogo Receptor 1. Neuron 2013, 77, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Hu, B.; Hahm, K.; Luo, Y.; Kam Hui, E.S.; Yuan, Q.; Wong, W.M.; Wang, L.; Su, H.; Chu, T.H.; et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 2007, 13, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Kotter, M.R.; Franklin, R.J. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain 2009, 132, 288–295. [Google Scholar] [CrossRef]
- Reichert, F.; Rotshenker, S. Deficient activation of microglia during optic nerve degeneration. J. Neuroimmunol. 1996, 70, 153–161. [Google Scholar] [CrossRef]
- Vargas, M.E.; Watanabe, J.; Singh, S.J.; Robinson, W.H.; Barres, B.A. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc. Natl. Acad. Sci. USA 2010, 107, 11993–11998. [Google Scholar] [CrossRef]
- Zhang, L.; Johnson, D.; Johnson, J.A. Deletion of Nrf2 impairs functional recovery, reduces clearance of myelin debris and decreases axonal remyelination after peripheral nerve injury. Neurobiol. Dis. 2013, 54, 329–338. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Braun, P.E.; Jackson, D.L.; Kottis, V.; McKerracher, L. Laminin overrides the inhibitory effects of peripheral nervous system and central nervous system myelin-derived inhibitors of neurite growth. J. Neurosci. Res. 1995, 42, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Vallieres, N.; Berard, J.L.; David, S.; Lacroix, S. Systemic injections of lipopolysaccharide accelerates myelin phagocytosis during Wallerian degeneration in the injured mouse spinal cord. Glia 2006, 53, 103–113. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Kang, J.H.; Theotokis, P.; Grigoriadis, N.; Petratos, S. Can We Design a Nogo Receptor-Dependent Cellular Therapy to Target MS? Cells 2019, 8, 1. https://doi.org/10.3390/cells8010001
Kim MJ, Kang JH, Theotokis P, Grigoriadis N, Petratos S. Can We Design a Nogo Receptor-Dependent Cellular Therapy to Target MS? Cells. 2019; 8(1):1. https://doi.org/10.3390/cells8010001
Chicago/Turabian StyleKim, Min Joung, Jung Hee Kang, Paschalis Theotokis, Nikolaos Grigoriadis, and Steven Petratos. 2019. "Can We Design a Nogo Receptor-Dependent Cellular Therapy to Target MS?" Cells 8, no. 1: 1. https://doi.org/10.3390/cells8010001
APA StyleKim, M. J., Kang, J. H., Theotokis, P., Grigoriadis, N., & Petratos, S. (2019). Can We Design a Nogo Receptor-Dependent Cellular Therapy to Target MS? Cells, 8(1), 1. https://doi.org/10.3390/cells8010001