1. Introduction
In the last few years, a remarkable shift in the mesenchymal stem cell (MSC) field has occurred [
1]. The focus of the researchers has switched from the differentiation and growth factor-secreting properties of MSC to the therapeutic potential of their extracellular vesicles (EV) [
2]. This term was adopted by the International Society for Extracellular Vesicles (ISEV) [
3,
4] to comprise the different class of membranous vesicles secreted by virtually all cell types into the extracellular environment. The most studied among them are exosomes and microvesicles. The former of endosomal origin, with a size roughly ranging from 40 to 150 nm [
5], the latter budding from the plasma membrane, with a broader size range of 100–1000 nm [
6]. In particular, EV from both adult [
7] and perinatal MSC [
8] have drawn attention for their immunomodulatory functions and regenerative capacity [
9,
10,
11,
12,
13]. Based on these features, MSC EV are currently under investigation in many pathological contexts. Similarly to cell therapies, translation to the clinical application of vesicular therapies entails the definition of clinical-grade identity parameters and the development of rapid and simple assays indicative of retention of biological activity after production and storage. These tests will be needed to guarantee the quality of EV-based therapeutics and to ensure consistent outcomes in different clinical centers [
14,
15]. Nevertheless, specific markers for EV of diverse mesenchymal origin are still lacking, as well as EV preparations quality assays, whereas more effort is dedicated to the exploration of functional properties and fields of therapeutic application.
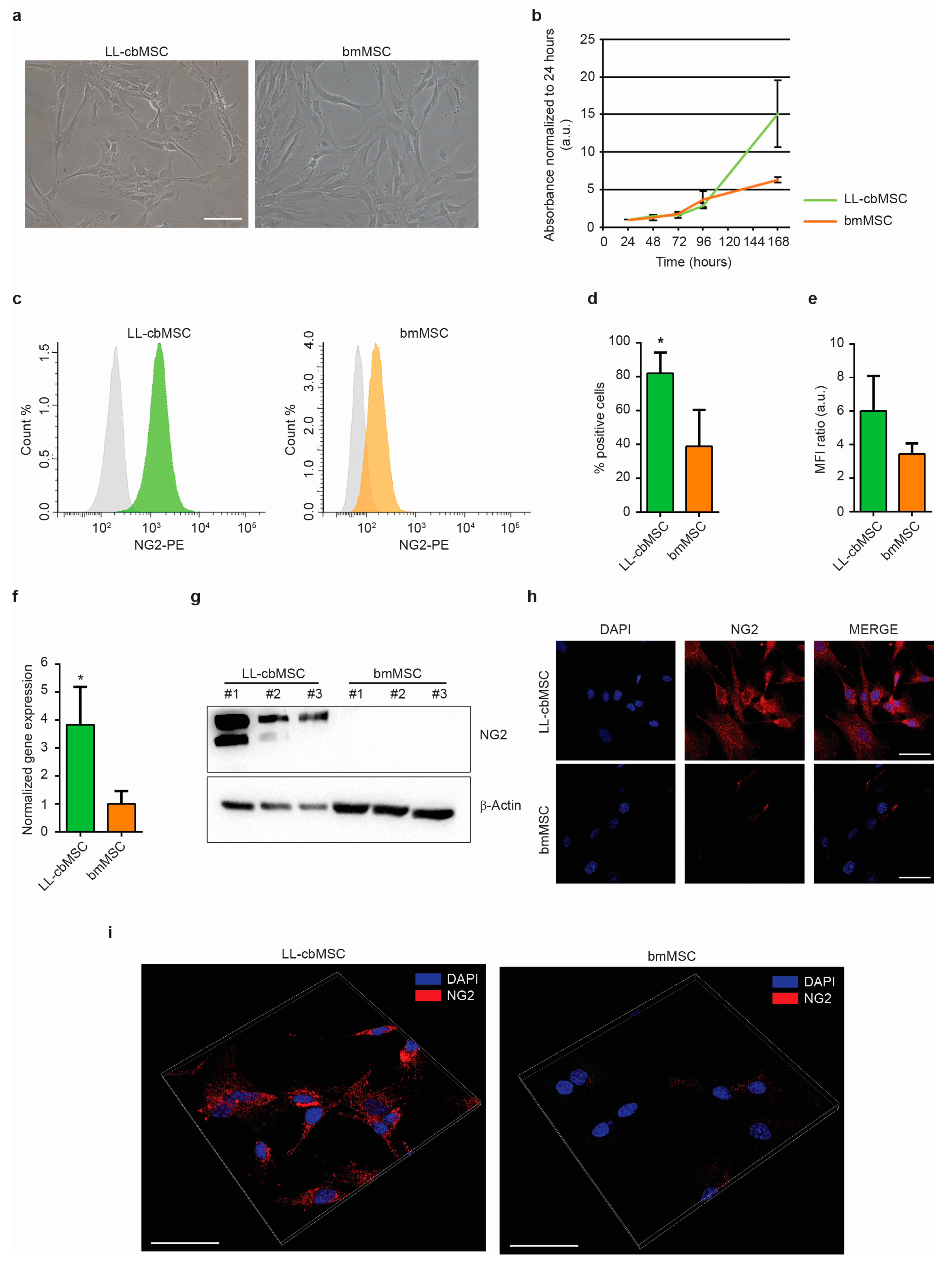
In a previous study, we addressed the expression of stemness-associated surface antigens in perinatal and adult MSC [
16] and evidenced neuron-glial antigen 2 (NG2) as a promising candidate marker of perinatal MSC versus adult MSC. This type 1 membrane protein is highly expressed by pericytes [
17], which were indicated as the in vivo progenitors of MSC [
18], and has a prominent role in the support of angiogenesis [
19,
20]. In detail, an NG2 knockout resulted in significant deficits in blood vessel formation during development, but also in tumor vascularization in adult organisms. In the present study, we want to investigate if EV released by perinatal and adult MSC preserve the parental cell differential expression of the NG2 antigen. Furthermore, we want to test whether NG2 can be used as an identity and quality marker for perinatal MSC. To this aim, long-living cord blood MSC (LL-cbMSC) and bone marrow MSC (bmMSC) were used, as representative of perinatal and adult MSC populations, respectively. NG2 expression was observed and validated in parental cells and generated EV. The abundance of the antigen was remarkably higher in LL-cbMSC than bmMSC. Intriguingly, the difference was maintained in the secreted EV. Furthermore, we challenged this EV-shuttled antigen in a potency assay experimental setting. LL-cbMSC but not bmMSC EV showed remarkable NG2-dependent pro-angiogenic properties, which demonstrated the functional relevance of the NG2 dose and the importance of the EV MSC source type.
2. Materials and Methods
2.1. Reagents
The following reagents were used in this study, listed in alphabetical order: αMEM-GlutaMAX (32571028; Thermo Fisher Scientific, Waltham, MA, USA); angiostatin (4919-100; BioVision, Milpitas, CA, USA); bFGF (RFGFB50; Thermo Fisher Scientific); CellTrace CFSE Cell Proliferation Kit (C34554; Thermo Fisher Scientific); DAPI (10236276001; Roche, Basel, Switzerland); DMEM without phenol red (31053028; Thermo Fisher Scientific); donkey anti-rabbit IgG HRP-linked secondary antibody (NA934-1ML; GE Healthcare, Chicago, IL, USA); endomed medium (F2245; Biochrome, Berlin, Germany); fetal bovine serum (FBS, 10270; Thermo Fisher Scientific); glutaraldehyde (G-5882; Sigma-Aldrich, Saint Louis, MI, USA); goat anti-mouse IgG HRP-linked secondary antibody (170-6516; Bio-Rad, Hercules, CA, USA); goat anti-rabbit IgG AF568-conjugated secondary antibody (A11011; Thermo Fisher Scientific); mouse anti-human β-actin antibody (A5441; Sigma-Aldrich); mouse anti-human CD63 antibody (CBL553; Millipore, Burlington, MA, USA); mouse anti-human CD81 antibody (555675; BD, Franklin Lakes, NJ, USA); mouse anti-human PE-conjugated NG2 antibody (IM3454U; Beckman Coulter, Brea, CA, USA); mouse IgG1 PE-conjugated isotype control (A07796; Beckman Coulter); nonfat milk (Blotting-Grade Blocker, 1706404; Bio-Rad); normal goat serum (566380; Sigma-Aldrich); paraformaldehyde (15710; Electron Microscopy Sciences, Hatfiled, PA, USA); PBS (ECB4004L; Euroclone, Pero, Italy); PDGF-AA (100-13A; Peprotech, Rocky Hill, NJ, USA); Restore PLUS Western blot stripping buffer (46430; Thermo Fisher Scientific); TRIzol (15596-026; Thermo Fisher Scientific); Triton X-100 (T8787; Sigma-Aldrich); TrypLE Select enzyme (12563011; Thermo Fisher Scientific); rabbit anti-human NG2 antibody (sc-20162; Santa Cruz Biotechnology, Dallas, TX, USA); RIPA (R0278; Sigma-Aldrich); SuperScript IV VILO (11756500; Thermo Fisher Scientific); SYBR Select master mix (4472937; Thermo Fisher Scientific); Thiazolyl Blue tetrazolium bromide (M2128; Sigma-Aldrich).
2.2. Cell Culture
LL-cbMSC (
n = 3) and bmMSC (
n = 3) used in this work were randomly selected from mesenchymal cell samples previously obtained and fully characterized for appropriate MSC identity following the International Society for Cellular Therapy guidelines [
21,
22], as described in detail in recent publications [
16,
23,
24,
25,
26]. LL-cbMSC were isolated from discarded cb units of healthy donors (at term 40 ± 2 weeks gestational age;
n = 2 male donors,
n = 1 female donor) not suitable for transplantation due to insufficient volume or white blood cell count, whereas bmMSC were isolated from healthy individuals undergoing bone fracture repair (51 ± 9 years old;
n = 2 male donors,
n = 1 female donor). Written informed consent was obtained from all donors involved in the research. No sensitive data of the donors were disclosed. The authors state that this study was performed according to the amended Declaration of Helsinki. Medium changes were performed twice a week with αMEM-GlutaMAX (Thermo Fisher Scientific) supplemented with 20% FBS (Thermo Fisher Scientific). Cell cultures were maintained at 37 °C, 5% CO
2 in a humidified atmosphere. At 80% confluence, the cells were harvested using TrypLE Select enzyme (Thermo Fisher Scientific) and seeded at 4 × 10
3 cells/cm
2 in T175 cm
2 flasks for expansion.
2.3. Microculture Tetrazolium Assay
Microculture tetrazolium (MTT) assay was performed as elsewhere described [
24] to study MSC proliferation. Briefly, 4000 cells/cm
2 were seeded into 96-well plates in quadruplicates and analysis was performed at 24, 48, 72, 96, 168 h time points. Thiazolyl Blue tetrazolium bromide (Sigma-Aldrich) was used at 0.5 mg/mL in DMEM without phenol red (Thermo Fisher Scientific) and the formazan crystals generated were dissolved in 96% ethanol. Optical density was measured at 570 nm after subtraction of 650 nm background on a GENios microplate reader (TECAN, Männedorf, Switzerland). Population doubling time was calculated as follows: PDT = [(t2 − t1) × log(2)]/[(log(A2) − log(A1)], where t1 and t2 are two time points of exponential growth, while A1 and A2 are the respective absorbance values normalized to 24 h time point.
2.4. Standard Flow Cytometry
Cell flow cytometry analysis was performed as elsewhere described [
16]. Briefly, at least 100,000 cells were stained with 10 µL PE-conjugated NG2 antibody (Beckman Coulter) or mouse IgG1 PE-conjugated isotype control (Beckman Coulter) in a total volume of 200 μL of PBS (Sigma-Aldrich) for 20 min in the dark at room temperature (RT). Next, cells were washed with PBS (Sigma-Aldrich), centrifuged at 350×
g for 7 min at RT, suspended in 300 μL of PBS (Sigma-Aldrich) and analyzed on a FACSCanto II cytometer (BD). At least 10,000 events were acquired and plotted against forward scatter (FSC)-height and FSC-area (FSC-A) to exclude cell doublets (P1 gate). P1 events were plotted against FSC-A and side scatter (SSC-A) to exclude debris and necrotic cells (P2 gate). Analytical data, including percentage of PE-positive events and mean fluorescence intensity (MFI) of P2-gated events, were analyzed in Excel. The MFI ratio was calculated as MFI (stained sample)/MFI (unstained sample).
2.5. qPCR
Total RNA was isolated from 100,000 LL-cbMSC (
n = 3) and bmMSC (
n = 3) using TRIzol (Thermo Fisher Scientific), and its quality and quantity were evaluated on a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). For the qPCR assay, cDNA was synthesized from 500 ng of total RNA with SuperScript IV VILO (Thermo Fisher Scientific). Next, qPCR was carried out using SYBR Select master mix (Thermo Fisher Scientific), according to the manufacturer’s protocols, on a CFX96 thermal cycler (Bio-Rad). The relative expression levels of the selected targets were determined using the ΔΔC
t method and normalized to GAPDH mRNA levels. The primers were designed using the NCBI primer-BLAST tool (
https://www.ncbi.nlm.nih.gov/). The primer sequences used in the qPCR analysis were as follows: forward 5′- AGGTGAAGGTCGGAGTCAAC-3′, reverse 5′-CCATGTAGTTGAGGTCAATGAAG-3′ for GAPDH; forward 5′-CCTTGCTGTGGCTGTGTCTT-3′, reverse 5′-GGACCCAGAGACCTTTGTTCTT-3′ for NG2.
2.6. Extracellular Vesicles
LL-cbMSC and bmMSC extracellular vesicles (EV) were isolated following the serial centrifugations protocol developed by Thery et al. [
27] for cell culture supernatant, with slight modifications. To harvest EV, cell cultures were rinsed three times with PBS (Sigma-Aldrich) to remove FBS EV and then incubated with FBS-free αMEM-GlutaMAX (Thermo Fisher Scientific) for 24 h. A total of 270 mL of cell culture supernatant was collected. To obtain pre-cleared EV-containing cell culture supernatants, residual cells and apoptotic bodies were removed by serial centrifugations. First, the samples were centrifuged at 350×
g for 10 min at RT, and the supernatant was collected. Second, the supernatant was centrifuged at 4700×
g for 15 min at RT, and the supernatant was collected again. Pre-cleared samples thus obtained were analyzed by nanoparticle tracking analysis (see below) or extracellular vesicle flow cytometry (see below), immediately or after storage at 4 °C for up to 2 weeks. For other applications needing concentrated EV, pre-cleared samples were further processed immediately or after storage at 4 °C for up to 2 weeks. Samples were diluted 1:1 with 0.1 µm-filtered PBS (Sigma-Aldrich) and centrifuged at 100,000×
g for 2 h at 4 °C on a Sorvall WX 80+ ultracentrifuge (75000080; Thermo Fisher Scientific), using a carbon fiber composite F37L-8x100 fixed rotor (096-087056; Thermo Fisher Scientific) and 70 mL polycarbonate tubes (010-1333; Thermo Fisher Scientific). To remove any contaminant protein aggregates generated by ultracentrifugation and obtain sufficiently pure EV preparations, EV pellets were suspended in 0.1 µm-filtered PBS (Sigma-Aldrich) and centrifuged again at 100,000×
g for 1 h at 4 °C, using 10.4 mL polycarbonate tubes (010-1382; Thermo Fisher Scientific) and suspended in 200 μL of 0.1 µm-filtered PBS (Sigma-Aldrich). Concentrated EV preparations thus obtained were used for transmission electron microscopy analysis (see below), Western analysis (see below), or for the in vitro model of endothelial cell growth support (see below), immediately or after storage at 4 °C for up to 1 week.
2.7. Western Analysis
Proteins were extracted from 1 × 10
6 pelleted MSC or from concentrated EV preparations in RIPA buffer (Sigma-Aldrich) without centrifugation. Protein concentration was quantified using Pierce BCA Protein Assay Kit (23227; Thermo Fisher Scientific). For tetraspanins evaluation, 5 µg of cellular and vesicular proteins were separated on Novex WedgeWell 4–12% Tris-Glycine Gels (XP04122BOX; Thermo Fisher Scientific), under non-reducing conditions. Blotting was performed via the iBlot 2 gel transfer device (Thermo Fisher Scientific) using iBlot polyvinylidene difluoride (PVDF) regular stacks (Thermo Fisher Scientific). To detect β-actin in tetraspanin blots, the membranes were stripped 10 min at RT with Restore PLUS Western blot stripping buffer (46430; Thermo Fisher Scientific). To detect NG2 protein, cellular (40 μg) and vesicular (6 μg) proteins were separated on a 6% polyacrylamide gel and blotted onto a PVDF membrane overnight at 4 °C in wet conditions. Immunoblotting conditions for all Western analyses were as follows. Membranes were blocked with 5% nonfat milk (Bio-Rad) and incubated with primary antibodies as follows: anti-NG2 (Santa Cruz Biotechnology) at 1:500 dilution for 2 h at RT (cellular samples) or overnight at 4 °C (vesicular samples); anti-β-actin (Sigma-Aldrich) at 1:5000 dilution overnight at 4 °C; anti-CD63 (Millipore) at 1:100 dilution overnight at 4 °C; anti-CD81 (BD) at 1:250 dilution overnight at 4 °C. After incubation with donkey anti-rabbit IgG HRP-linked secondary antibody (GE Healthcare) at 1:3000 dilution, or goat anti-mouse IgG HRP-linked secondary antibody (Bio-Rad) at 1:3000 dilution, proteins of interest were visualized with the Amersham ECL Prime Western blotting detection kit (RPN2232; GE Healthcare) or SuperSignal West Femto trial kit for vesicular NG2 detection (34095; Thermo Fisher Scientific). Chemiluminescence images were obtained on a Chemidoc XRS+ system (Bio-Rad). Uncropped images of all membranes are shown in
Supplementary Figure S2.
2.8. Immunofluorescence
Cells grown on coverslips were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) in PBS (Sigma-Aldrich) for 20 min, permeabilized in 0.3% Triton X-100 (Sigma-Aldrich) for 15 min, washed three times with PBS (Sigma-Aldrich) and blocked with PBS (Sigma-Aldrich) containing 5% normal goat serum (Sigma-Aldrich) and 0.3% Triton X-100 (blocking buffer) for 1 h at RT. Cells were incubated overnight at 4 °C with primary antibody NG2 (Santa Cruz Biotechnology) diluted 1:50 in blocking buffer, washed three times with PBS (Sigma-Aldrich) and then incubated with goat anti-rabbit IgG AF568-conjugated secondary antibody (Thermo Fisher Scientific) diluted 1:1000 in blocking buffer for 1 h at RT. Finally, the nuclei were stained with DAPI (Roche) for 10 min. Images were acquired using a Leica TCS SP8 X confocal microscope with HCX PL APO 63x/1.4 objective. Confocal z-stacks were acquired with sections of 0.3 µm.
2.9. Nanoparticle Tracking Analysis
The total number and size distribution of EV was estimated by nanoparticle tracking analysis (NTA), performed on an NS300 NanoSight instrument (Malvern Panalytical, Malvern, UK). PBS-diluted pre-cleared EV-containing cell culture supernatants were analyzed, acquiring 5 videos of 60 s in flow mode. Camera level and dilution were adjusted so that all measurements complied with the target quality parameters of 20–120 particles/frame and 3–6 threshold range. Particle concentration and size distribution were calculated by NanoSight NTA 3.3 software (Malvern Panalytical).
2.10. Extracellular Vesicle Flow Cytometry
EV flow cytometry analysis was performed as outlined in Ragni et al. [
10]. Briefly, 50 μL of pre-cleared EV-containing cell culture supernatant was incubated or not with 10 μM CellTrace CFSE cell proliferation kit (Thermo Fisher Scientific) for 30 min in the dark at RT. Staining was stopped by adding 300 μL of PBS (Sigma-Aldrich), and EV were analyzed immediately on a FACSCantoII cytometer (BD), setting FSC-A and SSC-A on a logarithmic scale and the FSC-A threshold in the 200–350 range. Events were acquired in low-speed mode and plotted against FSC-A and SSC-A to clearly distinguish electronic noise from EV events (P1 gate). P1 gate-selected events were then plotted against SSC-A and FL1 channel signals to evaluate CFSE positivity compared to an unstained sample.
2.11. Electron Microscopy
Electron microscopy analysis was performed as already described [
10]. Briefly, for scanning electron microscopy (SEM) the cells were fixed in PBS (Sigma-Aldrich) 2% glutaraldehyde (Sigma-Aldrich) and dehydrated with ethanol solutions. Next, samples were sputter-coated with an SCD040 Balzer Sputterer (Oerlikon Balzers, Balzers, Liechtenstein). Samples were analyzed with an SEM Philips 505 microscope (Philips, Amsterdam, Netherlands). Transmission electron microscopy was performed on concentrated EV preparations resuspended in PBS (Sigma-Aldrich) to analyze their ultrastructural morphology. According to proper dilutions, the samples were adsorbed to 300 mesh carbon-coated copper grids (Electron Microscopy Sciences) for 5 min in a humidified chamber at room temperature. EVs on grids were then fixed in 2% glutaraldehyde (Sigma-Aldrich) in PBS for 10 min and then briefly rinsed in milli-Q water. Grids with adhered EV were examined with a Philips CM 100 transmission electron microscope TEM at 80 kV, after negative staining with 2% phosphotungstic acid, brought to pH 7.0 with NaOH. Images were captured by a Kodak digital camera.
2.12. Bead-Based Extracellular Vesicle Flow Cytometry
EV immunophenotyping was performed on pre-cleared EV-containing cell culture supernatants with the Human MACSPlex exosome kit (130-108-813; Miltenyi Biotec, Bergisch Gladbach, Germany), following the manufacturer’s instructions. The kit consists of a cocktail of 39 fluorescently labeled bead populations, each coated with an antibody specific for one out of 37 surface epitopes or with the appropriate antibody out of two isotype controls. Briefly, 120 μL of sample was incubated overnight on a tube rotating mixer at RT in the dark with antibody-coated MACSPlex Exosome Capture Beads. The following bead-linked antibodies included in the kit were used: HLA-DRDPDQ REA, CD105 mouse IgG1, CD49e mouseIgG2b, CD9 mouse IgG1, HLA-ABC REA, CD63 mouse IgG1k, CD81 REA, NG2 (MCSP) mIgG1, CD146 mouse IgG1, CD44 mouse IgG1, CD29 mouse IgG1k, CD45 mouse IgG2a, CD31 mouse IgG1, CD14 mouse IgG2a, REA control, mouse IgG1 control. After a washing step with MACSPlex Buffer, the samples were incubated for 1 h on a tube rotating mixer at RT in the dark with a MACSPlex exosome detection reagent cocktail, comprising APC-conjugated antibodies against CD9, CD63, and CD81. The formation of complexes between antigen-specific capture beads, EV, and the detection reagent was evaluated on a FACSCanto II cytometer (BD). Single beads were visualized plotting acquired events against FSC-A and SSC-A (P1 gate). P1 gate-selected beads were then plotted against FITC-channel and PE-channel signals to detect 39 bead populations. Next, each bead population was analyzed for the APC signal to determine antigen expression compared to appropriate isotype control.
2.13. In Vitro Model of Endothelial Cell Growth Support
Human umbilical vein endothelial cells (HUVEC) (C2517A; Lonza, Basilea, Switzerland) were seeded at 10,000 cells/cm2 in endomed medium (F2245; Biochrome, Berlin, Germany) directly in the 96-wells of a RTCA E-plate (05232368001; ACEA Biosciences, San Diego, CA, USA) in the presence or not of concentrated LL-cbMSC or bmMSC EV preparations, 5 μg/mL angiostatin (BioVision), 40 ng/mL bFGF (Thermo Fisher Scientific), and 20 ng/mL PDGF-AA (Peprotech). The E-plate was loaded onto an RTCA SP xCELLigence system (ACEA Biosciences). The xCELLigence system estimates cell proliferation measuring cellular impedance thanks to microelectrodes fused at the bottom of the wells. The automatic output of the system is named the cell index parameter. The cultures were monitored in real-time during 48 h performing cell index calculation every hour. Cell index values were used to plot results. Where specified, concentrated MSC EV preparations were incubated with NG2 antibody (Beckman Coulter) for 1 h at RT, before administration to HUVEC. Untreated HUVEC were used as the internal control.
4. Discussion
Clinical translation of innovative advanced therapy medicinal products (ATMP) entails a great effort dedicated to dealing with cellular derivatives as a proper pharmaceutical. Standardized and scalable processes are needed, along with appropriate and straightforward assays to define ATMP identity and quality. The exact classification of extracellular vesicles (EV) within or outside the ATMP framework is still under debate [
14]. Nonetheless, it is highly reasonable that vesicular therapies will need similar identity and quality assays as those requested for cellular therapies [
15]. With regard to terminology, other groups working in the clinical translation of vesicular therapies have proposed “small EV” as a more appropriate term to refer to EV released by MSC, by reason of their size profile compared to other classes of EV lacking the same therapeutic properties [
31].
In this frame, we investigated the potential application of neural/glial antigen 2 (NG2) as a specific marker for long-living cord blood mesenchymal stem cells (LL-cbMSC) EV, which showed an average size compatible with the “small EV” classification introduced above. Interestingly, NG2 antigen abundance on EV membranes mirrored that of parental cell plasma membranes. In detail, LL-cbMSC and secreted EV showed much higher positivity for NG2 than bmMSC and bmMSC EV. NG2 was reported to modulate cell growth via intracellular pathways [
32], in addition to more studied extracellular effects on angiogenesis, through the promotion of endothelial cell motility and proliferation [
20,
29,
30,
33]. Based on these properties and previous studies [
34], we set up a simple and straightforward assay to address whether MSC EV-shuttled NG2 could elicit similar effects on endothelial cells in vitro. In correlation with the higher expression of NG2, LL-cbMSC EV, but not bmMSC EV, showed positive effects on endothelial cell growth. Possible mechanisms of action of soluble NG2 were proposed by others [
29,
30]. In detail, NG2 was shown to directly interact with kringle domains contained in plasminogen and its bioactive fragments plasmin and angiostatin, leading to sequestration of these proteins and modulation of their inhibitory effects on endothelial cells. Moreover, NG2 was also shown to harbor binding sites specific for bFGF and PDGF-AA growth factors, through which more efficient signaling by these mitogenic factors is achieved in target cells. Therefore, NG2 could be used as an indicator not only of LL-cbMSC EV identity but also of their quality, meant as the preservation of EV biological activity through all phases of isolation, production, and storage.
A similar approach, linking identity-defining EV antigen with quality of EV preparation, was adopted by Witwer et al. in the same context of MSC EV production [
31]. In their work, they focused on CD73 surface antigen and its ecto-5-prime-nucleotidase enzymatic activity, which catalyzes the conversion of AMP to adenosine. This activity can be easily addressed by commercially available assays [
35]. CD73 is also a key MSC surface marker, as stated by the International Society for Cellular Therapy [
21,
22]. These features allow the implementation of CD73 as sentinel to monitor protein denaturation and loss of enzyme activity during the preparation or storage of MSC EV for diverse clinical applications.
Another marker found to be more expressed in LL-cbMSC than bmMSC was CD105 or endoglin, another type 1 glycoprotein shared between MSC and endothelial cells that forms part of the TGFβ receptor complex. The functional relevance of this surface protein on EV has been already studied by others in the context of metastasis, where CD105
+ microvesicles generated by cancer cells triggered angiogenesis supporting the formation of a pre-metastatic niche [
36]. Surprisingly, both class I and II HLA antigens were not expressed on MSC EV membranes. This result was unexpected because HLA-ABC is highly expressed in MSC [
16], whereas HLA-DRDPDQ is only expressed by antigen-presenting cells. This feature of EV could be a remarkable advantage compared to parental MSC when considering the immune response potentially elicited by cellular therapies. Furthermore, this result suggests that there is an active mechanism sorting membrane proteins to MSC EV, or that HLA antigens are specifically excluded. Another relevant result concerned the expression of CD63, CD81, and CD9. These tetraspanins are the most well-known markers for EV, initially proposed to be specific for exosomes. Later studies showed a broader and more complex pattern of expression, extended to other classes of EV, including microvesicles [
37,
38]. MSC EV showed a robust and bright signal from CD63 and CD81, in contrast to a significantly dimmer CD9 signal. These data may indicate either a lower antigen density or a lower number of CD9
+ MSC EV. Finally, CD63 and CD81 analyses clearly evidenced the enrichment of these antigens in EV compared to parental MSC. Thus, the use of CD63 and CD81 as broad identity markers for MSC EV could be evaluated.
In conclusion, we propose NG2 as surface antigen marker to specifically address LL-cbMSC EV identity and quality, thanks to its preferential expression in LL-cbMSC EV compared to bmMSC EV and to the retention of its functionality in shuttling EV. These features are required to set up consistent assays for pre-clinical and clinical studies, answering one of the urgent needs in the emergent field of MSC-based EV therapeutics.