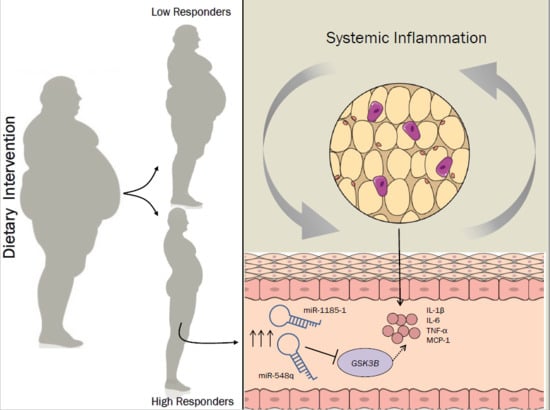
miR-1185-1 and miR-548q Are Biomarkers of Response to Weight Loss and Regulate the Expression of GSK3B
Abstract
1. Introduction
2. Experimental Section
2.1. RNA Isolation and Reverse Transcription
2.2. Microarray Analyses and miRNA-Seq
2.3. Bioinformatic Study
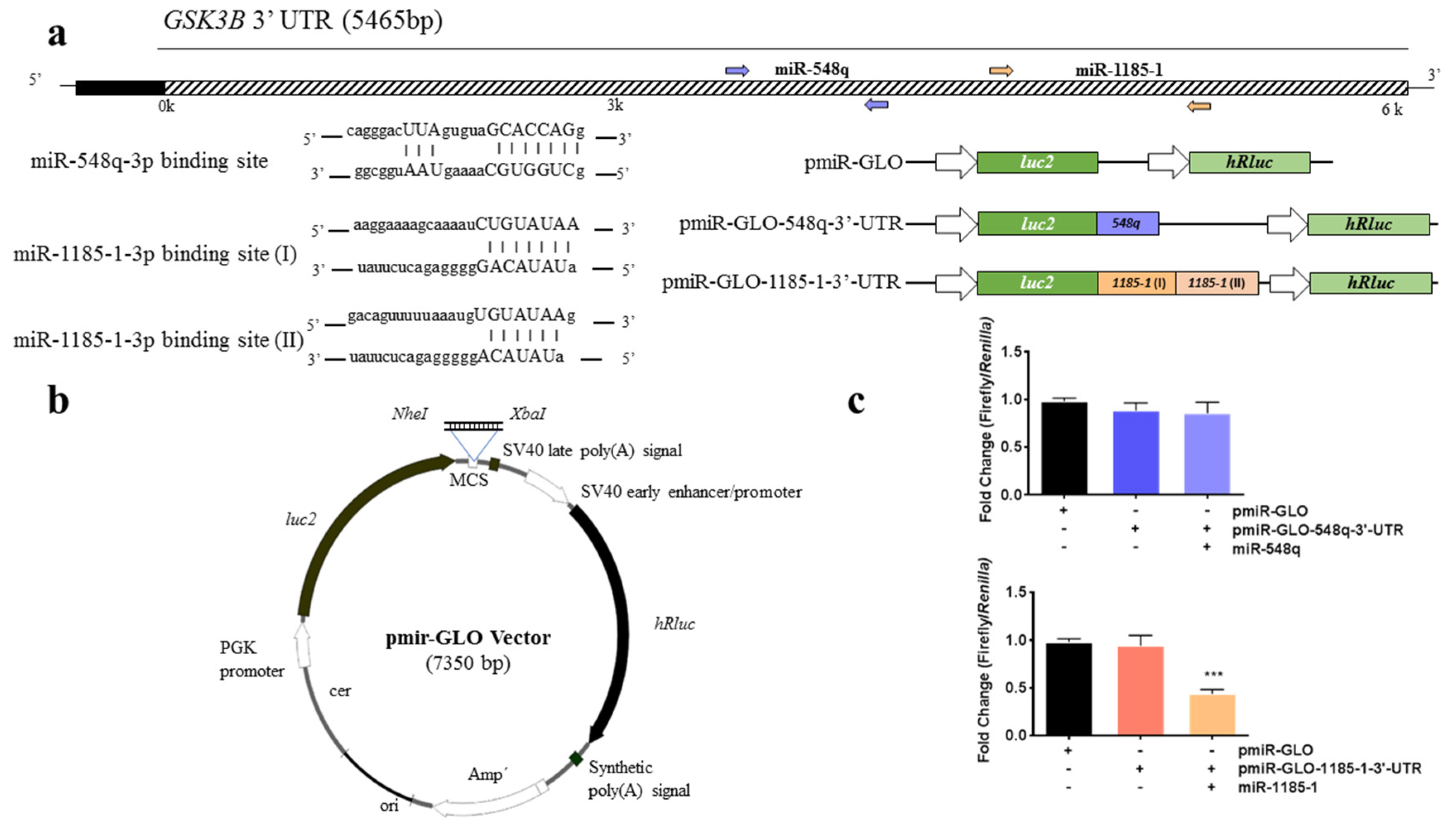
2.4. Luciferase Reporter Constructs
2.5. Cell Culture
mirVana miRNA Mimic Transfections
2.6. Dual-Luciferase Reporter Assays
2.7. Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Dietary Intervention
3.2. miR-548q and miR-1185-1 Are Overexpressed in High Responders to the Weight Loss Intervention
3.3. GSK3B Is a Putative Target Gene for miR-548q and miR-1185-1
3.4. miR-1185-1 Binds to the 3′-UTR of GSK3B
3.5. miR-548q and miR-1185-1 Decrease the Endogenous GSK3B mRNA Levels
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Goni, L.; Cuervo, M.; Milagro, F.I.; Martinez, J.A. Future Perspectives of Personalized Weight Loss Interventions Based on Nutrigenetic, Epigenetic, and Metagenomic Data. J. Nutr. 2016, 905S–912S. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Davis, C.D. The emerging role of microRNAs and nutrition in modulating health and disease. Annu. Rev. Nutr. 2014, 34, 305–336. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Gai, C.; Deregibus, M.C.; Tetta, C.; Camussi, G. Noncoding RNAs Carried by Extracellular Vesicles in Endocrine Diseases. Int. J. Endocrinol. 2018, 2018, 1–18. [Google Scholar] [CrossRef]
- Houshmand-Oeregaard, A.; Schrolkamp, M.; Kelstrup, L.; Hansen, N.S.; Hjort, L.; Thuesen, A.C.B.; Broholm, C.; Mathiesen, E.R.; Clausen, T.D.; Vaag, A.; et al. Increased expression of microRNA-15a and microRNA-15b in skeletal muscle from adult offspring of women with diabetes in pregnancy. Hum. Mol. Genet. 2018, 27, 1763–1771. [Google Scholar] [CrossRef]
- Marques-Rocha, J.L.; Samblas, M.; Milagro, F.I.; Bressan, J.; Martinez, J.A.; Marti, A. Noncoding RNAs, cytokines, and inflammation-related diseases. Faseb. J. 2015, 29, 3595–3611. [Google Scholar] [CrossRef]
- Yu, J.; Lv, Y.; Di, W.; Liu, J.; Kong, X.; Sheng, Y.; Huang, M.; Lv, S.; Qi, H.; Gao, M.; et al. MiR-27b-3p Regulation in Browning of Human Visceral Adipose Related to Central Obesity. Obesity 2018, 26, 387–396. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wang, L.H.; Su, D.J.; Zhu, D.; Li, Q.M.; Chi, M.H. MicroRNA-29b promotes the adipogenic differentiation of human adipose tissue-derived stromal cells. Obesity 2016, 24, 1097–1105. [Google Scholar] [CrossRef]
- Liu, W.; Cao, H.; Ye, C.; Chang, C.; Lu, M.; Jing, Y.; Zhang, D.; Yao, X.; Duan, Z.; Xia, H.; et al. Hepatic miR-378 targets p110alpha and controls glucose and lipid homeostasis by modulating hepatic insulin signalling. Nat. Commun. 2014, 5, 5684–5697. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Wu, W.; Yin, B.; Liu, X.; Ren, F. MicroRNA-463-3p/ABCG4: A new axis in glucose-stimulated insulin secretion. Obesity 2016, 24, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Derghal, A.; Djelloul, M.; Airault, C.; Pierre, C.; Dallaporta, M.; Troadec, J.D.; Tillement, V.; Tardivel, C.; Bariohay, B.; Trouslard, J.; et al. Leptin is required for hypothalamic regulation of miRNAs targeting POMC 3’UTR. Front. Cell Neurosci. 2015, 9, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Pescador, N.; Perez-Barba, M.; Ibarra, J.M.; Corbaton, A.; Martinez-Larrad, M.T.; Serrano-Rios, M. Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS ONE 2013, 8, e77251. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Danielson, K.M.; Benton, M.C.; Ziegler, O.; Shah, R.; Stubbs, R.S.; Das, S.; Macartney-Coxson, D. miRNA Signatures of Insulin Resistance in Obesity. Obesity 2017, 25, 1734–1744. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Wu, X.; Jiang, L.; Yang, S.; Ding, Z.; Fang, Z.; Hua, H.; Kirby, M.S.; Shou, J. A circulating microRNA signature as noninvasive diagnostic and prognostic biomarkers for nonalcoholic steatohepatitis. BMC Genom. 2018, 19, 188–209. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Roy, D.; Bhattacharyya, M.; Bandyopadhyay, S. Biological networks in Parkinson’s disease: An insight into the epigenetic mechanisms associated with this disease. BMC Genom. 2017, 18, 721–751. [Google Scholar] [CrossRef]
- Landrier, J.F.; Derghal, A.; Mounien, L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells 2019, 8, 859. [Google Scholar] [CrossRef]
- Parr, E.B.; Camera, D.M.; Burke, L.M.; Phillips, S.M.; Coffey, V.G.; Hawley, J.A. Circulating MicroRNA Responses between ‘High’ and ‘Low’ Responders to a 16-Wk Diet and Exercise Weight Loss Intervention. PLoS ONE 2016, 11, e0152545. [Google Scholar] [CrossRef]
- Milagro, F.I.; Miranda, J.; Portillo, M.P.; Fernandez-Quintela, A.; Campión, J.; Martínez, J.A. High-Throughput Sequencing of microRNAs in Peripheral Blood Mononuclear Cells: Identification of Potential Weight Loss Biomarkers. PLoS ONE 2013, 8, e54319. [Google Scholar] [CrossRef]
- Garcia-Lacarte, M.; Martinez, J.A.; Zulet, M.A.; Milagro, F.I. Implication of miR-612 and miR-1976 in the regulation of TP53 and CD40 and their relationship in the response to specific weight-loss diets. PLoS ONE 2018, 13, e0201217. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic. Acids. Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wu, Y.; Zhang, X.; Liao, Y.; Sibanda, V.L.; Liu, W.; Guo, A.-Y. Comprehensive analysis of human small RNA sequencing data provides insights into expression profiles and miRNA editing. Rna. Biol. 2014, 11, 1375–1385. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Varma, V.; Yao-Borengasser, A.; Rasouli, N.; Nolen, G.T.; Phanavanh, B.; Starks, T.; Gurley, C.; Simpson, P.; McGehee, R.E., Jr.; Kern, P.A.; et al. Muscle inflammatory response and insulin resistance: Synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1300–E1310. [Google Scholar] [CrossRef]
- Wang, X.; Ge, A.; Cheng, M.; Guo, F.; Zhao, M.; Zhou, X.; Liu, L.; Yang, N. Increased hypothalamic inflammation associated with the susceptibility to obesity in rats exposed to high-fat diet. Exp. Diabetes Res. 2012, 2012, 847246–847254. [Google Scholar] [CrossRef]
- Chang, R.C.; Ying, W.; Bazer, F.W.; Zhou, B. MicroRNAs Control Macrophage Formation and Activation: The Inflammatory Link between Obesity and Cardiovascular Diseases. Cells 2014, 3, 702–712. [Google Scholar] [CrossRef]
- Feng, J.; Li, A.; Deng, J.; Yang, Y.; Dang, L.; Ye, Y.; Li, Y.; Zhang, W. miR-21 attenuates lipopolysaccharide-induced lipid accumulation and inflammatory response: Potential role in cerebrovascular disease. Lipids Health Dis. 2014, 13, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Fordham, J.B.; Naqvi, A.R.; Nares, S. miR-24 Regulates Macrophage Polarization and Plasticity. J. Clin. Cell Immunol. 2015, 6, 362–381. [Google Scholar] [PubMed]
- Tahamtan, A.; Teymoori-Rad, M.; Nakstad, B.; Salimi, V. Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment. Front. Immunol. 2018, 9, 1377–1415. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef]
- Steinbrecher, K.A.; Wilson, W., 3rd; Cogswell, P.C.; Baldwin, A.S. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol. Cell. Biol. 2005, 25, 8444–8455. [Google Scholar] [CrossRef]
- Beurel, E. Regulation by Glycogen Synthase Kinase-3 of Inflammation and T Cells in CNS Diseases. Front. Mol. Neurosci. 2011, 4, 18–42. [Google Scholar] [CrossRef]
- Martin, M.; Rehani, K.; Jope, R.S.; Michalek, S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005, 6, 777–784. [Google Scholar] [CrossRef]
- Eldar-Finkelman, H.; Schreyer, S.A.; Shinohara, M.M.; LeBoeuf, R.C.; Krebs, E.G. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes 1999, 48, 1662–1666. [Google Scholar] [CrossRef]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Meng, Y.; Zhang, C.; Di, L. GSK3-activated STAT5 regulates expression of SFRPs to modulate adipogenesis. Faseb. J. 2018, 32, 4714–4726. [Google Scholar] [CrossRef]
- Patricia, S.-B.; Wolfgang, W.; Ralf, K. Simultaneous Cre-mediated conditional knockdown of two genes in mice. Genesis 2008, 46, 144–151. [Google Scholar]
- Pearce, N.J.; Arch, J.R.S.; Clapham, J.C.; Coghlan, M.P.; Corcoran, S.L.; Lister, C.A.; Llano, A.; Moore, G.B.; Murphy, G.J.; Smith, S.A.; et al. Development of glucose intolerance in male transgenic mice overexpressing human glycogen synthase kinase-3β on a muscle-specific promoter. Metabolism 2004, 53, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yang, W.K.; Song, J.H.; Ra, Y.M.; Jeong, J.-H.; Choe, W.; Kang, I.; Kim, S.-S.; Ha, J. Anti-obesity effects of 3-hydroxychromone derivative, a novel small-molecule inhibitor of glycogen synthase kinase-3. Biochem. Pharm. 2013, 85, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, C.; Esteller, M. Chapter Seven-MicroRNAs and Epigenetics. In Advances in Cancer Research; Croce, C.M., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 135, pp. 189–220. [Google Scholar]
- Tang, X.; Zheng, D.; Hu, P.; Zeng, Z.; Li, M.; Tucker, L.; Monahan, R.; Resnick, M.B.; Liu, M.; Ramratnam, B. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the β-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic. Acids Res. 2014, 42, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wang, R.; Li, D.; Lin, X.J.; Wei, Q.K.; Yuan, Y.; Wang, Q.; Chen, W.; Zhuang, S.M. A novel GSK-3 beta-C/EBP alpha-miR-122-insulin-like growth factor 1 receptor regulatory circuitry in human hepatocellular carcinoma. Hepatology 2010, 52, 1702–1712. [Google Scholar] [CrossRef]
- Ji, J.; Yamashita, T.; Wang, X.W. Wnt/beta-catenin signaling activates microRNA-181 expression in hepatocellular carcinoma. Cell Biosci. 2011, 1, 4–15. [Google Scholar] [CrossRef]
- Schwanhausser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef]
Sample Availability: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. |



| GSK3B-miR-548q-F | 5′-TTAGCTAGCACAGTAGGTACCGGCCTGTA-3′ | 668 bp |
| GSK3B-miR-548q -R | 5′-TTATCTAGAGGTGGCACTCCGTGCAGT-3′ | |
| GSK3B-miR-1185-1-F | 5′-TTTGCTAGCCCGATGGATCACTTGGGCCT-3′ | 856 bp |
| GSK3B-miR-1185-1-R | 5′-TTATCTAGAGGAGGTACAGCCCCACTGTT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Lacarte, M.; Mansego, M.L.; Zulet, M.A.; Martinez, J.A.; Milagro, F.I. miR-1185-1 and miR-548q Are Biomarkers of Response to Weight Loss and Regulate the Expression of GSK3B. Cells 2019, 8, 1548. https://doi.org/10.3390/cells8121548
Garcia-Lacarte M, Mansego ML, Zulet MA, Martinez JA, Milagro FI. miR-1185-1 and miR-548q Are Biomarkers of Response to Weight Loss and Regulate the Expression of GSK3B. Cells. 2019; 8(12):1548. https://doi.org/10.3390/cells8121548
Chicago/Turabian StyleGarcia-Lacarte, Marcos, Maria L. Mansego, M. Angeles Zulet, J. Alfredo Martinez, and Fermin I. Milagro. 2019. "miR-1185-1 and miR-548q Are Biomarkers of Response to Weight Loss and Regulate the Expression of GSK3B" Cells 8, no. 12: 1548. https://doi.org/10.3390/cells8121548
APA StyleGarcia-Lacarte, M., Mansego, M. L., Zulet, M. A., Martinez, J. A., & Milagro, F. I. (2019). miR-1185-1 and miR-548q Are Biomarkers of Response to Weight Loss and Regulate the Expression of GSK3B. Cells, 8(12), 1548. https://doi.org/10.3390/cells8121548