Relevance of Neurotrophin Receptors CD271 and TrkC for Prognosis, Migration, and Proliferation in Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Preparation and Immunohistochemistry
2.3. Immunofluorescence
2.4. Determination of HPV-Mediated Carcinomas
2.4.1. DNA Isolation
2.4.2. Polymerase Chain Reaction
2.4.3. p16 Immunohistochemistry
2.5. Cell Lines
2.6. Migration Assay and Cell Culture
2.7. Fluorescence-Activated Cell Sorting (FACS)
2.8. Statistical Analysis
3. Results
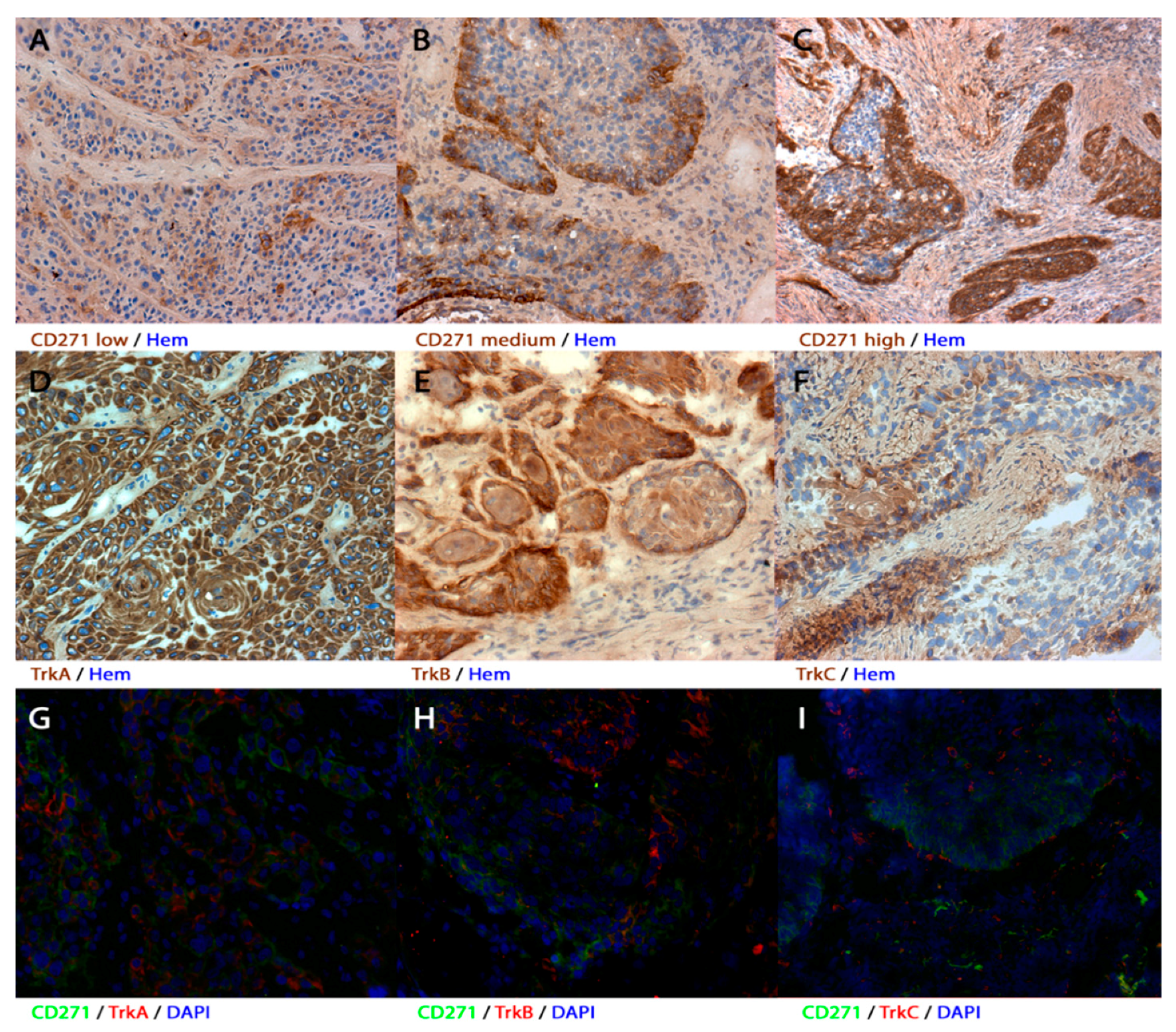
3.1. Expression of CD271, TrkA, TrkB, and TrkC in Human Primary HNSCC
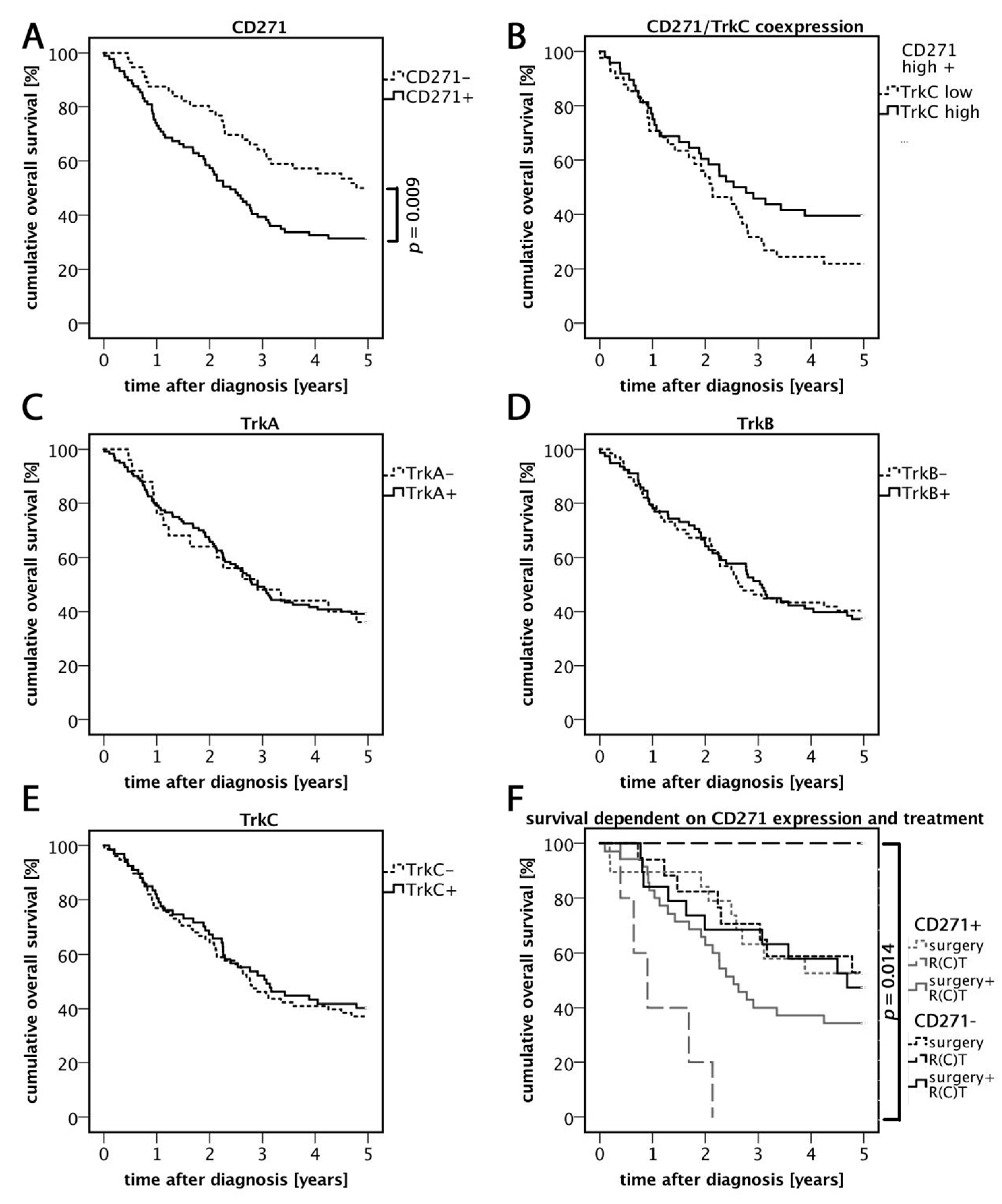
3.2. High Expression of CD271 Correlates with Reduced Overall Survival and an Increased Number of Distant Metastases
3.3. Relevance of CD271 Expression in Response to Treatment
3.4. TrkC/CD271 Expression is Associated with Less Distant Metastases
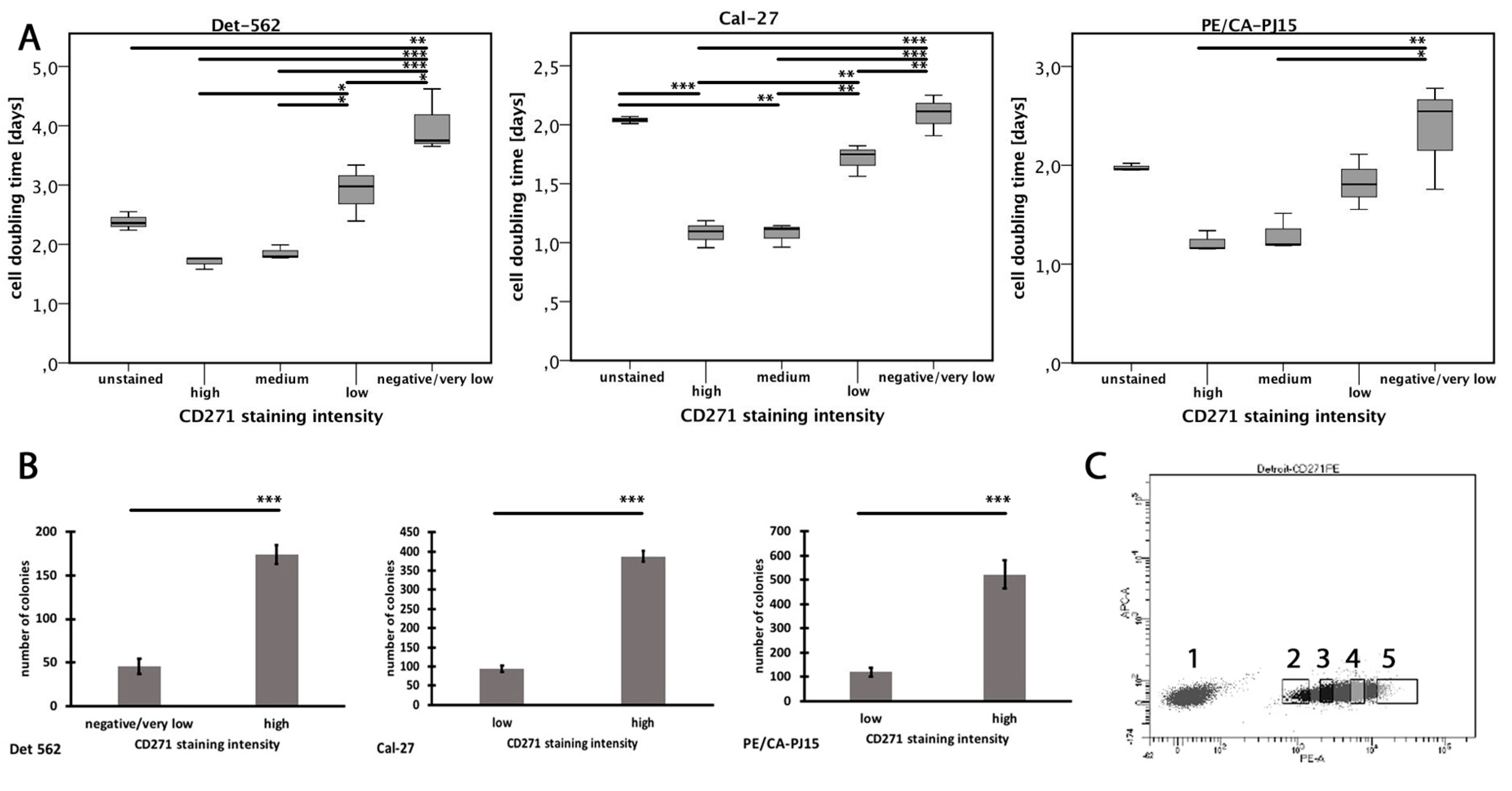
3.5. Expression of CD271 is Associated with High Cell Replication
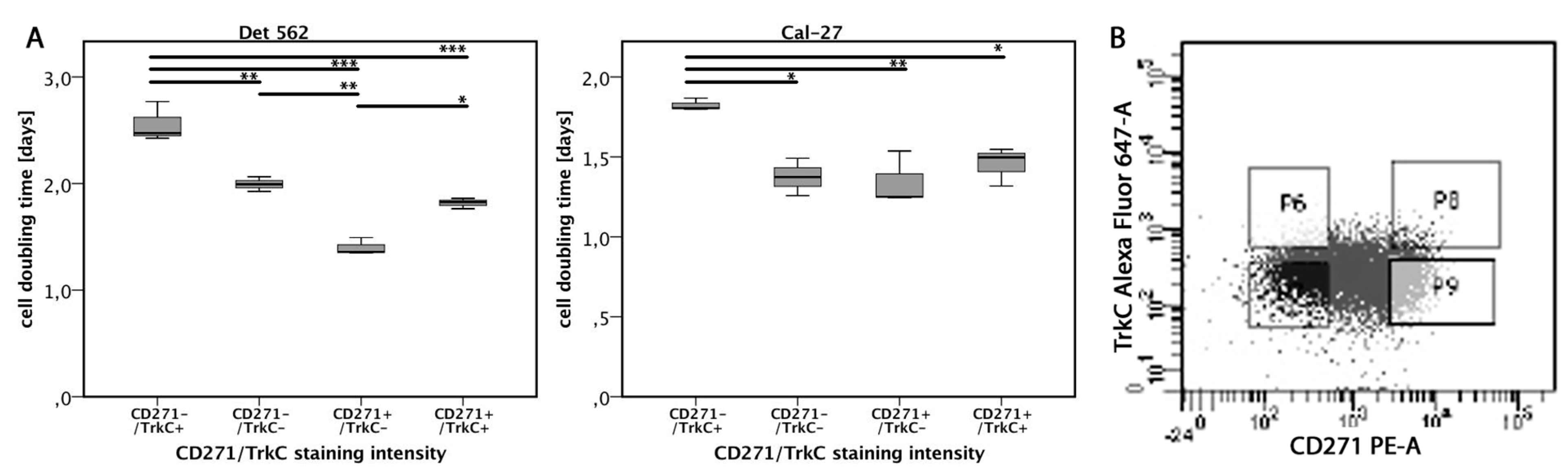
3.6. TrkC Co-Expression Reduces Proliferation in CD271high Cells
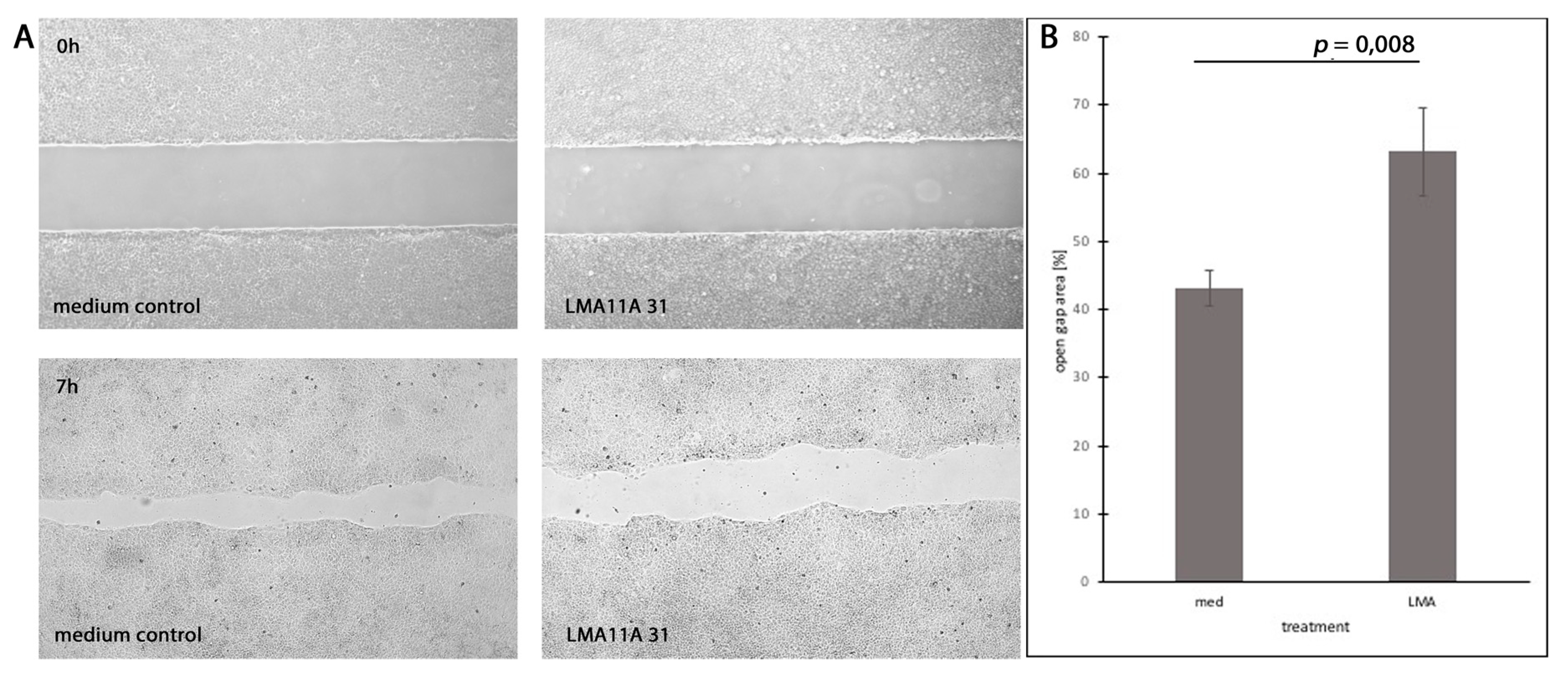
3.7. Dependence of Cell Motility and Mitotic Rates on Stimulation and Inhibition of Neurotrophic Receptors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. North Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.B.; Sessions, R.B.; Hong, W.K. Head and Neck Cancer: A Multidisciplinary Approach, 3rd ed.; Lipppincott Williams & Wilkins: Philadelphia, PA, USA, 2009; 960p. [Google Scholar]
- Di Donato, M.; Cernera, G.; Migliaccio, A.; Castoria, G. Nerve growth factor induces proliferation and aggressiveness in prostate cancer cells. Cancers 2019, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Sauca, O.; Chung, M.K.; Shin, J.H.; Karamboulas, C.; Kwok, S.; Jung, Y.H.; Oakley, R.; Tysome, J.R.; Farnebo, L.O.; Kaplan, M.J.; et al. CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget 2014, 5, 6854–6866. [Google Scholar] [CrossRef]
- Yuanlong, H.; Haifeng, J.; Xiaoyin, Z.; Jialin, S.; Jie, L.; Li, Y.; Huahong, X.; Jiugang, S.; Yanglin, P.; Kaichun, W.; et al. The inhibitory effect of p75 neurotrophin receptor on growth of human hepatocellular carcinoma cells. Cancer Lett. 2008, 268, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, C.; de Vos, A.M. Nerve growth factor: Structure and function. Cell Mol. Life Sci. 2001, 58, 748–759. [Google Scholar] [CrossRef]
- Bothwell, M. Functional interactions of neurotrophins and neurotrophin receptors. Annu. Rev. Neurosci. 1995, 18, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Chao, M.V. Neurotrophin receptor structure and interactions. Pharm. Acta Helv. 2000, 74, 253–260. [Google Scholar] [CrossRef]
- Miriam Bibel, Y.-A.B. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000, 14, 2919–2937. [Google Scholar] [CrossRef]
- Radke, J.; Rossner, F.; Redmer, T. CD271 determines migratory properties of melanoma cells. Sci. Rep. 2017, 7, 9834. [Google Scholar] [CrossRef]
- Mochizuki, M.; Tamai, K.; Imai, T.; Sugawara, S.; Ogama, N.; Nakamura, M.; Matsuura, K.; Yamaguchi, K.; Satoh, K.; Sato, I.; et al. CD271 regulates the proliferation and motility of hypopharyngeal cancer cells. Sci. Rep. 2016, 6, 30707. [Google Scholar] [CrossRef] [PubMed]
- Lehraiki, A.; Cerezo, M.; Rouaud, F.; Abbe, P.; Allegra, M.; Kluza, J.; Marchetti, P.; Imbert, V.; Cheli, Y.; Bertolotto, C.; et al. Increased CD271 expression by the NF-kB pathway promotes melanoma cell survival and drives acquired resistance to BRAF inhibitor vemurafenib. Cell Discov. 2015, 1, 15030. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shin, J.H.; Eggold, J.T.; Chung, M.K.; Zhang, L.H.; Lee, J.; Sunwoo, J.B. ESM1 mediates NGFR-induced invasion and metastasis in murine oral squamous cell carcinoma. Oncotarget 2016, 7, 70738–70749. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Jung, Y.H.; Lee, J.K.; Cho, S.Y.; Murillo-Sauca, O.; Uppaluri, R.; Shin, J.H.; Sunwoo, J.B. CD271 confers an invasive and metastatic phenotype of head and neck squamous cell carcinoma through the upregulation of slug. Clin. Cancer Res. 2018, 24, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yue, D.; Chen, X.; Wang, L.; Li, J.; Ping, Y.; Gao, Q.; Wang, D.; Zhang, T.; Li, F.; et al. Epigenetic regulation of CD271, a potential cancer stem cell marker associated with chemoresistance and metastatic capacity. Oncol. Rep. 2015, 33, 425–432. [Google Scholar] [CrossRef]
- Linggi, M.S.; Burke, T.L.; Williams, B.B.; Harrington, A.; Kraemer, R.; Hempstead, B.L.; Yoon, S.O.; Carter, B.D. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem. 2005, 280, 13801–13808. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Tamai, K.; Oizumi, S.; Oyama, K.; Yamaguchi, K.; Sato, I.; Satoh, K.; Matsuura, K.; Saijo, S.; Sugamura, K.; et al. CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS ONE 2013, 8, e62002. [Google Scholar] [CrossRef]
- Roh, J.; Muelleman, T.; Tawfik, O.; Thomas, S.M. Perineural growth in head and neck squamous cell carcinoma: A review. Oral Oncol. 2015, 51, 16–23. [Google Scholar] [CrossRef]
- Khotskaya, Y.B.; Holla, V.R.; Farago, A.F.; Mills Shaw, K.R.; Meric-Bernstam, F.; Hongm, D.S. Targeting TRK family proteins in cancer. Pharmacol. Ther. 2017, 173, 58–66. [Google Scholar] [CrossRef]
- Dechant, G.; Barde, Y.A. Signalling through the neurotrophin receptor p75NTR. Curr Opin Neurobiol. 1997, 7, 413–418. [Google Scholar] [CrossRef]
- Casaccia-Bonnefil, P.; Kong, H.; Chao, M.V. Neurotrophins: The biological paradox of survival factors eliciting apoptosis. Cell Death Differ. 1998, 5, 357–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Tsunoda, S.; Mori, Y.; Ito, T.; Kikuchi, K.; Wang, T.C.; Yasumoto, S.; Shimada, Y. The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin. Cancer Res. 2006, 12, 5096–5103. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Petig, A.L.; Stockfleth, E.; Stucker, M. Expression of SOX10, ABCB5 and CD271 in melanocytic lesions and correlation with survival data of patients with melanoma. Clin. Exp. Dermatol. 2016, 41, 709–716. [Google Scholar] [CrossRef]
- Sasahira, T.; Kirita, T.; Kuniyasu, H. Update of molecular pathobiology in oral cancer: A review. Int. J. Clin. Oncol. 2014, 19, 431–436. [Google Scholar] [CrossRef]
- Yilmaz, T.; Jiffar, T.; de la Garza, G.; Lin, H.; Milas, Z.; Takahashi, Y.; Hanna, E.; MacIntyre, T.; Brown, J.L.; Myers, J.N.; et al. Theraputic targeting of Trk supresses tumor proliferation and enhances cisplatin activity in HNSCC. Cancer Biol. Ther. 2010, 10, 644–653. [Google Scholar] [CrossRef]
- Zhu, L.; Werner, J.A.; Mandic, R. Implications of tropomyosin-related kinase B (TrkB) in head and neck cancer. Anticancer Res. 2007, 27, 3121–3126. [Google Scholar]
- Kupferman, M.E.; Jiffar, T.; El-Naggar, A.; Yilmaz, T.; Zhou, G.; Xie, T.; Feng, L.; Wang, J.; Holsinger, F.C.; Yu, D.; et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene 2010, 29, 2047–2059. [Google Scholar] [CrossRef]
- Dudas, J.; Dietl, W.; Romani, A.; Reinold, S.; Glueckert, R.; Schrott-Fischer, A.; Dejaco, D.; Johnson Chacko, L.; Tuertscher, R.; Schartinger, V.H.; et al. Nerve growth factor (NGF)-receptor survival axis in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 1771. [Google Scholar] [CrossRef]
- Wolfer, S.; Elstner, S.; Schultze-Mosgau, S. Degree of keratinization is an independent prognostic factor in oral squamous cell carcinoma. J. Oral Maxillofac. Surg. 2018, 76, 444–454. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, Z.C.; Zhang, T.X.; Liu, S.; Liu, X.; Liu, J.J.; Niu, Y. TrkC expression predicts favorable clinical outcome in invasive ductal carcinoma of breast independent of NT-3 expression. Am. J. Cancer Res. 2014, 4, 811–823. [Google Scholar] [PubMed]
- Luo, Y.; Kaz, A.M.; Kanngurn, S.; Welsch, P.; Morris, S.M.; Wang, J.; Lutterbaugh, J.D.; Markowitz, S.D.; Grady, W.M. NTRK3 is a potential tumor suppressor gene commonly inactivated by epigenetic mechanisms in colorectal cancer. PLoS Genet. 2013, 9, e1003552. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Kim, G.M.; Kim, M.S.; Lim, M.H.; Yun, C.; Jeong, J.; Nam, J.S.; Kim, S.J. TrkC plays an essential role in breast tumor growth and metastasis. Carcinogenesis 2010, 31, 1939–1947. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngo, M.; Han, A.; Lakatos, A.; Sahoo, D.; Hachey, S.J.; Weiskopf, K.; Beck, A.H.; Weissman, I.L.; Boiko, A.D. Antibody therapy targeting CD47 and CD271 effectively suppresses melanoma metastasis in patient-derived xenografts. Cell Rep. 2016, 16, 1701–1716. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.; Liu, G. Overexpression of RhoA promotes the proliferation and migration of cervical cancer cells. Biosci. Biotechnol. Biochem. 2014, 78, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.L.; Lun, X.; Rahn, J.J.; Liacini, A.; Wang, L.; Hamilton, M.G.; Parney, I.F.; Hempstead, B.L.; Robbins, S.M.; Forsyth, P.A.; et al. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 2007, 5, e212. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Tsujioka, M.; Honda, S.; Tanaka, M.; Shimizu, S. Autophagy suppresses cell migration by degrading GEF-H1, a RhoA GEF. Oncotarget 2016, 7, 34420–34429. [Google Scholar] [CrossRef] [PubMed]
| Patient Data | Cases | [%] |
|---|---|---|
| Gender | 184 | 100.0 |
| male | 161 | 87.5 |
| female | 23 | 12.5 |
| Age (years) | 184 | 100.0 |
| median ± standard error | 57.09 ± 9.027 | |
| youngest | 29 | |
| oldest | 79 | |
| 5 year OS (days) | 163 | 88.6 |
| median ± standard error | 1137 ± 647 | |
| 5 year DFS (days) | 56 | 30.4 |
| median ± standard error | 720 ± 623 | |
| Tumor Location | 184 | 100.0 |
| oropharynx | 54 | 29.3 |
| hypopharynx | 53 | 28.8 |
| larynx | 77 | 41.8 |
| T stage | 158 | 85.9 |
| T1 | 25 | 15.8 |
| T2 | 69 | 43.7 |
| T3 | 32 | 20.3 |
| T4 | 32 | 20.3 |
| N stage | 174 | 94.6 |
| N0 | 70 | 40.2 |
| N1 | 25 | 14.4 |
| N2 | 63 | 36.2 |
| N3 | 16 | 9.2 |
| M stage | 143 | 77.7 |
| M0 | 117 | 81.8 |
| M1 | 26 | 18.2 |
| UICC stage | 174 | 94.6 |
| UICC 0 | 1 | 0.6 |
| UICC 1 | 17 | 9.8 |
| UICC 2 | 33 | 19.0 |
| UICC 3 | 27 | 15.5 |
| UICC 4 | 96 | 55.2 |
| G stage | 172 | 93.5 |
| G1 | 12 | 7.0 |
| G2 | 125 | 72.7 |
| G3 | 35 | 20.3 |
| Alcohol and Tobacco Consumption | 106 | 57.6 |
| none | 14 | 13.2 |
| tobacco | 16 | 15.1 |
| alcohol | 8 | 7.5 |
| tobacco + alcohol | 68 | 64.2 |
| Therapy | 120 | 65.2 |
| surgery | 47 | 39.2 |
| R(C)T | 8 | 6.7 |
| surgery + R(C)T | 65 | 54.2 |
| HPV-Mediated | 19 | 10.3 |
| HPV 16 | 18 | 94.7 |
| HPV 18 | 1 | 5.3 |
| Keratinization | 184 | 100.0 |
| no | 107 | 41.8 |
| yes | 77 | 58.2 |
| Expression | Overall | Oropharynx | Hypopharynx | Larynx | HPV-mediated | M0 | M1 | 5 year OS (days) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | (%) | Cases | (%) | Cases | (%) | Cases | (%) | Cases | (%) | Cases | (%) | Cases | (%) | Median ± standard error | |
| CD271+ | 113 | 61 | 34 | 18 | 39 | 21 | 40 | 22 | 13 | 12 | 63 | 44 | 21I | 15 | 1024 ± 64II |
| CD271− | 71 | 39 | 20 | 11 | 14 | 8 | 37III | 20 | 6 | 8 | 54 | 38 | 5 | 3 | 1325 ± 77 |
| TrkA+ | 149 | 81 | 49 | 27 | 41 | 22 | 59 | 32 | 13 | 9 | 96 | 67 | 21 | 15 | 1137 ± 55 |
| TrkA− | 35 | 19 | 5 | 3 | 12 | 7 | 18 | 10 | 6 | 17 | 21 | 15 | 5 | 3 | 1136 ± 127 |
| TrkB+ | 102 | 55 | 27 | 15 | 32 | 17 | 43 | 23 | 11 | 11 | 67 | 47 | 11 | 8 | 1136 ± 68 |
| TrkB− | 82 | 45 | 27 | 15 | 21 | 11 | 34 | 18 | 8 | 10 | 50 | 35 | 15 | 10 | 1137 ± 77 |
| TrkC+ | 83 | 45 | 24 | 13 | 24 | 13 | 35 | 19 | 6 | 7 | 59 | 41 | 8 | 6 | 1174 ± 76 |
| TrkC− | 101 | 55 | 30 | 16 | 29 | 16 | 42 | 23 | 13 | 13 | 58 | 41 | 18 | 13 | 1106 ± 69 |
| CD271+/TrkA+ | 98 | 87 | 32 | 28 | 32 | 28 | 34 | 30 | 7 | 7 | 54 | 64 | 19 | 23 | 1014 ± 69 |
| CD271+/TrkA− | 15 | 13 | 2 | 2 | 7 | 6 | 6 | 3 | 6IV | 40 | 9 | 11 | 2 | 2 | 1103 ± 185 |
| CD271+/TrkB+ | 64 | 57 | 17 | 15 | 25 | 22 | 22 | 12 | 6 | 9 | 36 | 43 | 10 | 12 | 969 ± 88 |
| CD271+/TrkB− | 49 | 43 | 17 | 15 | 14 | 12 | 18 | 10 | 7 | 14 | 27 | 32 | 11 | 13 | 1091 ± 95 |
| CD271+/TrkC+ | 58 | 51 | 16 | 14 | 20 | 18 | 22 | 12 | 5 | 9 | 38 | 45 | 7V | 8 | 1101 ± 94VI |
| CD271+/TrkC– | 55 | 49 | 18 | 16 | 19 | 17 | 18 | 10 | 8 | 15 | 25 | 30 | 14 | 17 | 944 ± 88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foerster, Y.; Stöver, T.; Wagenblast, J.; Diensthuber, M.; Balster, S.; Gabrielpillai, J.; Petzold, H.; Geissler, C. Relevance of Neurotrophin Receptors CD271 and TrkC for Prognosis, Migration, and Proliferation in Head and Neck Squamous Cell Carcinoma. Cells 2019, 8, 1167. https://doi.org/10.3390/cells8101167
Foerster Y, Stöver T, Wagenblast J, Diensthuber M, Balster S, Gabrielpillai J, Petzold H, Geissler C. Relevance of Neurotrophin Receptors CD271 and TrkC for Prognosis, Migration, and Proliferation in Head and Neck Squamous Cell Carcinoma. Cells. 2019; 8(10):1167. https://doi.org/10.3390/cells8101167
Chicago/Turabian StyleFoerster, Yannick, Timo Stöver, Jens Wagenblast, Marc Diensthuber, Sven Balster, Jennis Gabrielpillai, Hannah Petzold, and Christin Geissler. 2019. "Relevance of Neurotrophin Receptors CD271 and TrkC for Prognosis, Migration, and Proliferation in Head and Neck Squamous Cell Carcinoma" Cells 8, no. 10: 1167. https://doi.org/10.3390/cells8101167
APA StyleFoerster, Y., Stöver, T., Wagenblast, J., Diensthuber, M., Balster, S., Gabrielpillai, J., Petzold, H., & Geissler, C. (2019). Relevance of Neurotrophin Receptors CD271 and TrkC for Prognosis, Migration, and Proliferation in Head and Neck Squamous Cell Carcinoma. Cells, 8(10), 1167. https://doi.org/10.3390/cells8101167





