Specification of BMP Signaling
Abstract
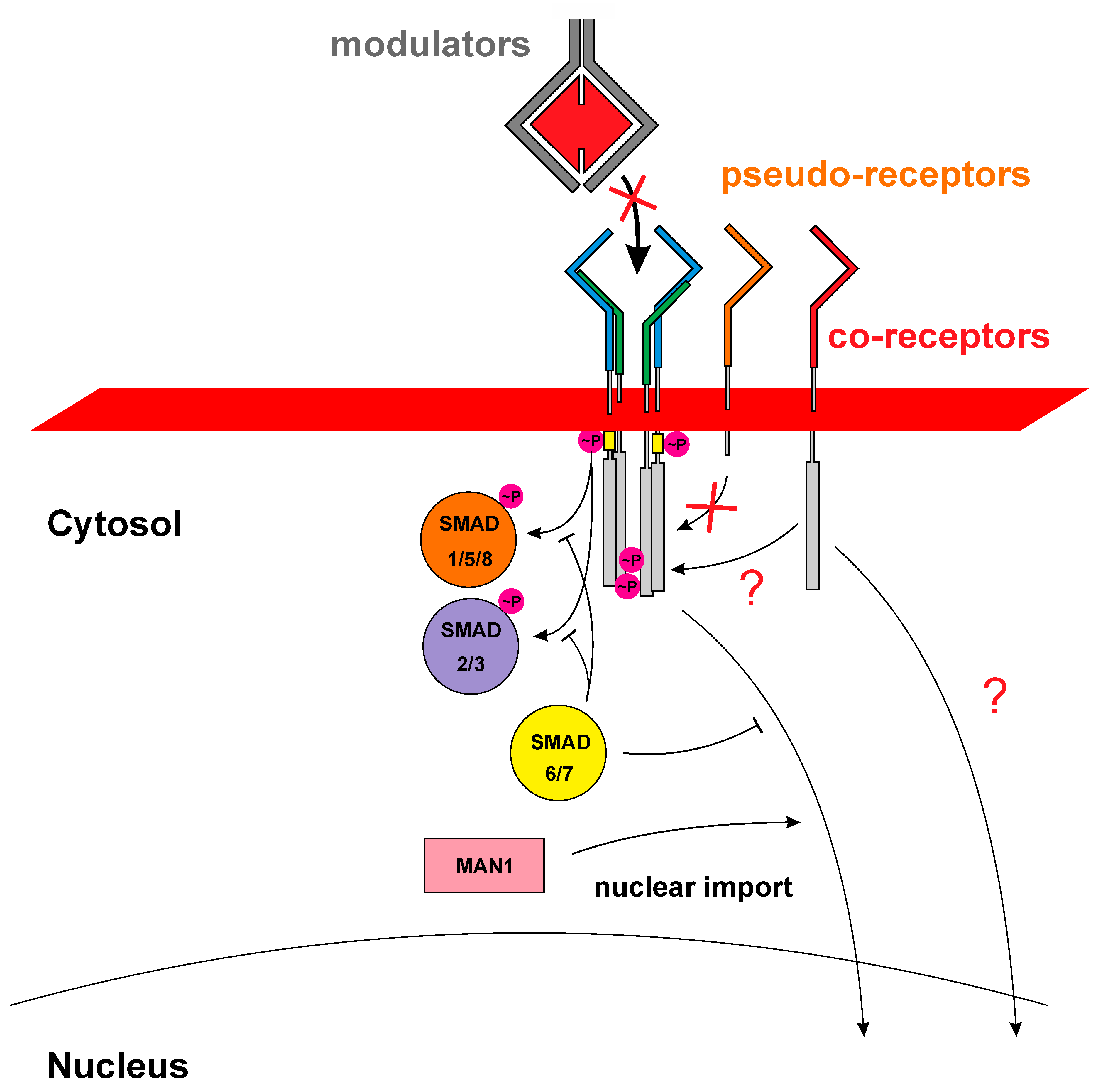
:1. The SMAD Dilemma: Many Growth Factors but Just Two Principal Signaling Pathways
2. The Ligand-Receptor Promiscuity Dilemma
3. The Beginning–Correlating Cellular Binding Sites and Receptors
4. Do Type II Receptors Matter for TGFβ/BMP Signal Specification?
5. BMP Type I Receptor Mysteries–Binding Does Not Mean Receptor Activation
6. Heterodimeric Ligands–An Unexplored World
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyazawa, K.; Miyazono, K. Regulation of TGF-beta Family Signaling by Inhibitory Smads. Cold Spring Harb. Perspect. Biol. 2017, 9, a022095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreira, A.C.O.; Zambuzzi, W.F.; Rossi, M.C.; Astorino Filho, R.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Promising Molecules for Bone Healing, Bioengineering, and Regenerative Medicine. Vitam. Horm. 2015, 99, 293–322. [Google Scholar] [PubMed]
- de Vinuesa, A.G.; Abdelilah-Seyfried, S.; Knaus, P.; Zwijsen, A.; Bailly, S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 2016, 27, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Puerto, M.C.; Iyengar, P.V.; García de Vinuesa, A.; ten Dijke, P.; Sanchez-Duffhues, G. Bone morphogenetic protein receptor signal transduction in human disease. J. Pathol 2019, 247, 9–20. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [Green Version]
- Lowery, J.W.; Rosen, V. The BMP Pathway and Its Inhibitors in the Skeleton. Physiol Rev. 2018, 98, 2431–2452. [Google Scholar] [CrossRef] [Green Version]
- Persson, U.; Izumi, H.; Souchelnytskyi, S.; Itoh, S.; Grimsby, S.; Engström, U.; Heldin, C.H.; Funa, K.; ten Dijke, P. The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. Febs Lett 1998, 434, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Piek, E.; Westermark, U.; Kastemar, M.; Heldin, C.H.; van Zoelen, E.J.; Nistér, M.; Ten Dijke, P. Expression of transforming-growth-factor (TGF)-beta receptors and Smad proteins in glioblastoma cell lines with distinct responses to TGF-beta1. Int. J. Cancer 1999, 80, 756–763. [Google Scholar] [CrossRef]
- Korchynskyi, O.; ten Dijke, P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 2002, 277, 4883–4891. [Google Scholar] [CrossRef] [Green Version]
- Dennler, S.; Itoh, S.; Vivien, D.; ten Dijke, P.; Huet, S.; Gauthier, J.M. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998, 17, 3091–3100. [Google Scholar] [CrossRef] [Green Version]
- Attisano, L.; Lee-Hoeflich, S.T. The Smads. Genome Biol. 2001, 2, reviews3010-1. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Hata, A.; Lo, R.S.; Wotton, D.; Shi, Y.; Pavletich, N.; Massagué, J. Determinants of specificity in TGF-beta signal transduction. Genes Dev. 1998, 12, 2144–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, B.Y.; Chacko, B.M.; Lam, S.S.; de Caestecker, M.P.; Correia, J.J.; Lin, K. Structural basis of Smad1 activation by receptor kinase phosphorylation. Mol. Cell. 2001, 8, 1303–1312. [Google Scholar] [CrossRef]
- Baburajendran, N.; Palasingam, P.; Ng, C.K.L.; Jauch, R.; Kolatkar, P.R. Crystal optimization and preliminary diffraction data analysis of the Smad1 MH1 domain bound to a palindromic SBE DNA element. Acta Cryst. Sect. F: Struct. Biol. Cryst. Commun. 2009, 65, 1105–1109. [Google Scholar] [CrossRef] [Green Version]
- Chai, N.; Li, W.X.; Wang, J.; Wang, Z.X.; Yang, S.M.; Wu, J.W. Structural basis for the Smad5 MH1 domain to recognize different DNA sequences. Nucleic Acids Res. 2015, 43, 9051–9064. [Google Scholar] [CrossRef] [Green Version]
- Martin-Malpartida, P.; Batet, M.; Kaczmarska, Z.; Freier, R.; Gomes, T.; Aragón, E.; Zou, Y.; Wang, Q.; Xi, Q.; Ruiz, L.; et al. Structural basis for genome wide recognition of 5-bp GC motifs by SMAD transcription factors. Nat. Commun. 2017, 8, 2070. [Google Scholar] [CrossRef]
- Wrighton, K.H.; Lin, X.; Feng, X.H. Phospho-control of TGF-beta superfamily signaling. Cell Res. 2009, 19, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, C.; Zaromytidou, A.I.; Xi, Q.; Gao, S.; Yu, J.; Fujisawa, S.; Barlas, A.; Miller, A.N.; Manova-Todorova, K.; Macias, M.J.; et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 2009, 139, 757–769. [Google Scholar]
- Hata, A.; Chen, Y.G. TGF-beta Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef]
- Xu, P.; Liu, J.; Derynck, R. Post-translational regulation of TGF-beta receptor and Smad signaling. FEBS Lett. 2012, 586, 1871–1884. [Google Scholar] [CrossRef] [Green Version]
- Dick, A.; Meier, A.; Hammerschmidt, M. Smad1 and Smad5 have distinct roles during dorsoventral patterning of the zebrafish embryo. Dev. Dyn. 1999, 216, 285–298. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H.; Tang, D.; Huang, S.; Zuscik, M.J.; Chen, D. Smad1 plays an essential role in bone development and postnatal bone formation. Osteoarthritis Cartil. 2011, 19, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Li, J.H.; Garcia, G.; Mu, W.; Piek, E.; Böttinger, E.P.; Chen, Y.; Zhu, H.J.; Kang, D.H.; Schreiner, G.F.; et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004, 66, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umans, L.; Cox, L.; Tjwa, M.; Bito, V.; Vermeire, L.; Laperre, K.; Sipido, K.; Moons, L.; Huylebroeck, D.; Zwijsen, A. Inactivation of Smad5 in endothelial cells and smooth muscle cells demonstrates that Smad5 is required for cardiac homeostasis. Am. J. Pathol. 2007, 170, 1460–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McReynolds, L.J.; Gupta, S.; Figueroa, M.E.; Mullins, M.C.; Evans, T. Smad1 and Smad5 differentially regulate embryonic hematopoiesis. Blood 2007, 110, 3881–3890. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.A.; Pietenpol, J.A.; Moses, H.L. A tale of two proteins: Differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling. J. Cell. Biochem. 2007, 101, 9–33. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Ren, X.; Tian, Y.; Chen, Z.; Xu, X.; Du, Y.; Jiang, C.; Fang, Y.; Liu, Z.; et al. Smad2 and Smad3 have differential sensitivity in relaying TGFbeta signaling and inversely regulate early lineage specification. Sci. Rep. 2016, 6, 21602. [Google Scholar] [CrossRef] [Green Version]
- Goumans, M.J; Mummery, C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int. J. Dev. Biol. 2000, 44, 253–265. [Google Scholar]
- Weinstein, M.; Yang, X.; Deng, C. Functions of mammalian Smad genes as revealed by targeted gene disruption in mice. Cytokine Growth Factor Rev. 2000, 11, 49–58. [Google Scholar] [CrossRef]
- Mulder, K.M. Role of Ras and Mapks in TGFbeta signaling. Cytokine Growth Factor Rev. 2000, 11, 23–35. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Atfi, A.; Djelloul, S.; Chastre, E.; Davis, R.; Gespach, C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J. Biol. Chem. 1997, 272, 1429–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glise, B.; Noselli, S. Coupling of Jun amino-terminal kinase and Decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 1997, 11, 1738–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulder, K.M.; Morris, S.L. Activation of p21ras by transforming growth factor beta in epithelial cells. J. Biol. Chem. 1992, 267, 5029–5031. [Google Scholar] [PubMed]
- Hanafusa, H.; Ninomiya-Tsuji, J.; Masuyama, N.; Nishita, M.; Fujisawa, J.I.; Shibuya, H.; Matsumoto, K.; Nishida, E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J. Biol. Chem. 1999, 274, 27161–27167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, S.; Iguchi, M.; Watanabe, K.; Hoshino, R.; Tsujimoto, M.; Kohno, M. Specific activation of the p38 mitogen-activated protein kinase signaling pathway and induction of neurite outgrowth in PC12 cells by bone morphogenetic protein-2. J. Biol. Chem. 1999, 274, 26503–26510. [Google Scholar] [CrossRef] [Green Version]
- Lou, J.; Tu, Y.; Li, S.; Manske, P.R. Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem. Biophys. Res. Commun. 2000, 268, 757–762. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirai, T.; Morishita, S.; Uchida, S.; Saeki-Miura, K.; Makishima, F. Makishima, p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp. Cell Res. 1999, 250, 351–363. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Shirakabe, K.; Shibuya, H.; Irie, K.; Oishi, I.; Ueno, N.; Taniguchi, T.; Nishida, E.; Matsumoto, K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 1995, 270, 2008–2011. [Google Scholar] [CrossRef]
- Shibuya, H.; Iwata, H.; Masuyama, N.; Gotoh, Y.; Yamaguchi, K.; Irie, K.; Matsumoto, K.; Nishida, E.; Ueno, N. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 1998, 17, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, K.; Nagai, S.I.; Ninomiya-Tsuji, J.; Nishita, M.; Tamai, K.; Irie, K.; Ueno, N.; Nishida, E.; Shibuya, H.; Matsumoto, K. Matsumoto, XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999, 18, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caestecker, M.P.; Parks, W.T.; Frank, C.J.; Castagnino, P.; Bottaro, D.P.; Roberts, A.B.; Lechleider, R.J. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998, 12, 1587–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gingery, A.; Bradley, E.W.; Pederson, L.; Ruan, M.; Horwood, N.J.; Oursler, M.J. TGF-beta coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp. Cell Res. 2008, 314, 2725–2738. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.; Preobrazhenska, O.; Wodarczyk, C.; Medler, Y.; Winkel, A.; Shahab, S.; Huylebroeck, D.; Gross, G.; Verschueren, K. Transforming growth factor-beta-activated kinase-1 (TAK1), a MAP3K, interacts with Smad proteins and interferes with osteogenesis in murine mesenchymal progenitors. J. Biol. Chem. 2005, 280, 27271–27283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassel, S.; Schmitt, S.; Hartung, A.; Roth, M.; Nohe, A.; Petersen, N.; Ehrlich, M.; Henis, Y.I.; Sebald, W.; Knaus, P. Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. JBJS 2003, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Kotzsch, A.; Nickel, J.; Harth, S.; Seher, A.; Mueller, U.; Sebald, W.; Mueller, T.D. A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct. Biol. 2007, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massague, J. Receptors for the TGF-beta family. Cell 1992, 69, 1067–1070. [Google Scholar] [CrossRef]
- Yadin, D.; Knaus, P.; Mueller, T.D. Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 2016, 27, 13–34. [Google Scholar] [CrossRef]
- Hinck, A.P.; Mueller, T.D.; Springer, T.A. Springer, Structural Biology and Evolution of the TGF-beta Family. Cold Spring Harb. Perspect. Biol. 2016, 8, a022103. [Google Scholar] [CrossRef] [Green Version]
- Mueller, T.D.; Nickel, J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012, 586, 1846–1859. [Google Scholar] [CrossRef]
- Nickel, J.; Sebald, W.; Groppe, J.C.; Mueller, T.D. Intricacies of BMP receptor assembly. Cytokine Growth Factor Rev. 2009, 20, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, K.; Seher, A.; Schmitz, W.; Mueller, T.D.; Sebald, W.; Nickel, J. Receptor oligomerization and beyond: A case study in bone morphogenetic proteins. BMC Biol. 2009, 7, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baardsnes, J.; Hinck, C.S.; Hinck, A.P.; O’Connor-McCourt, M.D. TbetaR-II discriminates the high- and low-affinity TGF-beta isoforms via two hydrogen-bonded ion pairs. Biochemistry 2009, 48, 2146–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.; Pobre, E.G.; Mulivor, A.W.; Grinberg, A.V.; Castonguay, R.; Monnell, T.E.; Solban, N.; Ucran, J.A.; Pearsall, R.S.; Underwood, K.W.; et al. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol. Cancer Ther. 2010, 9, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Gray, P.C.; Greenwald, J.; Blount, A.L.; Kunitake, K.S.; Donaldson, C.J.; Choe, S.; Vale, W. Identification of a binding site on the type II activin receptor for activin and inhibin. J. Biol. Chem. 2000, 275, 3206–3212. [Google Scholar] [CrossRef] [Green Version]
- Paralkar, V.M.; Hammonds, R.G.; Reddi, A.H. Identification and characterization of cellular binding proteins (receptors) for recombinant human bone morphogenetic protein 2B, an initiator of bone differentiation cascade. Proc. Natl. Acad. Sci. USA 1991, 88, 3397–3401. [Google Scholar] [CrossRef] [Green Version]
- Baarends, W.M.; Van Helmond, M.J.; Post, M.; van Der Schoot, P.J.; Hoogerbrugge, J.W.; de Winter, J.P.; Uilenbroek, J.T.; Karels, B.; Wilming, L.G.; Meijers, J.H. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the mullerian duct. Development 1994, 120, 189–197. [Google Scholar]
- Gouédard, L.; Chen, Y.G.; Thevenet, L.; Racine, C.; Borie, S.; Lamarre, I.; Josso, N.; Massagué, J.; di Clemente, N. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mullerian hormone and its type II receptor. J. Biol. Chem. 2000, 275, 27973–27978. [Google Scholar] [CrossRef] [Green Version]
- Orvis, G.D.; Jamin, S.P.; Kwan, K.M.; Mishina, Y.; Kaartinen, V.M.; Huang, S.; Roberts, A.B.; Umans, L.; Huylebroeck, D.; Zwijsen, A.; et al. Functional redundancy of TGF-beta family type I receptors and receptor-Smads in mediating anti-Mullerian hormone-induced Mullerian duct regression in the mouse. Biol. Reprod. 2008, 78, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Visser, J.A.; Olaso, R.; Verhoef-Post, M.; Kramer, P.; Themmen, A.P.; Ingraham, H.A. The serine/threonine transmembrane receptor ALK2 mediates Mullerian inhibiting substance signaling. Mol. Endocrinol. 2001, 15, 936–945. [Google Scholar]
- Lin, H.Y.; Wang, X.F.; Ng-Eaton, E.; Weinberg, R.A.; Lodish, H.F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell 1992, 68, 775–785. [Google Scholar] [CrossRef]
- Radaev, S.; Zou, Z.; Huang, T.; Lafer, E.M.; Hinck, A.P.; Sun, P.D. Ternary complex of transforming growth factor-beta1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. J. Biol. Chem. 2010, 285, 14806–14814. [Google Scholar] [CrossRef] [Green Version]
- Groppe, J.; Hinck, C.S.; Samavarchi-Tehrani, P.; Zubieta, C.; Schuermann, J.P.; Taylor, A.B.; Schwarz, P.M.; Wrana, J.L.; Hinck, A.P. Cooperative assembly of TGF-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell 2008, 29, 157–168. [Google Scholar] [CrossRef]
- Hart, P.J.; Deep, S.; Taylor, A.B.; Shu, Z.; Hinck, C.S.; Hinck, A.P. Crystal structure of the human TbetaR2 ectodomain--TGF-beta3 complex. Nat. Struct. Biol. 2002, 9, 203–208. [Google Scholar]
- Zúñiga, J.E.; Groppe, J.C.; Cui, Y.; Hinck, C.S.; Contreras-Shannon, V.; Pakhomova, O.N.; Yang, J.; Tang, Y.; Mendoza, V.; López-Casillas, F.; et al. Assembly of TbetaRI: TbetaRII: TGFbeta ternary complex in vitro with receptor extracellular domains is cooperative and isoform-dependent. J. Mol. Biol. 2005, 354, 1052–1068. [Google Scholar] [CrossRef]
- Mazerbourg, S.; Klein, C.; Roh, J.; Kaivo-Oja, N.; Mottershead, D.G.; Korchynskyi, O.; Ritvos, O.; Hsueh, A.J. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol. Endocrinol. 2004, 18, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Rosendahl, A.; Sideras, P.; ten Dijke, P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002, 21, 1743–1753. [Google Scholar] [CrossRef]
- Maring, J.A.; van Meeteren, L.A.; Goumans, M.J.; ten Dijke, P. Interrogating TGF-beta Function and Regulation in Endothelial Cells. Methods Mol. Biol. 2016, 1344, 193–203. [Google Scholar]
- Ramachandran, A.; Vizan, P.; Das, D.; Chakravarty, P.; Vogt, J.; Rogers, K.W.; Müller, P.; Hinck, A.P.; Sapkota, G.P.; Hill, C.S. TGF-beta uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. Elife 2018, 7, e31756. [Google Scholar] [CrossRef]
- Chang, C. Agonists and Antagonists of TGF-beta Family Ligands. Cold Spring Harb. Perspect. Biol. 2016, 8, a021923. [Google Scholar] [CrossRef] [Green Version]
- Nickel, J.; Ten Dijke, P.; Mueller, T.D. TGF-beta family co-receptor function and signaling. Acta Biochim. Biophys. Sin. 2018, 50, 12–36. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Lin, X.; Feng, X.H. Posttranslational Regulation of Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022087. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Estévez-Salmerón, L.D.; Stroschein, S.L.; Zhu, X.; He, J.; Zhou, S.; Luo, K. The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-{beta} superfamily of cytokines. J. Biol. Chem. 2005, 280, 15992–16001. [Google Scholar] [CrossRef] [Green Version]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef]
- Schmelzer, C.H.; Burton, L.E.; Tamony, C.M.; Schwall, R.H.; Mason, A.J.; Liegeois, N. Purification and characterization of recombinant human activin B. Biochim. Biophys. Acta 1990, 1039, 135–141. [Google Scholar] [CrossRef]
- Huylebroeck, D.; Nimmen, K.V.; Waheed, A.; Figura, K.V.; Marmenout, A.; Fransen, L.; Waele, P.D.; Jaspar, J.M.; Franchimont, P.; Stunnenberg, H.; et al. Expression and processing of the activin-A/erythroid differentiation factor precursor: A member of the transforming growth factor-beta superfamily. Mol. Endocrinol. 1990, 4, 1153–1165. [Google Scholar] [CrossRef] [Green Version]
- Brunner, A.M.; Gentry, L.E.; Cooper, J.A.; Purchio, A.F. Recombinant type 1 transforming growth factor beta precursor produced in Chinese hamster ovary cells is glycosylated and phosphorylated. Mol. Cell Biol. 1988, 8, 2229–2232. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.A.; Rosen, V.; D’Alessandro, J.S.; Bauduy, M.; Cordes, P.; Harada, T.; Israel, D.I.; Hewick, R.M.; Kerns, K.M.; LaPan, P. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA 1990, 87, 2220–2224. [Google Scholar] [CrossRef] [Green Version]
- Madisen, L.; Farrand, A.L.; Lioubin, M.N.; Marzowski, J.; Knox, L.B.; Webb, N.R.; Lim, J.; Purchio, A.F. Expression and characterization of recombinant TGF-beta 2 proteins produced in mammalian cells. DNA 1989, 8, 205–212. [Google Scholar] [CrossRef]
- Graycar, J.L.; Miller, D.A.; Arrick, B.A.; Lyons, R.M.; Moses, H.L.; Derynck, R. Human transforming growth factor-beta 3: Recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and -beta 2. Mol. Endocrinol. 1989, 3, 1977–1986. [Google Scholar] [CrossRef]
- Frolik, C.A.; Wakefield, L.M.; Smith, D.M.; Sporn, M.B. Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J. Biol. Chem. 1984, 259, 10995–11000. [Google Scholar]
- Massague, J. Subunit structure of a high-affinity receptor for type beta-transforming growth factor. Evidence for a disulfide-linked glycosylated receptor complex. J. Biol. Chem. 1985, 260, 7059–7066. [Google Scholar]
- Kondo, S.; Hashimoto, M.; Etoh, Y.; Murata, M.; Shibai, H.; Muramatsu, M. Identification of the two types of specific receptor for activin/EDF expressed on Friend leukemia and embryonal carcinoma cells. Biochem. Biophys. Res. Commun. 1989, 161, 1267–1272. [Google Scholar] [CrossRef]
- Massague, J.; Like, B. Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J. Biol. Chem. 1985, 260, 2636–2645. [Google Scholar]
- Mathews, L.S.; Vale, W.W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell 1991, 65, 973–982. [Google Scholar] [CrossRef]
- Ichijo, H.; Franzén, P.; Schulz, P.; Saras, J.; Toyoshima, H.; Heldin, C.H.; Miyazono, K. Activin receptor-like kinases: A novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene 1993, 8, 2879–2887. [Google Scholar]
- Attisano, L.; Carcamo, J.; Ventura, F.; Weis, F.M.; Massague, J.; Wrana, J.L. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993, 75, 671–680. [Google Scholar] [CrossRef]
- Attisano, L.; Wrana, J.L.; Cheifetz, S.; Massague, J. Novel activin receptors: Distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell 1992, 68, 97–108. [Google Scholar] [CrossRef]
- Nishitoh, H.; Ichijo, H.; Kimura, M.; Matsumoto, T.; Makishima, F.; Yamaguchi, A.; Yamashita, H.; Enomoto, S.; Miyazono, K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J. Biol. Chem. 1996, 271, 21345–21352. [Google Scholar] [CrossRef] [Green Version]
- Storm, E.E.; Kingsley, D.M. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development 1996, 122, 3969–3979. [Google Scholar]
- Yi, S.E.; Daluiski, A.; Pederson, R.; Rosen, V.; Lyons, K.M. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 2000, 127, 621–630. [Google Scholar]
- Erlacher, L.; Mccartney, J.; Piek, E.; Ten Dijke, P.; Yanagishita, M.; Oppermann, H.; Luyten, F.P. Cartilage-derived morphogenetic proteins and osteogenic protein-1 differentially regulate osteogenesis. J. Bone Miner. Res. 1998, 13, 383–392. [Google Scholar] [CrossRef]
- Nickel, J.; Kotzsch, A.; Sebald, W.; Mueller, T.D. A single residue of GDF-5 defines binding specificity to BMP receptor IB. J. Mol. Biol. 2005, 349, 933–947. [Google Scholar] [CrossRef]
- Seemann, P.; Schwappacher, R.; Kjaer, K.W.; Krakow, D.; Lehmann, K.; Dawson, K.; Stricker, S.; Pohl, J.; Plöger, F.; Staub, E.; et al. Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J. Clin. Invest. 2005, 115, 2373–2381. [Google Scholar] [CrossRef] [Green Version]
- Bessa, P.C.; Cerqueira, M.T.; Rada, T.; Gomes, M.E.; Neves, N.M.; Nobre, A.; Reis, R.L.; Casal, M. Expression, purification and osteogenic bioactivity of recombinant human BMP-4, -9, -10, -11 and -14. Protein Expr Purif 2009, 63, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Kotzsch, A.; Nickel, J.; Seher, A.; Sebald, W.; Müller, T.D. Crystal structure analysis reveals a spring-loaded latch as molecular mechanism for GDF-5-type I receptor specificity. EMBO J. 2009, 28, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Ide, H.; Katoh, M.; Sasaki, H.; Yoshida, T.; Aoki, K.; Nawa, Y.; Osada, Y.; Sugimura, T.; Terada, M. Cloning of human bone morphogenetic protein type IB receptor (BMPR-IB) and its expression in prostate cancer in comparison with other BMPRs. Oncogene 1997, 14, 1377–1382. [Google Scholar] [CrossRef]
- Zou, H.; Wieser, R.; Massagué, J.; Niswander, L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997, 11, 2191–2203. [Google Scholar] [CrossRef] [Green Version]
- Nikaido, M.; Tada, M.; Ueno, N. Restricted expression of the receptor serine/threonine kinase BMPR-IB in zebrafish. Mech. Dev. 1999, 82, 219–222. [Google Scholar] [CrossRef]
- Akiyama, S.; Katagiri, T.; Namiki, M.; Yamaji, N.; Yamamoto, N.; Miyama, K.; Shibuya, H.; Ueno, N.; Wozney, J.M.; Suda, T. Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp. Cell Res. 1997, 235, 362–369. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.Y.; Danielson, P.D.; Shah, P.C.; Rockwell, S.; Lechleider, R.J.; Martin, J.; Manganaro, T.; Donahoe, P.K. The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell 1996, 86, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Chaikuad, A.; Alfano, I.; Kerr, G.; Sanvitale, C.E.; Boergermann, J.H.; Triffitt, J.T.; von Delft, F.; Knapp, S.; Knaus, P.; Bullock, A.N. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J. Biol. Chem. 2012, 287, 36990–36998. [Google Scholar] [CrossRef] [Green Version]
- Huse, M.; Chen, Y.G.; Massagué, J.; Kuriyan, J. Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell 1999, 96, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Groppe, J.C.; Wu, J.; Shore, E.M.; Kaplan, F.S. In vitro analyses of the dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs 2011, 194, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Wieser, R.; Wrana, J.L.; Massague, J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995, 14, 2199–2208. [Google Scholar] [CrossRef]
- Souchelnytskyi, S.; Ten Dijke, P.; Miyazono, K.; Heldin, C.H. Phosphorylation of Ser165 in TGF-beta type I receptor modulates TGF-beta1-induced cellular responses. EMBO J. 1996, 15, 6231–6240. [Google Scholar] [CrossRef]
- Rosenzweig, B.L.; Imamura, T.; Okadome, T.; Cox, G.N.; Yamashita, H.; Ten Dijke, P.; Heldin, C.H.; Miyazono, K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 7632–7636. [Google Scholar] [CrossRef] [Green Version]
- Rudarakanchana, N.; Flanagan, J.A.; Chen, H.; Upton, P.D.; Machado, R.; Patel, D.; Trembath, R.C.; Morrell, N.W. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum. Mol. Genet. 2002, 11, 1517–1525. [Google Scholar] [CrossRef] [Green Version]
- Hassel, S.; Eichner, A.; Yakymovych, M.; Hellman, U.; Knaus, P.; Souchelnytskyi, S. Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 1346–1358. [Google Scholar] [CrossRef]
- Perron, J.C.; Dodd, J. ActRIIA and BMPRII Type II BMP receptor subunits selectively required for Smad4-independent BMP7-evoked chemotaxis. PLoS ONE 2019, 4, e8198. [Google Scholar] [CrossRef]
- Attisano, L.; Wrana, J.L.; Montalvo, E.; Massague, J. Activation of signalling by the activin receptor complex. Mol. Cell Biol. 1996, 16, 1066–1073. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.P.; Yeo, C.Y.; Lee, Y.; Schrewe, H.; Whitman, M.; Li, E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002, 16, 2749–2754. [Google Scholar] [CrossRef] [Green Version]
- New, H.V.; Kavka, A.I.; Smith, J.C.; Green, J.B.A. Differential effects on Xenopus development of interference with type IIA and type IIB activin receptors. Mech. Dev. 1997, 61, 175–186. [Google Scholar] [CrossRef]
- Allendorph, G.P.; Vale, W.W.; Choe, S. Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc. Natl. Acad. Sci. USA 2006, 103, 7643–7648. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Hong, K.H.; Yun, J.; Oh, S.P. Generation of activin receptor type IIB isoform-specific hypomorphic alleles. Genesis 2006, 44, 487–494. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, R.; Chen, D.; Oyajobi, B.O.; Zhao, M. Functional redundancy of type II BMP receptor and type IIB activin receptor in BMP2-induced osteoblast differentiation. J. Cell. Physiol. 2012, 227, 952–963. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.O.; Sieber, C.; Bhushan, R.; Börgermann, J.H.; Graf, D. and Knaus, P. BMPs: From bone to body morphogenetic proteins. Sci. Signal. 2010, 3, mr1. [Google Scholar]
- Greenwald, J.; Groppe, J.; Gray, P.; Wiater, E.; Kwiatkowski, W.; Vale, W.; Choe, S. The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol. Cell 2003, 11, 605–617. [Google Scholar] [CrossRef]
- Sako, D.; Grinberg, A.V.; Liu, J.; Davies, M.V.; Castonguay, R.; Maniatis, S.; Andreucci, A.J.; Pobre, E.G.; Tomkinson, K.N.; Monnell, T.E.; et al. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. J. Biol. Chem. 2010, 285, 21037–21048. [Google Scholar] [CrossRef] [Green Version]
- Wiater, E.; Vale, W. Inhibin is an antagonist of bone morphogenetic protein signaling. J. Biol. Chem. 2003, 278, 7934–7941. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, E.; Paul, H.; Friedle, H.; Metz, A.; Scheucher, M.; Clement, J.H.; Knöchel, W. Antagonistic actions of activin A and BMP-2/4 control dorsal lip-specific activation of the early response gene XFD-1’ in Xenopus laevis embryos. EMBO J. 1996, 15, 6739–6749. [Google Scholar] [CrossRef]
- Candia, A.F.; Watabe, T.; Hawley, S.H.; Onichtchouk, D.; Zhang, Y.; Derynck, R.; Niehrs, C.; Cho, K.W. Cellular interpretation of multiple TGF-beta signals: Intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development 1997, 124, 4467–4480. [Google Scholar]
- Olsen, O.E.; Wader, K.F.; Hella, H.; Mylin, A.K.; Turesson, I.; Nesthus, I.; Waage, A.; Sundan, A.; Holien, T. Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun. Signal. 2015, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Seher, A.; Lagler, C.; Stühmer, T.; Müller-Richter, U.D.A.; Kübler, A.C.; Sebald, W.; Müller, T.D.; Nickel, J. Utilizing BMP-2 muteins for treatment of multiple myeloma. PLoS ONE 2017, 12, e0174884. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Ten Dijke, P.; Heldin, C.H.; Miyazono, K. Bone morphogenetic protein receptors. Bone 1996, 19, 569–574. [Google Scholar] [CrossRef]
- ten Dijke, P.; Yamashita, H.; Sampath, T.K.; Reddi, A.H.; Estevez, M.; Riddle, D.L.; Ichijo, H.; Heldin, C.H.; Miyazono, K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 1994, 269, 16985–16988. [Google Scholar]
- Klammert, U.; Mueller, T.D.; Hellmann, T.V.; Wuerzler, K.K.; Kotzsch, A.; Schliermann, A.; Schmitz, W.; Kuebler, A.C.; Sebald, W.; Nickel, J. GDF-5 can act as a context-dependent BMP-2 antagonist. BMC Biol. 2015, 13, 77. [Google Scholar] [CrossRef] [Green Version]
- Macias-Silva, M.; Hoodless, P.A.; Tang, S.J.; Buchwald, M.; Wrana, J.L. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 1998, 273, 25628–25636. [Google Scholar] [CrossRef] [Green Version]
- Ebisawa, T.; Tada, K.; Kitajima, I.; Tojo, K.; Sampath, T.K.; Kawabata, M.; Miyazono, K.; Imamura, T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 1999, 112, 3519–3527. [Google Scholar]
- Saremba, S.; Nickel, J.; Seher, A.; Kotzsch, A.; Sebald, W.; Mueller, T.D. Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. FEBS J. 2008, 275, 172–183. [Google Scholar] [CrossRef]
- Harth, S. Molecular recognition in BMP ligand-receptor interactions. Ph.D. Thesis, Julius-Maximilians University, Wuerzburg, Germany, 2010. [Google Scholar]
- Harth, S.; Kotzsch, A.; Hu, J.; Sebald, W.; Mueller, T.D. A selection fit mechanism in BMP receptor IA as a possible source for BMP ligand-receptor promiscuity. PLoS ONE 2010, 5, e13049. [Google Scholar] [CrossRef] [PubMed]
- Traeger, L.; Gallitz, I.; Sekhri, R.; Bäumer, N.; Kuhlmann, T.; Kemming, C.; Holtkamp, M.; Müller, J.C.; Karst, U.; Canonne-Hergaux, F.; et al. ALK3 undergoes ligand-independent homodimerization and BMP-induced heterodimerization with ALK2. Free Radic. Biol. Med. 2018, 129, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Danielpour, D.; Sporn, M.B. Differential inhibition of transforming growth factor beta 1 and beta 2 activity by alpha 2-macroglobulin. J. Biol. Chem. 1990, 265, 6973–6977. [Google Scholar]
- De Crescenzo, G.; Pham, P.L.; Durocher, Y.; D O’Connor-McCourt, M. Transforming growth factor-beta (TGF-beta) binding to the extracellular domain of the type II TGF-beta receptor: Receptor capture on a biosensor surface using a new coiled-coil capture system demonstrates that avidity contributes significantly to high affinity binding. J. Mol. Biol. 2003, 328, 1173–1183. [Google Scholar]
- Ling, N.; Ying, S.Y.; Ueno, N.; Shimasaki, S.; Esch, F.; Hotta, M.; Guillemin, R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature 1986, 321, 779–782. [Google Scholar] [CrossRef]
- Pangas, S.A.; Woodruff, T.K. Activin signal transduction pathways. Trends Endocrinol. Metab. 2000, 11, 309–314. [Google Scholar] [CrossRef]
- Künnapuu, J.; Tauscher, P.M.; Tiusanen, N.; Nguyen, M.; Löytynoja, A.; Arora, K.; Shimmi, O. Cleavage of the Drosophila screw prodomain is critical for a dynamic BMP morphogen gradient in embryogenesis. Dev. Biol. 2014, 389, 149–159. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, M.B.; Umulis, D.; Othmer, H.G.; Blair, S.S. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 2006, 133, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Schmid, B.; Furthauer, M.; Connors, S.A.; Trout, J.; Thisse, B.; Thisse, C.; Mullins, M.C. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 2000, 127, 957–967. [Google Scholar]
- Shimmi, O.; Umulis, D.; Othmer, H.; O’Connor, M.B. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 2005, 120, 873–886. [Google Scholar] [CrossRef] [Green Version]
- Hazama, M.; Aono, A.; Ueno, N.; Fujisawa, Y. Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem. Biophys. Res. Commun. 1995, 209, 859–866. [Google Scholar] [CrossRef]
- Israel, D.I.; Nove, J.; Kerns, K.M.; Kaufman, R.J.; Rosen, V.; Cox, K.A.; Wozney, J.M. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 1996, 13, 291–300. [Google Scholar] [CrossRef]
- McNatty, K.P.; Juengel, J.L.; Reader, K.L.; Lun, S.; Myllymaa, S.; Lawrence, S.B.; Western, A.; Meerasahib, M.F.; Mottershead, D.G.; Groome, N.P.; et al. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function. Reproduction 2005, 129, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Mottershead, D.G.; Harrison, C.A.; Mueller, T.D.; Stanton, P.G.; Gilchrist, R.B.; McNatty, K.P. Growth differentiation factor 9: Bone morphogenetic protein 15 (GDF9:BMP15) synergism and protein heterodimerization. Proc. Natl. Acad. Sci. USA 2013, 110, E2257. [Google Scholar] [CrossRef] [Green Version]
- Mottershead, D.G.; Sugimura, S.; Al-Musawi, S.L.; Li, J.J.; Richani, D.; White, M.A.; Martin, G.A.; Trotta, A.P.; Ritter, L.J.; Shi, J.; et al. Cumulin, an Oocyte-secreted Heterodimer of the Transforming Growth Factor-beta Family, Is a Potent Activator of Granulosa Cells and Improves Oocyte Quality. J. Biol. Chem. 2015, 290, 24007–24020. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Li, Q.; Wigglesworth, K.; Rangarajan, A.; Kattamuri, C.; Peterson, R.T.; Eppig, J.J.; Thompson, T.B.; Matzuk, M.M. Growth differentiation factor 9: Bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl. Acad. Sci. USA 2013, 110, E776–E785. [Google Scholar] [CrossRef] [Green Version]
- Valera, E.; Isaacs, M.J.; Kawakami, Y.; Belmonte, J.C.I.; Choe, S. BMP-2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS ONE 2010, 5, e11167. [Google Scholar] [CrossRef] [Green Version]
- Aono, A.; Hazama, M.; Notoya, K.; Taketomi, S.; Yamasaki, H.; Tsukuda, R.; Sasaki, S.; Fujisawa, Y. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem. Biophys. Res. Commun. 1995, 210, 670–677. [Google Scholar] [CrossRef]
- C Miao, C.; Qin, D.; Cao, P.; Lu, P.; Xia, Y.; Li, M.; Sun, M.; Zhang, W.; Yang, F.; Zhang, Y. BMP2/7 heterodimer enhances osteogenic differentiation of rat BMSCs via ERK signaling compared with respective homodimers. J. Cell. Biochem. 2018, 120, 8754–8763. [Google Scholar] [CrossRef]
- Morimoto, T.; Kaito, T.; Matsuo, Y.; Sugiura, T.; Kashii, M.; Makino, T.; Iwasaki, M.; Yoshikawa, H. The bone morphogenetic protein-2/7 heterodimer is a stronger inducer of bone regeneration than the individual homodimers in a rat spinal fusion model. Spine J. 2015, 15, 1379–1390. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.; Zheng, Y.; Fan, Y.; Gu, Z. BMP2/7 heterodimer is a stronger inducer of bone regeneration in peri-implant bone defects model than BMP2 or BMP7 homodimer. Dent. Mater. J. 2012, 31, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Kaneko, E.; Maeda, J.; Ueno, N. Mesoderm induction by BMP-4 and -7 heterodimers. Biochem. Biophys. Res. Commun. 1997, 232, 153–156. [Google Scholar] [CrossRef]
- Little, S.C.; Mullins, M.C. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat. Cell Biol. 2009, 11, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, M.J.; Kawakami, Y.; Allendorph, G.P.; Yoon, B.H.; Belmonte, J.C.I.; Choe, S. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol. Endocrinol. 2010, 24, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Seeherman, H.J.; Berasi, S.P.; Brown, C.T.; Martinez, R.X.; Juo, Z.S.; Jelinsky, S.; Cain, M.J.; Grode, J.; Tumelty, K.E.; Bohner, M.; et al. A BMP/activin A chimera is superior to native BMPs and induces bone repair in nonhuman primates when delivered in a composite matrix. Sci. Transl. Med. 2019, 11, eaar4953. [Google Scholar] [CrossRef]
- Sampath, T.K.; Coughlin, J.E.; Whetstone, R.M.; Banach, D.; Corbett, C.; Ridge, R.J.; Ozkaynak, E.; Oppermann, H.; Rueger, D.C. Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming growth factor-beta superfamily. J. Biol. Chem. 1990, 265, 13198–13205. [Google Scholar]
- Wang, E.A.; Rosen, V.; Cordes, P.; Hewick, R.M.; Kriz, M.J.; Luxenberg, D.P.; Sibley, B.S.; Wozney, J.M. Purification and characterization of other distinct bone-inducing factors. Proc. Natl. Acad. Sci. USA 1988, 85, 9484–9488. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Neugebauer, J.; McKnite, A.; Tilak, A.; Christian, J.L. BMP7 functions predominantly as a heterodimer with BMP2 or BMP4 during mammalian embryogenesis. Elife 2019, 8, e48872. [Google Scholar] [CrossRef]
- Tillet, E.; Ouarné, M.; Desroches-Castan, A.; Mallet, C.; Subileau, M.; Didier, R.; Lioutsko, A.; Belthier, G.; Feige, J.J.; Bailly, S. A heterodimer formed by bone morphogenetic protein 9 (BMP9) and BMP10 provides most BMP biological activity in plasma. J. Biol. Chem. 2018, 293, 10963–10974. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-beta structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.G.; McCoy, J.C.; Czepnik, M.; Mills, M.J.; Hagg, A.; Walton, K.L.; Cotton, T.R.; Hyvönen, M.; Lee, R.T.; Gregorevic, P.; et al. Molecular characterization of latent GDF8 reveals mechanisms of activation. Proc. Natl. Acad. Sci. USA 2018, 115, E866–E875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Fischer, G.; Hyvönen, M. Structure and activation of pro-activin A. Nat. Commun. 2016, 7, 12052. [Google Scholar]
- Zhao, B.; Xu, S.; Dong, X.; Lu, C.; Springer, T.A. Prodomain-growth factor swapping in the structure of pro-TGF-beta1. J. Biol. Chem. 2018, 293, 1579–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.Q. Consequences of knocking out BMP signaling in the mouse. Genesis 2003, 35, 43–56. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nickel, J.; Mueller, T.D. Specification of BMP Signaling. Cells 2019, 8, 1579. https://doi.org/10.3390/cells8121579
Nickel J, Mueller TD. Specification of BMP Signaling. Cells. 2019; 8(12):1579. https://doi.org/10.3390/cells8121579
Chicago/Turabian StyleNickel, Joachim, and Thomas D. Mueller. 2019. "Specification of BMP Signaling" Cells 8, no. 12: 1579. https://doi.org/10.3390/cells8121579
APA StyleNickel, J., & Mueller, T. D. (2019). Specification of BMP Signaling. Cells, 8(12), 1579. https://doi.org/10.3390/cells8121579




