Articular Cartilage Regeneration in Osteoarthritis
Abstract
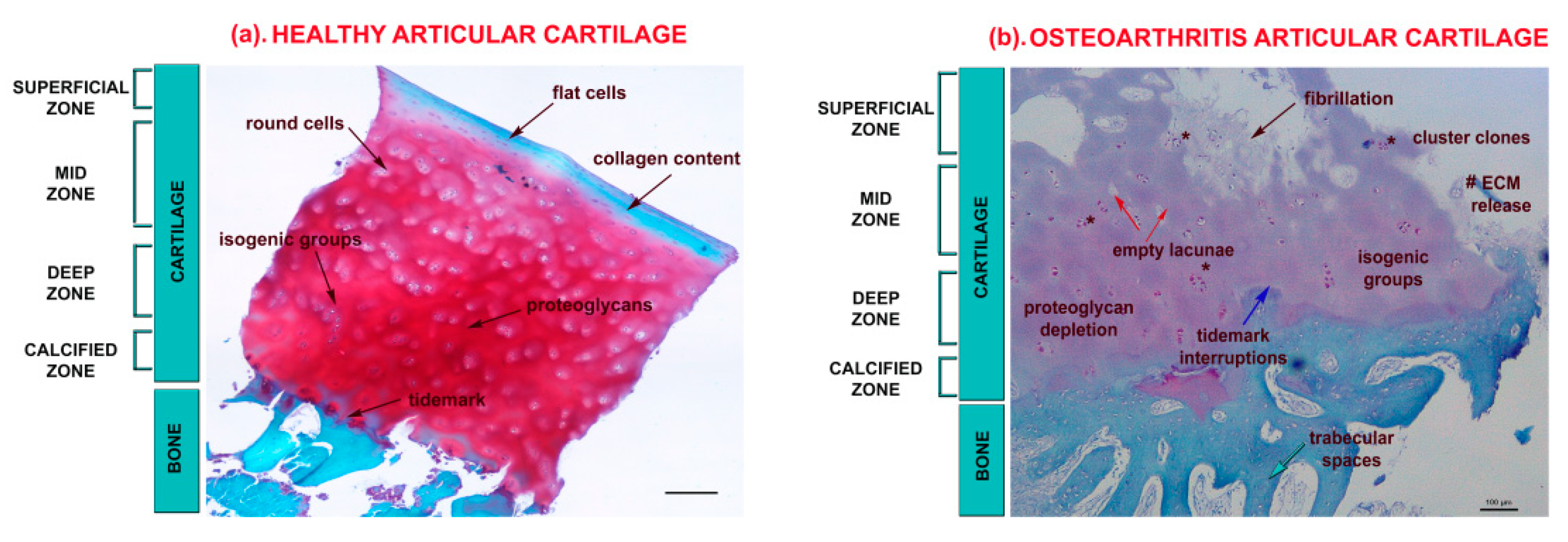
1. Articular Cartilage in Osteoarthritis
2. Osteoarthritis Treatments
3. Regenerative Medicine as a Novel Therapeutic Option
3.1. Autologous Chondrocyte Implantation
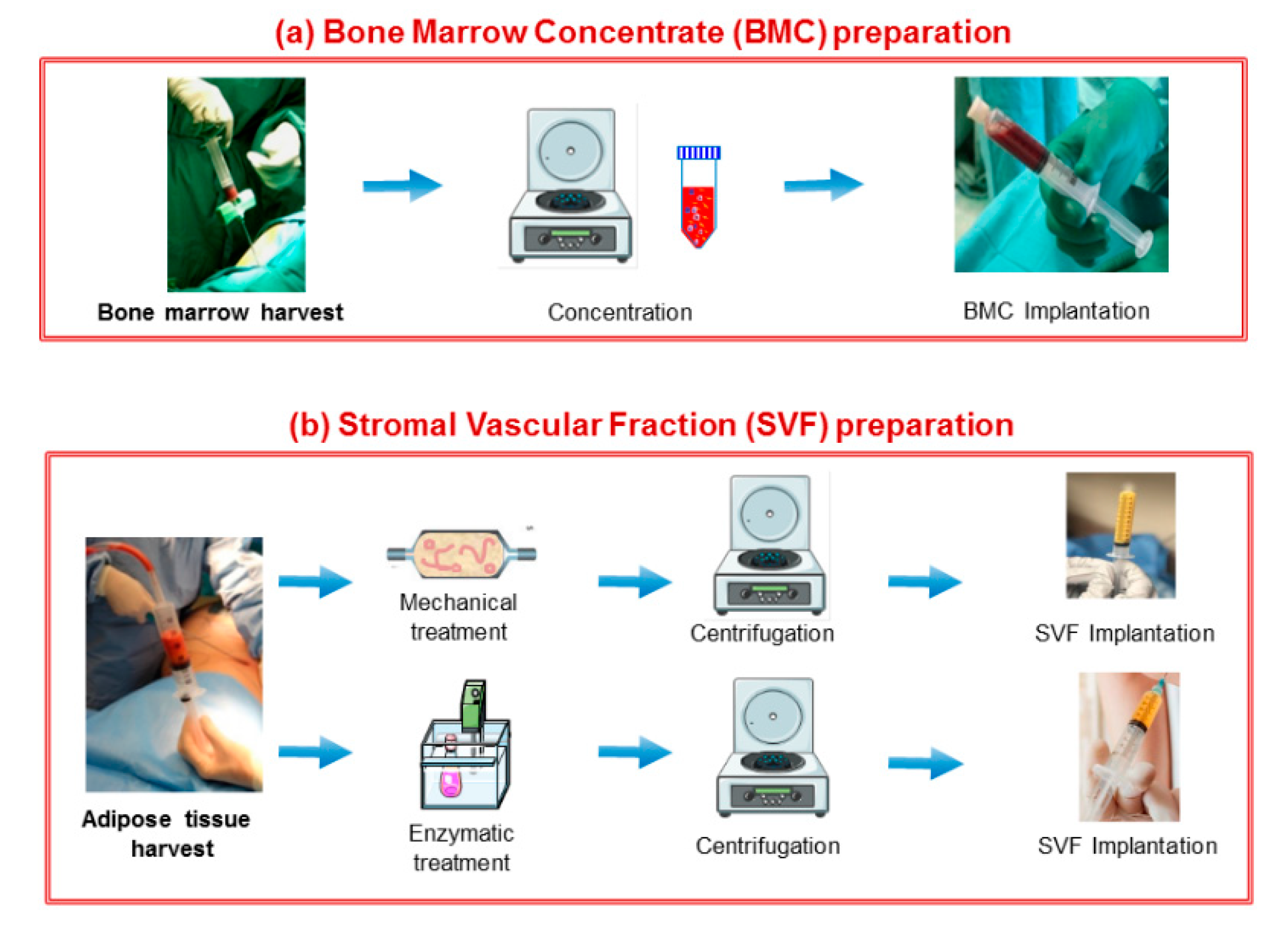
3.2. Stem/Stromal Cell-Based Therapy
3.3. Growth Factors
3.4. Gene Therapy
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kidd, B. Mechanisms of Pain in Osteoarthritis. Hss J. 2012, 8, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L. Advances in understanding cartilage remodeling. F1000Res 2015, 4, 642. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef]
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther 1998, 28, 203–215. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. 2017, 19, 1–12. [Google Scholar] [CrossRef]
- Saito, T.; Tanaka, S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-ΚB. Arthritis Res. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Ogando, J.; Tardáguila, M.; Díaz-Alderete, A.; Usategui, A.; Miranda-Ramos, V.; Martínez-Herrera, D.J.; de la Fuente, L.; García-León, M.J.; Moreno, M.C.; Escudero, S. Notch-regulated MIR-223 targets the aryl hydrocarbon receptor pathway and increases cytokine production in macrophages from rheumatoid arthritis patients. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M.; Van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ripmeester, E.G.J.; Timur, U.T.; Caron, M.M.J.; Welting, T.J.M. Recent insights into the contribution of the changing hypertrophic chondrocyte phenotype in the development and progression of osteoarthritis. Front. Bioeng. Biotechnol. 2018, 6, 18. [Google Scholar] [CrossRef]
- Sharma, A.R.; Jagga, S.; Lee, S.S.; Nam, J.S. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 19805–19830. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Altman, R.D.; Toupin April, K.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef]
- Fernandes, L.; Hagen, K.B.; Bijlsma, J.W.J.; Andreassen, O.; Christensen, P.; Conaghan, P.G.; Doherty, M.; Geenen, R.; Hammond, A.; Kjehen, I. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013, 72, 1125–1135. [Google Scholar] [CrossRef]
- Tiku, M.L.; Sabaawy, H.E. Cartilage regeneration for treatment of osteoarthritis: A paradigm for nonsurgical intervention. Ther. Adv. Musculoskelet. Dis. 2015, 7, 76–87. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Persson, M.S.M.; Sarmanova, A.; Doherty, M.Z.W. Comment on: Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: A met-analysis of randomized controlled trials: Reply. Rheumatology 2018, 57, 2060. [Google Scholar] [CrossRef]
- Ronn, K.; Reischl, N.; Gautier, E.J.M. Current surgical treatment of knee osteoarthritis. Dis. Model Mech. 2011, 2011, 454873. [Google Scholar] [CrossRef] [PubMed]
- De l’Escalopier, N.; Anract, P.; Biau, D. Surgical treatments for osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Goebel, L.K.H.; Orth, P.; Cucchiarini, M.; Madry, H. Subchondral drilling for articular cartilage repair: A systematic review of translational research. Dis. Model. Mech. 2018, 11, dmm034280. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Grigolo, B. Host environment: Scaffolds and signaling (tissue engineering) articular cartilage regeneration: Cells, scaffolds, and growth factors. Bio. Orthop. New Approach 2017, 87–103. [Google Scholar] [CrossRef]
- Burguera, E.F.; Gato Calvo, L.; Pereira, C.R.; Garcia, F.J.B.; Magalhaes, J.C.S. Regenerative Medicine Approaches for Osteoarthritis. In Osteoarthritis; SM Group: Oklahoma, OK, USA, 2016; pp. 1–15. [Google Scholar]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef]
- Francioli, S.; Cavallo, C.; Grigolo, B.; Martin, I.; Barbero, A. Engineered cartilage maturation regulates cytokine production and interleukin-1β response. Clin. Orthop. Relat. Res. 2011, 469, 2773–2784. [Google Scholar] [CrossRef]
- Kim, I. A brief overview of cell therapy and its product. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 201–202. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- Iwasa, J.; Engebretsen, L.; Shima, Y.M.; Ochi, M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 561–577. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Gene Delivery to Joints by Intra-Articular Injection. Hum. Gene Ther. 2018, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Carlos Rodriguez-Merchan, E.; Valentino, L.A. The role of gene therapy in cartilage repair. Arch. Bone Jt. Surg. 2019, 7, 79–90. [Google Scholar]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isakkson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Davies, R.L.; Kuiper, N.J. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef]
- Minas, T.; Gomoll, A.H.; Solhpour, S.; Rosenberger, R.; Probst, C.; Bryant, T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin. Orthop. Relat. Res. 2010, 468, 147–157. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous Chondrocyte Implantation. J. Bone Jt. Surg. Am. 2010, 92, 220–2233. [Google Scholar] [CrossRef]
- Ogura, T.; Mosier, B.A.; Bryant, T.; Minas, T. A 20-year follow-up after first-generation autologous chondrocyte implantation. Am. J. Sports Med. 2017, 45, 2751–2761. [Google Scholar] [CrossRef]
- EudraLex. Good Manufacturing Practice (GMP) Guidelines; PharmaLogika, 2009; Volume 4. [Google Scholar]
- Roseti, L.; Bassi, A.; Grigolo, B.; Fornasari, P.M. Development of human chondrocyte-based medicinal products for autologous cell therapy. Biomater. Sci. Eng. 2011, 349–368. [Google Scholar] [CrossRef]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol. Assess. 2017, 21, 1–294. [Google Scholar] [CrossRef]
- Ilic, D.; Polak, J.M. Stem cells in regenerative medicine: Introduction. Br. Med. Bull. 2011, 98, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Kenihan, M.A. Mesenchymal Stem Cell Therapy in Osteoarthritis and Regenerative Medicine. Curr. Sport. Med. Rep. 2018, 17, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Brown, M.; Scholes, C.; Hafsi, K.; Marenah, M.; Li, J.; Hassan, F.; Maffulli, N.; Murrell, W.D. Efficacy and safety of culture-expanded, mesenchymal stem/stromal cells for the treatment of knee osteoarthritis: A systematic review protocol. J. Orthop. Surg. Res. 2019, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.M.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. J. Orthop. Surg. Res. 2019, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh Soltani, S.; Forogh, B.; Ahmadbeigi, N.; Hadizadeh Kharazi, H.; Fallahzadeh, K.; Kashani, L.; Karami, M.; Kheyrollah, Y.; Vasei, M. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: A pilot study. Cytotherapy 2019, 21, 54–63. [Google Scholar] [CrossRef]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, W.; Liu, H.; Cui, Y.; Mao, Q.; Fei, Z.X.C. Curative effect of human umbilical cord mesenchymal stem cells by intra-articular injection for degenerative knee osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2016, 30, 1472–1477. [Google Scholar]
- Nam, Y.; Rim, Y.A.; Lee, J.; Ju, J.H. Current therapeutic strategies for stem cell-based cartilage regeneration. Stem Cells Int. 2018, 2018, 8490489. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Caplan, A.I. There Is No “Stem Cell Mess”. Tissue Eng. Part B Rev. 2019, 25, 291–293. [Google Scholar] [CrossRef]
- Block, T.J.; Garza, J.R. Regenerative Cells for the Management of Osteoarthritis and Joint Disorders: A Concise Literature Review. Aesthet. Surg. J. 2017, 37, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, H.; Xie, Y.; Sang, L.; Liu, J.; Chen, B. Effect of mesenchymal stromal cells for articular cartilage degeneration treatment: A meta-analysis. Cytotherapy 2015, 17, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M.; Karli, D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Phys. 2010, 11, 343–353. [Google Scholar]
- Davatchi, F.; Sadeghi Abdollahi, B.; Mohyeddin, M.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2016, 19, 219–225. [Google Scholar] [CrossRef]
- Soler, R.; Orozco, L.; Munar, A.; Huguet, M.; Lopez, R.; Vives, J.; Coll, R.; Codinach, M.; Garcia-Lopez, J. Final results of a phase I–II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 2016, 4, 647–654. [Google Scholar] [CrossRef]
- Al-Najar, M.; Khalil, H.; Al-Ajlouni, J.; Al-Antary, E.; Hamdan, M.; Rahmeh, R.; Alhattab, D.; Samara, O.; Yasin, M.; Al Abdullah, A. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: A phase I/II study. J. Orthop. Surg. Res. 2017, 12, 190. [Google Scholar] [CrossRef]
- Wong, K.L.; Lee, K.B.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: A prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Núñez-Córdoba, J.M.; López-Elío, S.; Andreu, E.; Sánchez-Guijo, F.; Aquerreta, J.D.; Bondía, J.M.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2018, 16, 213. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. 2016, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, B.; El Araby, M.M.; D’Angelo, A.; Iacono, F.; Nannini, A.; Vitale, N.D.; Marcacci, M.; Respizzi, S.; Kon, E. Adipose-Derived Stem Cell Treatments and Formulations. Clin. Sports Med. 2019, 38, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Kasir, R.; Vernekar, N.V.; Laurencin, C.T. Regenerative engineering of cartilage using adipose-derived stem cells. Regen. Eng. Transl. Med. 2015, 1, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue is a Source of Multipotent Stem Cells. Mol. Biol Cell 2002, 12, 4279–4295. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. 2019, 10, 143. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1651–1652. [Google Scholar] [CrossRef]
- Pers, Y.M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Shin, J.S.; Shim, H.; Yoon, K.S. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am. J. Sports Med. 2017, 45, 2774–2783. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.S. Usage of Human Mesenchymal Stem Cells in Cell-based Therapy: Advantages and Disadvantages. Dev. Reprod. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Kfoury, Y.; Scadden, D.T. Mesenchymal Cell Contributions to the Stem Cell Niche. Cell Stem Cell 2015, 16, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Mayoly, A.; Iniesta, A.; Curvale, C.; Kachouh, N.; Jaloux, C.; Eraud, J.; Vogtensperger, M.; Veran, J.; Grimaud, F.; Jouve, E. Development of autologous platelet-richmeh plasma mixed-microfat as an advanced therapy medicinal product for intra-articular injection of radio-carpal osteoarthritis: From validation data to preliminary clinical results. Int. J. Mol. Sci. 2019, 20, E1111. [Google Scholar] [CrossRef] [PubMed]
- Mehranfar, S.; Abdi Rad, I.; Mostafav, E.; Akbarzadeh, A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: An overview of clinical trials. Artif. Cells Nanomed. Biotechnol. 2019, 47, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Varma, H.S.; Dadarya, B.V.A. The new avenues in the management of osteoarthritis of knee stem cells. Indian Med. Assoc. 2010, 108, 583–585. [Google Scholar]
- Chahla, J.; Cinque, M.E.; Schon, J.M.; Liechti, D.J.; Matheny, L.M.; LaPrade, R.F.; Clanton, T.O. Bone marrow aspirate concentrate for the treatment of osteochondral lesions of the talus: A systematic review of outcomes. J. Exp. Orthop. 2016, 3, 33. [Google Scholar] [CrossRef]
- Peretti, G.M.; Ulivi, M.; De Girolamo, L.; Meroni, V.; Lombardo, M.D.; Mangiavini, L. Evaluation of the use of autologous micro-fragmented adipose tissue in the treatment of knee osteoarthritis: Preliminary results. J. Biol. Regul. Homeost. Agents 2018, 32, 193–199. [Google Scholar]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The Role of Growth Factors in Cartilage Repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef]
- Sasaki, T.; Akagi, R.; Akatsu, Y.; Fukawa, T.; Hoshi, H.; Yamamoto, Y.; Enomoto, T.; Sato, Y.; Nakagawa, R.; Takahashi, K.; et al. The effect of systemic administration of G-CSF on a full-thickness cartilage defect in a rabbit model MSC proliferation as presumed mechanism G-CSF for cartilage repair. BJR 2017, 6, 123–131. [Google Scholar] [CrossRef]
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 140–147. [Google Scholar] [CrossRef]
- Southworth, T.M.; Naveen, N.B.; Tauro, T.M.; Leong, N.L.; Cole, B.J. The Use of Platelet-Rich Plasma in Symptomatic Knee Osteoarthritis. J. Knee Surg. 2019, 32, 37–45. [Google Scholar] [CrossRef]
- Sánchez, M.; Delgado, D.; Sánchez, P.; Muiños-López, E.; Paiva, B.; Granero-Moltó, F.; Prósper, F.; Pompei, O.; Pérez, J.C. Combination of Intra-Articular and Intraosseous Injections of Platelet Rich Plasma for Severe Knee Osteoarthritis: A Pilot Study. Biomed. Res. Int. 2016, 2016, 4868613. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.; Mathias, M.; Andrade, R.; Bastos, R.; Balduino, A.; Schott, V.; Rodeo, S.; Espregueira-Mendes, J. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Choi, Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Guo, T.; Zhao, J.; Chang, Z.; Ding, Z.; Hong, H.; Chen, J.; Zhang, J. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-771 for chondrocytes proliferation. Biomaterials 2006, 27, 1095–1103. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, S.B.; Wu, F.L.; Mao, Z.B.; Yu, C.L. Transfection of primary chondrocytes using chitosan-pEGFP nanoparticles. J. Control Release 2006, 112, 223–228. [Google Scholar] [CrossRef]
- Kim, M.K.; Ha, C.W.; In, Y.; Cho, S.D.; Choi, E.S.; Ha, J.K.; Lee, J.H.; Yoo, J.D.; Bin, S.I.; Choi, C.H.; et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum. Gene. Clin. Dev. 2018, 27, 10. [Google Scholar]
- McKay, J.; Frantzen, K.; Vercruyssen, N.; Hafsi, K.; Opitz, T.; Davis, A.; Murrell, W. Rehabilitation following regenerative medicine treatment for knee osteoarthritis-current concept review. J. Clin. Orthop. Trauma 2019, 10, 59–66. [Google Scholar] [CrossRef]
- Van Pham, P. Clinical trials for stem cell transplantation: When are they needed? Stem Cell Res. Ther. 2016, 7, 65. [Google Scholar] [CrossRef]
- Buttgereit, F.; Burmester, G.R.; Bijlsma, J.W.J. Non-surgical management of knee osteoarthritis: Where are we now and where do we need to go? RMD Open 2015, 1, e000027. [Google Scholar] [CrossRef]
- Marc, J.F. Regenerative medicine in osteoarthritis a new chance for knee osteoarthritis patients. Int. J. Clin. Rheumtol. 2018, 13, 278–279. [Google Scholar] [CrossRef]
- Clouet, J.; Vinatier, C.; Merceron, C.; Pot-vaucel, M.; Maugars, Y.; Weiss, P.; Grimandi, G.; Guicheux, J. From osteoarthritis treatments to future regenerative therapies for cartilage. Drug Discov. Today 2009, 14, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Pham, C.T.N. Intra-articular drug delivery systems for joint diseases. Curr. Opin. Pharm. 2018, 40, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Wang, B. Cartilage repair by mesenchymal stem cells: Clinical trial update and perspectives. J. Orthop. Transl. 2017, 9, 76e88. [Google Scholar] [CrossRef]
- Schubert, C.; van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. PT 2014, 39, 704–711. [Google Scholar]
- Roseti, L.; Cavallo, C.; Desando, G.; Parisi, V.; Petretta, M.; Bartolotti, I.; Grigolo, B. Three-Dimensional Bioprinting of Cartilage by the Use of Stem Cells: A Strategy to Improve Regeneration. Materials 2018, 11, 1749. [Google Scholar] [CrossRef]
- Wuelling, M.; Vortkamp, A. A newly discovered stem cell that keeps bones growing. Nature 2019, 567, 178–179. [Google Scholar] [CrossRef]
- Mizuhashi, K.; Ono, W.; Matsushita, Y.; Sakagami, N.; Takahashi, A.; Saunders, T.L.; Nagasawa, T.; Kronenberg, H.M.; Ono, N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 2018, 563, 254–258. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Zhang, M.; Ionescu, A.; Ayturk, U.M.; Ono, N.; Kobayashi, A.; Kronenberg, H.; Warman, M.L.; Lassar, A.B. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015, 67, 1261–1273. [Google Scholar] [CrossRef]
- Lefebvre, V.; Bhattaram, P. Prg4-expressing cells: Articular stem cells or differentiated progeny in the articular chondrocyte lineage? Arthritis Rheumatol. 2015, 67, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Chagin, A.S.; Medvedeva, E.V. Cartilage stem cells identified, but can they heal? Nat. Rev. Rheumatol. 2017, 13, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Fellows, C.R.; Williams, R.; Davies, I.R.; Gohil, K.; Baird, D.M.; Fairclough, J.; Rooney, P.; Archer, C.W.; Khan, I.M. Characterisation of a divergent progenitor cell sub-populations in human osteoarthritic cartilage: The role of telomere erosion and replicative senescence. Sci. Rep. 2017, 7, 41421. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Baboolal, T.G.; Jones, E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 719–730. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.K.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017, 8, 15040. [Google Scholar] [CrossRef]
- Rhee, D.K.; Marcelino, J.; Baker, M.; Gong, Y.; Smits, P.; Lefebvre, V.; Jay, G.D.; Stewart, M.; Wang, H.; Warman, M.L.; et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Invest. 2005, 115, 622–631. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. https://doi.org/10.3390/cells8111305
Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B. Articular Cartilage Regeneration in Osteoarthritis. Cells. 2019; 8(11):1305. https://doi.org/10.3390/cells8111305
Chicago/Turabian StyleRoseti, Livia, Giovanna Desando, Carola Cavallo, Mauro Petretta, and Brunella Grigolo. 2019. "Articular Cartilage Regeneration in Osteoarthritis" Cells 8, no. 11: 1305. https://doi.org/10.3390/cells8111305
APA StyleRoseti, L., Desando, G., Cavallo, C., Petretta, M., & Grigolo, B. (2019). Articular Cartilage Regeneration in Osteoarthritis. Cells, 8(11), 1305. https://doi.org/10.3390/cells8111305







