Sex Differences in Liver, Adipose Tissue, and Muscle Transcriptional Response to Fasting and Refeeding in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Plasma Assays
2.4. Relative Quantitation Real-Time PCR
2.5. Statistical Analysis
3. Results
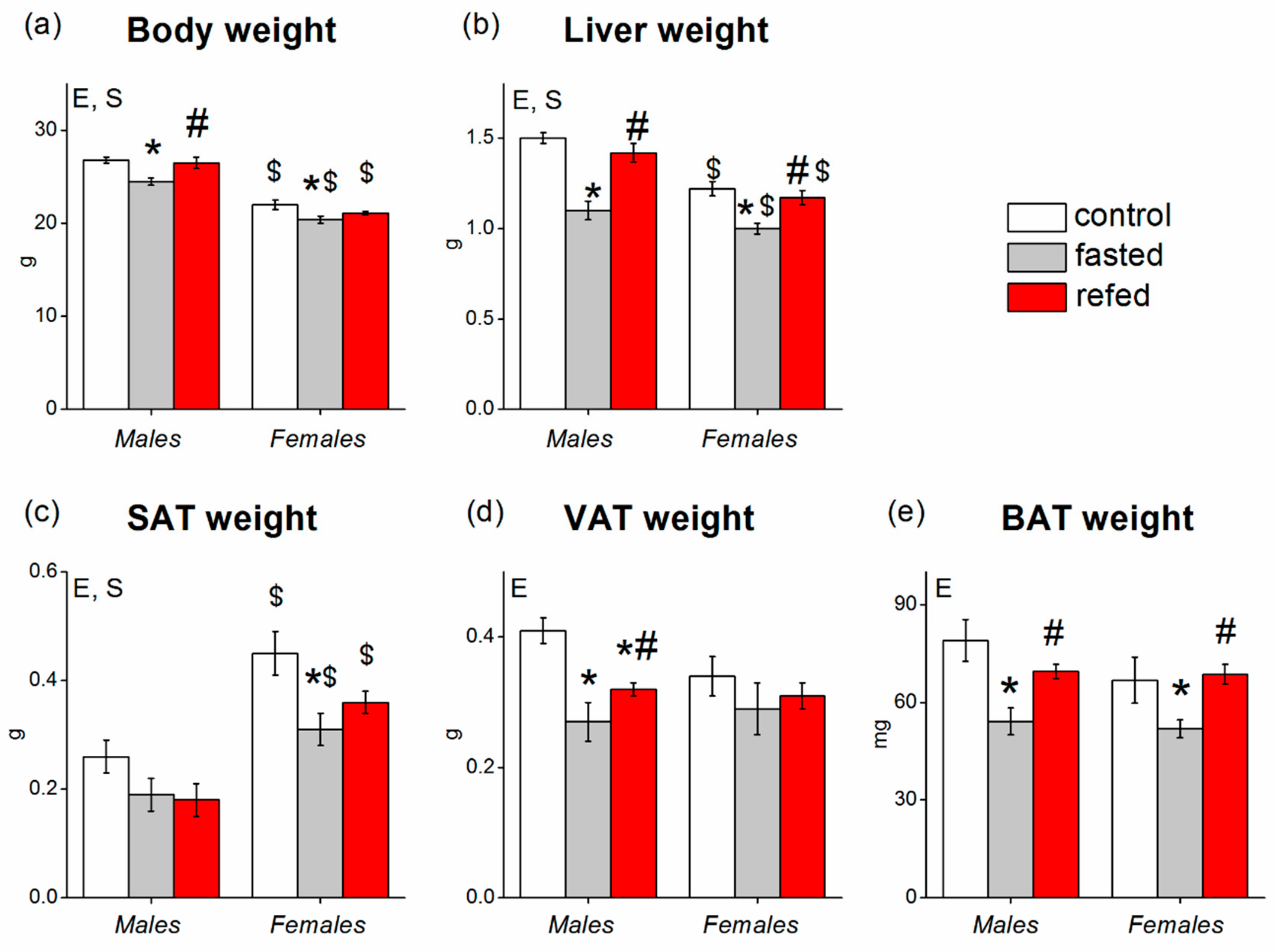
3.1. Food Intake, Body Weight, Tissue Weights, and Blood Biochemistry
3.2. Liver Gene Expression
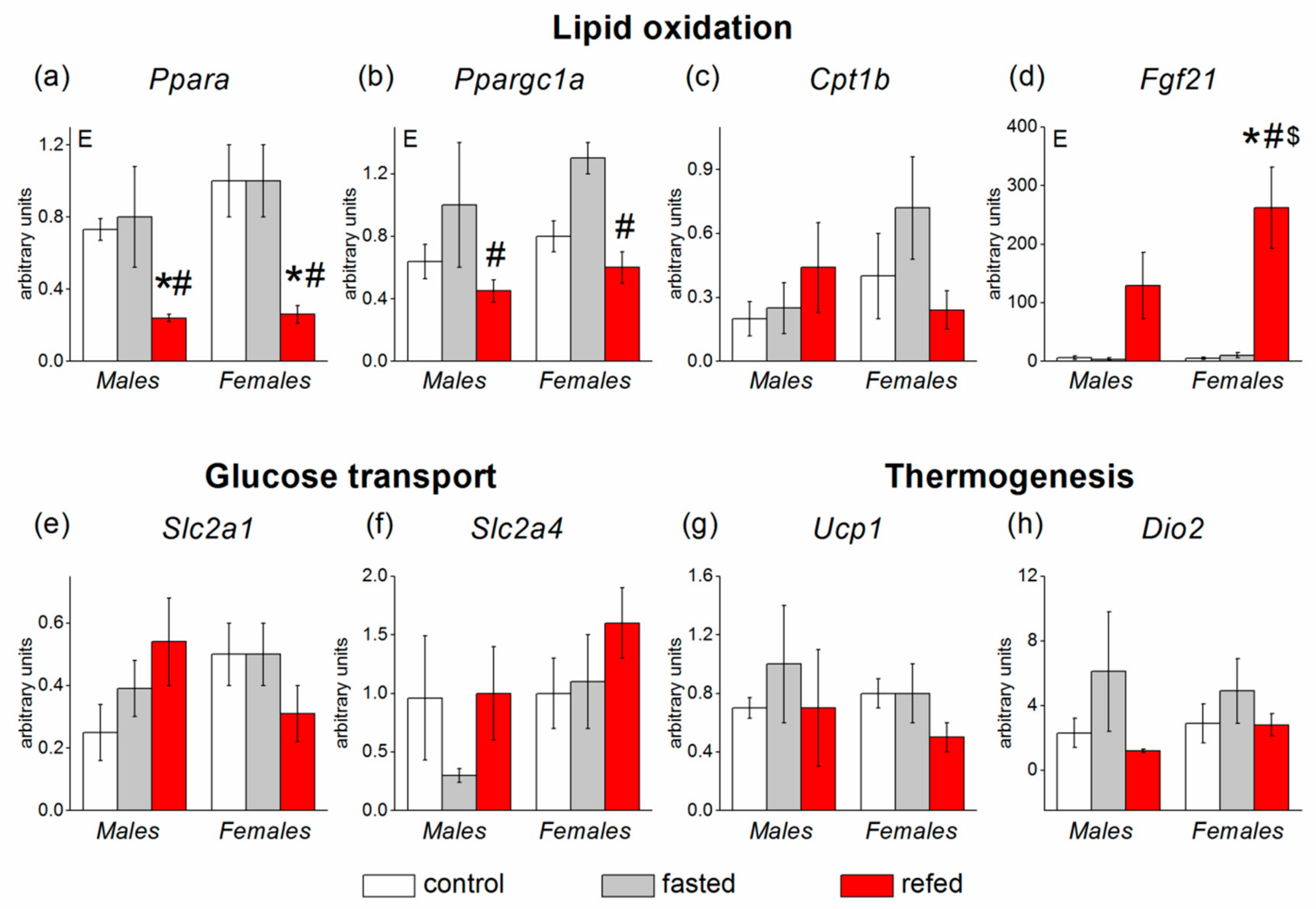
3.3. Adipose Tissue Gene Expression
3.4. Muscle Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lundsgaard, A.-M.; Kiens, B. Gender differences in skeletal muscle substrate metabolism—Molecular mechanisms and insulin sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 2015, 6, 14. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Gasparin, F.R.S.; Carreño, F.O.; Mewes, J.M.; Gilglioni, E.H.; Pagadigorria, C.L.S.; Natali, M.R.M.; Utsunomiya, K.S.; Constantin, R.P.; Ouchida, A.T.; Curti, C.; et al. Sex differences in the development of hepatic steatosis in cafeteria diet-induced obesity in young mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, C.; Yassin, K.; Wahlström, E.; Cheung, L.; Lindberg, J.; Brismar, K.; Ostenson, C.-G.; Norstedt, G.; Tollet-Egnell, P. Sex-different hepatic glycogen content and glucose output in rats. BMC Biochem. 2010, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Benz, V.; Bloch, M.; Wardat, S.; Böhm, C.; Maurer, L.; Mahmoodzadeh, S.; Wiedmer, P.; Spranger, J.; Foryst-Ludwig, A.; Kintscher, U. Sexual dimorphic regulation of body weight dynamics and adipose tissue lipolysis. PLoS ONE 2012, 7, e37794. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Inagaki, T.; Satapati, S.; Ding, X.; He, T.; Goetz, R.; Mohammadi, M.; Finck, B.N.; Mangelsdorf, D.J.; Kliewer, S.A.; et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA 2009, 106, 10853–10858. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wong, K.; Giles, A.; Jiang, J.; Lee, J.W.; Adams, A.C.; Kharitonenkov, A.; Yang, Q.; Gao, B.; Guarente, L.; et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology 2014, 146, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.G.; Jornayvaz, F.R.; Petersen, M.C.; Pesta, D.; Guigni, B.A.; Serr, J.; Zhang, D.; Kahn, M.; Samuel, V.T.; Jurczak, M.J.; et al. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology 2013, 154, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Planavila, A.; Redondo-Angulo, I.; Ribas, F.; Garrabou, G.; Casademont, J.; Giralt, M.; Villarroya, F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc. Res. 2015, 106, 19–31. [Google Scholar] [CrossRef]
- Bazhan, N.; Jakovleva, T.; Balyibina, N.; Dubinina, A.; Denisova, E.; Feofanova, N.; Makarova, E. Sex dimorphism in the Fgf21 gene expression in liver and adipose tissues is dependent on the metabolic condition. Online J. Biol. Sci. 2019, 19, 28–36. [Google Scholar] [CrossRef]
- Allard, C.; Bonnet, F.; Xu, B.; Coons, L.; Albarado, D.; Hill, C.; Fagherazzi, G.; Korach, K.S.; Levin, E.R.; Lefante, J.; et al. Activation of hepatic estrogen receptor-α increases energy expenditure by stimulating the production of fibroblast growth factor 21 in female mice. Mol. Metab. 2019, 22, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Lewitt, M.S.; Brismar, K. Gender difference in the leptin response to feeding in peroxisome-proliferator-activated receptor-alpha knockout mice. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1296–1300. [Google Scholar] [CrossRef][Green Version]
- Couillard, C.; Bergeron, N.; Prud’homme, D.; Bergeron, J.; Tremblay, A.; Bouchard, C.; Mauriège, P.; Després, J.P. Gender difference in postprandial lipemia: Importance of visceral adipose tissue accumulation. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2448–2455. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xie, Y.; Morrison, R.F.; Bucher, N.L.; Farmer, S.R. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Investig. 1998, 101, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Eimre, M.; Paju, K.; Peet, N.; Kadaja, L.; Tarrend, M.; Kasvandik, S.; Seppet, J.; Ivask, M.; Orlova, E.; Kõks, S. Increased mitochondrial protein levels and bioenergetics in the Musculus rectus femoris of Wfs1-deficient mice. Oxid. Med. Cell. Longev. 2018, 2018, 3175313. [Google Scholar] [CrossRef] [PubMed]
- Preidis, G.A.; Kim, K.H.; Moore, D.D. Nutrient-sensing nuclear receptors PPARα and FXR control liver energy balance. J. Clin. Investig. 2017, 127, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Lundåsen, T.; Hunt, M.C.; Nilsson, L.-M.; Sanyal, S.; Angelin, B.; Alexson, S.E.H.; Rudling, M. PPARalpha is a key regulator of hepatic FGF21. Biochem. Biophys. Res. Commun. 2007, 360, 437–440. [Google Scholar] [CrossRef]
- Paquette, A.; Wang, D.; Jankowski, M.; Gutkowska, J.; Lavoie, J.-M. Effects of ovariectomy on PPARα, SREBP-1c, and SCD-1 gene expression in the rat liver. Menopause 2008, 15, 1169–1175. [Google Scholar] [CrossRef]
- Kim, J.H.; Meyers, M.S.; Khuder, S.S.; Abdallah, S.L.; Muturi, H.T.; Russo, L.; Tate, C.R.; Hevener, A.L.; Najjar, S.M.; Leloup, C.; et al. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol. Metab. 2014, 3, 177–190. [Google Scholar] [CrossRef]
- Ding, H.; Zheng, S.; Garcia-Ruiz, D.; Hou, D.; Wei, Z.; Liao, Z.; Li, L.; Zhang, Y.; Han, X.; Zen, K.; et al. Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149-3p and suppression of PRDM16. Nat. Commun. 2016, 7, 11533. [Google Scholar] [CrossRef]
- Tang, H.-N.; Tang, C.-Y.; Man, X.-F.; Tan, S.-W.; Guo, Y.; Tang, J.; Zhou, C.-L.; Zhou, H.-D. Plasticity of adipose tissue in response to fasting and refeeding in male mice. Nutr. Metab. 2017, 14, 3. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Kristensen, K.; Hermann, P.A.; Katzenellenbogen, J.A.; Richelsen, B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004, 89, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Kajita, K.; Mune, T.; Ikeda, T.; Matsumoto, M.; Uno, Y.; Sugiyama, C.; Matsubara, K.; Morita, H.; Takemura, M.; Seishima, M.; et al. Effect of fasting on PPARgamma and AMPK activity in adipocytes. Diabetes Res. Clin. Pract. 2008, 81, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yoon, M. 17β-Estradiol inhibition of PPARγ-induced adipogenesis and adipocyte-specific gene expression. Acta Pharm. Sin. 2011, 32, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Lewitt, M.S.; Brismar, K.; Wang, J.; Wivall-Helleryd, I.L.; Sindelar, P.; Gonzalez, F.J.; Bergman, T.; Bobek, G.A. Responses of insulin-like growth factor (IGF)-I and IGF-binding proteins to nutritional status in peroxisome proliferator-activated receptor-alpha knockout mice. Growth Horm. IGF Res. 2001, 11, 303–313. [Google Scholar] [CrossRef]
- Haffner, S.M.; Miettinen, H.; Karhapää, P.; Mykkänen, L.; Laakso, M. Leptin concentrations, sex hormones, and cortisol in nondiabetic men. J. Clin. Endocrinol. Metab. 1997, 82, 1807–1809. [Google Scholar] [CrossRef]
- Elbers, J.M.; Asscheman, H.; Seidell, J.C.; Frölich, M.; Meinders, A.E.; Gooren, L.J. Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. J. Clin. Endocrinol. Metab. 1997, 82, 3267–3270. [Google Scholar] [CrossRef]
- Jenks, M.Z.; Fairfield, H.E.; Johnson, E.C.; Morrison, R.F.; Muday, G.K. Sex steroid hormones regulate leptin transcript accumulation and protein secretion in 3T3-L1 cells. Sci. Rep. 2017, 7, 8232. [Google Scholar] [CrossRef]
- Fukuda, H.; Iritani, N.; Sugimoto, T.; Ikeda, H. Transcriptional regulation of fatty acid synthase gene by insulin/glucose, polyunsaturated fatty acid and leptin in hepatocytes and adipocytes in normal and genetically obese rats. Eur. J. Biochem. 1999, 260, 505–511. [Google Scholar] [CrossRef]
- O’Brien, R.M.; Streeper, R.S.; Ayala, J.E.; Stadelmaier, B.T.; Hornbuckle, L.A. Insulin-regulated gene expression. Biochem. Soc. Trans. 2001, 29, 552–558. [Google Scholar] [CrossRef]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Yu, R.T.; Mangelsdorf, D.J.; Kliewer, S.A. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef]
- Muise, E.S.; Azzolina, B.; Kuo, D.W.; El-Sherbeini, M.; Tan, Y.; Yuan, X.; Mu, J.; Thompson, J.R.; Berger, J.P.; Wong, K.K. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol. Pharm. 2008, 74, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Pedersen, M.; Nielsen, A.R.; Krogh-Madsen, R.; Yfanti, C.; Akerstrom, T.; Nielsen, S.; Pedersen, B.K. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes 2009, 58, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Isensee, J.; Ruiz Noppinger, P. Sexually dimorphic gene expression in mammalian somatic tissue. Gend. Med. 2007, 4 (Suppl. B), 75–95. [Google Scholar] [CrossRef]





| Protein | Gene | Gene Expression Assay |
|---|---|---|
| Carnitine palmitoyltransferase 1a | Cpt1a | Mm01231183_m1 |
| Carnitine palmitoyltransferase 1b | Cpt1b | Mm00487191_g1 |
| Deiodinase, iodothyronine, type II | Dio2 | Mm00515664_m1 |
| Fatty acid synthase | Fasn | Mm00662319_m1 |
| Fibroblast growth factor 21 | Fgf21 | Mm00840165_g1 |
| Glucose-6-phosphatase, catalytic | G6pc | Mm00839363_m1 |
| Glucokinase | Gck | Mm00439129_m1 |
| Insulin receptor | Insr | Mm01211875_m1 |
| Lipase, hormone sensitive | Lipe | Mm00495359_m1 |
| Lipoprotein lipase | Lpl | Mm00434764_m1 |
| Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha | Ppargc1a | Mm01208835_m1 |
| Peroxisome proliferator activated receptor alpha | Ppara | Mm0040939_m1 |
| Peroxisome proliferator activated receptor gamma | Pparg | Mm00440940_m1 |
| Phosphoenolpyruvate carboxykinase 1, cytosolic | Pck1 | Mm01247058_m1 |
| Pyruvate kinase liver and red blood cell | Pklr | Mm00443090_m1 |
| Solute carrier family 2 (facilitated glucose transporter), member 1 (GLUT1) | Slc2a1 | Mm00441480_m1 |
| Solute carrier family 2 (facilitated glucose transporter), member 2 (GLUT2) | Slc2a2 | Mm00446229_m1 |
| Solute carrier family 2 (facilitated glucose transporter), member 4 (GLUT4) | Slc2a4 | Mm00436615_m1 |
| Uncoupling protein 1 (mitochondrial, proton carrier) | Ucp1 | Mm01244861_m1 |
| Uncoupling protein 3 (mitochondrial, proton carrier) | Ucp3 | Mm01163394_m1 |
| Beta-actin | Actb | Mm00607939_s1 |
| Males | Females | P | |||||
|---|---|---|---|---|---|---|---|
| Control | Fasting | Refeeding | Control | Fasting | Refeeding | ||
| Liver (%) | 5.58 ± 0.11 (n = 10) | 4.47 ± 0.21 (n = 9) * | 5.32 ± 0.11 (n = 9) # | 5.47 ± 0.11 (n = 10) | 4.90 ± 0.13 (n = 8) *,$ | 5.54 ± 0.14 (n = 9) # | E |
| SAT (%) | 0.98 ± 0.11 (n = 10) | 0.76 ± 0.13 (n = 9) | 0.68 ± 0.10 (n = 9) | 1.99 ± 0.18 (n = 10) $ | 1.48 ± 0.13 (n = 8) *,$ | 1.71 ± 0.11 (n = 9) $ | S |
| VAT (%) | 1.52 ± 0.07 (n = 10) | 1.10 ± 0.13 (n = 9) | 1.20 ± 0.05 (n = 9) | 1.52 ± 0.13 (n = 10) | 1.39 ± 0.21 (n = 8) | 1.48 ± 0.11 (n = 9) | |
| BAT (%) | 0.29 ± 0.02 (n = 10) | 0.22 ± 0.02 (n = 9) * | 0.26 ± 0.01 (n = 9) | 0.30 ± 0.03 (n = 10) | 0.25 ± 0.01 (n = 7) | 0.33 ± 0.02 (n = 9) #,$ | E, S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhan, N.; Jakovleva, T.; Feofanova, N.; Denisova, E.; Dubinina, A.; Sitnikova, N.; Makarova, E. Sex Differences in Liver, Adipose Tissue, and Muscle Transcriptional Response to Fasting and Refeeding in Mice. Cells 2019, 8, 1529. https://doi.org/10.3390/cells8121529
Bazhan N, Jakovleva T, Feofanova N, Denisova E, Dubinina A, Sitnikova N, Makarova E. Sex Differences in Liver, Adipose Tissue, and Muscle Transcriptional Response to Fasting and Refeeding in Mice. Cells. 2019; 8(12):1529. https://doi.org/10.3390/cells8121529
Chicago/Turabian StyleBazhan, Nadezhda, Tatiana Jakovleva, Natalia Feofanova, Elena Denisova, Anastasia Dubinina, Natalia Sitnikova, and Elena Makarova. 2019. "Sex Differences in Liver, Adipose Tissue, and Muscle Transcriptional Response to Fasting and Refeeding in Mice" Cells 8, no. 12: 1529. https://doi.org/10.3390/cells8121529
APA StyleBazhan, N., Jakovleva, T., Feofanova, N., Denisova, E., Dubinina, A., Sitnikova, N., & Makarova, E. (2019). Sex Differences in Liver, Adipose Tissue, and Muscle Transcriptional Response to Fasting and Refeeding in Mice. Cells, 8(12), 1529. https://doi.org/10.3390/cells8121529





