H2O2 as a Feedback Signal on Dual-Located WHIRLY1 Associates with Leaf Senescence in Arabidopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.2. Chlorophyll Fluorescence and Concentration Measurement
2.3. Measurement of H2O2 Content in Rosette Leaves
2.4. Staining of Hydrogen Peroxide
2.5. Quantitative RT-PCR Analysis (qRT-PCR)
2.6. In Vitro DNA-Binding Assays
2.7. Isolation and Detection of Plastid and Nuclear Proteins
2.8. ChIP-qPCR Assay
2.9. Statistical Analysis
3. Results
3.1. Ectopic Expression of a Plastid Isoform WHY1 Causes a Strong Leaf Senescence
3.2. Allocation of WHY1 into Plastid-Enhanced Production of Reactive Oxygen Species (ROS)
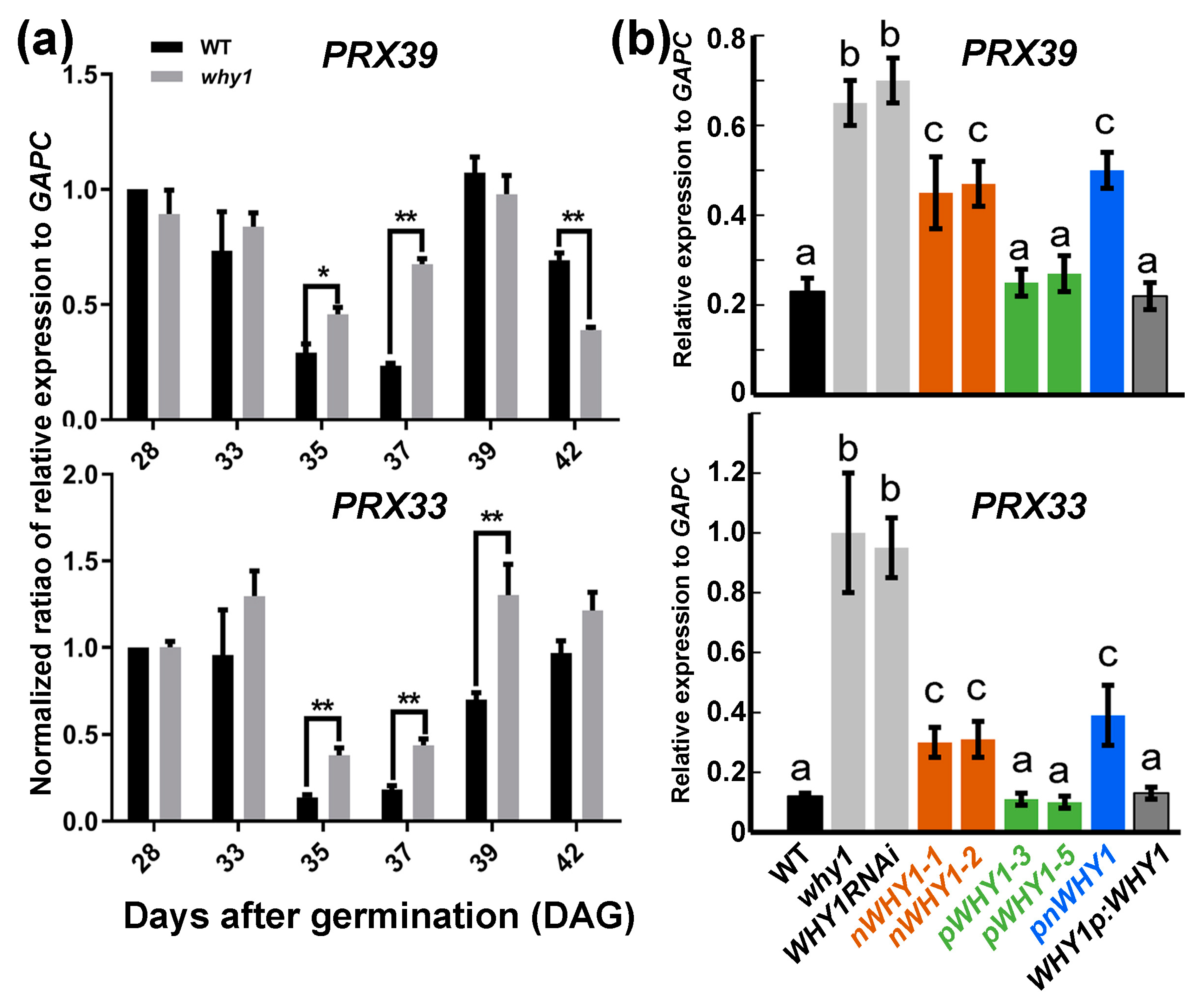
3.3. PRX33 and PRX39 were Downstream of WHY1 but with no Obvious Involvement in WHY1-Mediated ROS Pathway
3.4. H2O2 Treatments Altered WHY1 Protein Distribution Between Plastids and the Nucleus but Not Its mRNA Levels
3.5. H2O2 Induces the Enrichment of H3K9ac and RNAP II at WRKY53 Promoter Region
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kleine, T.; Voigt, C.; Leister, D. Plastid signalling to the nucleus: Messengers still lost in the mists? Trends Genet. 2009, 25, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kleine, T.; Leister, D. Retrograde signaling: Organelles go networking. Biochim. Biophys. Acta 2016, 1857, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.X.; Phua, S.Y.; Crisp, P.; McQuinn, R.; Pogson, B.J. Learning the languages of the chloroplast: Retrograde signaling and beyond. Annu. Rev. Plant Biol. 2016, 67, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Kim, C.; Landgraf, F.; Apel, K. Executer1- and executer2-dependent transfer of stress-related signals from the plastid to the nucleus of arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 10270–10275. [Google Scholar] [CrossRef] [Green Version]
- Bobik, K.; Burch-Smith, T.M. Chloroplast signaling within, between and beyond cells. Front. Plant Sci. 2015, 6, 781. [Google Scholar] [CrossRef] [Green Version]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for a sal1-pap chloroplast retrograde pathway that functions in drought and high light signaling in arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Savchenko, T.; Baidoo, E.E.; Chehab, W.E.; Hayden, D.M.; Tolstikov, V.; Corwin, J.A.; Kliebenstein, D.J.; Keasling, J.D.; Dehesh, K. Retrograde signaling by the plastidial metabolite mecpp regulates expression of nuclear stress-response genes. Cell 2012, 149, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Woodson, J.D.; Perez-Ruiz, J.M.; Chory, J. Heme synthesis by plastid ferrochelatase i regulates nuclear gene expression in plants. Curr. Biol. 2011, 21, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Nevarez, P.A.; Qiu, Y.; Inoue, H.; Yoo, C.Y.; Benfey, P.N.; Schnell, D.J.; Chen, M. Mechanism of dual targeting of the phytochrome signaling component hemera/ptac12 to plastids and the nucleus. Plant Physiol. 2017, 173, 1953–1966. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Luo, L.; Xu, J.; Xin, P.; Guo, H.; Wu, J.; Bai, L.; Wang, G.; Chu, J.; Zuo, J.; et al. Malate transported from chloroplast to mitochondrion triggers production of ros and pcd in arabidopsis thaliana. Cell Res. 2018, 28, 448–461. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Feng, P.; Xu, X.; Guo, H.; Ma, J.; Chi, W.; Lin, R.; Lu, C.; Zhang, L. A chloroplast envelope-bound phd transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2011, 2, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.J.; Vogel, M.O.; Viehhauser, A. Ap2/erebp transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma 2010, 245, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Wang, P.; Korkaric, M.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. Glk transcription factors coordinate expression of the photosynthetic apparatus in arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Seo, J.K.; Gao, S.; Cui, X.; Jin, H. Silencing of atrap, a target gene of a bacteria-induced small rna, triggers antibacterial defense responses through activation of lsu2 and down-regulation of glk1. New Phytol. 2017, 215, 1144–1155. [Google Scholar] [CrossRef] [Green Version]
- Townsend, P.D.; Dixon, C.H.; Slootweg, E.J.; Sukarta, O.C.A.; Yang, A.W.H.; Hughes, T.R.; Sharples, G.J.; Palsson, L.O.; Takken, F.L.W.; Goverse, A.; et al. The intracellular immune receptor rx1 regulates the DNA-binding activity of a golden2-like transcription factor. J. Biol. Chem. 2018, 293, 3218–3233. [Google Scholar] [CrossRef] [Green Version]
- Lv, R.; Li, Z.; Li, M.; Dogra, V.; Lv, S.; Liu, R.; Lee, K.P.; Kim, C. Uncoupled expression of nuclear and plastid photosynthesis-associated genes contributes to cell death in a lesion mimic mutant. Plant Cell 2019, 31, 210–230. [Google Scholar] [CrossRef] [Green Version]
- Krause, K.; Kilbienski, I.; Mulisch, M.; Rodiger, A.; Schafer, A.; Krupinska, K. DNA-binding proteins of the whirly family in arabidopsis thaliana are targeted to the organelles. FEBS Lett. 2005, 579, 3707–3712. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, E.; Miao, Y.; Mulisch, M.; Krupinska, K. Single-stranded DNA-binding protein whirly1 in barley leaves is located in plastids and the nucleus of the same cell. Plant Physiol. 2008, 147, 1800–1804. [Google Scholar] [CrossRef] [Green Version]
- Isemer, R.; Mulisch, M.; Schafer, A.; Kirchner, S.; Koop, H.U.; Krupinska, K. Recombinant whirly1 translocates from transplastomic chloroplasts to the nucleus. FEBS Lett. 2012, 586, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Li, Y.; Jiang, Y.; Wu, B.; Miao, Y. Phosphorylation of whirly1 by cipk14 shifts its localization and dual functions in arabidopsis. Mol. Plant 2017, 10, 749–763. [Google Scholar] [CrossRef] [Green Version]
- Desveaux, D.; Despres, C.; Joyeux, A.; Subramaniam, R.; Brisson, N. Pbf-2 is a novel single-stranded DNA binding factor implicated in pr-10a gene activation in potato. Plant Cell 2000, 12, 1477–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desveaux, D.; Subramaniam, R.; Despres, C.; Mess, J.N.; Levesque, C.; Fobert, P.R.; Dangl, J.L.; Brisson, N. A “whirly” transcription factor is required for salicylic acid-dependent disease resistance in arabidopsis. Dev. Cell 2004, 6, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Jiang, J.; Ren, Y.; Zhao, Z. The single-stranded DNA-binding protein whirly1 represses wrky53 expression and delays leaf senescence in a developmental stage-dependent manner in arabidopsis. Plant Physiol. 2013, 163, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupinska, K.; Dahnhardt, D.; Fischer-Kilbienski, I.; Kucharewicz, W.; Scharrenberg, C.; Trosch, M.; Buck, F. Identification of whirly1 as a factor binding to the promoter of the stress- and senescence-associated gene hvs40. J. Plant Growth Regul. 2014, 33, 91–105. [Google Scholar] [CrossRef]
- Zhuang, K.; Kong, F.; Zhang, S.; Meng, C.; Yang, M.; Liu, Z.; Wang, Y.; Ma, N.; Meng, Q. Whirly1 enhances tolerance to chilling stress in tomato via protection of photosystem ii and regulation of starch degradation. New Phytol. 2019, 221, 1998–2012. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.H.; Kwon, C.; Lee, M.M.; Chung, I.K. Single-stranded DNA binding factor atwhy1 modulates telomere length homeostasis in arabidopsis. Plant J. 2007, 49, 442–451. [Google Scholar] [CrossRef]
- Huang, D.; Lan, W.; Li, D.; Deng, B.; Lin, W.; Ren, Y.; Miao, Y. Whirly1 occupancy affects histone lysine modification and wrky53 transcription in arabidopsis developmental manner. Front. Plant Sci. 2018, 9, 1503. [Google Scholar] [CrossRef]
- Pfalz, J.; Liere, K.; Kandlbinder, A.; Dietz, K.J.; Oelmuller, R. Ptac2, −6, and −12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell 2006, 18, 176–197. [Google Scholar] [CrossRef] [Green Version]
- Prikryl, J.; Watkins, K.P.; Friso, G.; van Wijk, K.J.; Barkan, A. A member of the whirly family is a multifunctional rna- and DNA-binding protein that is essential for chloroplast biogenesis. Nucleic Acids Res. 2008, 36, 5152–5165. [Google Scholar] [CrossRef]
- Melonek, J.; Mulisch, M.; Schmitz-Linneweber, C.; Grabowski, E.; Hensel, G.; Krupinska, K. Whirly1 in chloroplasts associates with intron containing rnas and rarely co-localizes with nucleoids. Planta 2010, 232, 471–481. [Google Scholar] [CrossRef]
- Cappadocia, L.; Marechal, A.; Parent, J.S.; Lepage, E.; Sygusch, J.; Brisson, N. Crystal structures of DNA-whirly complexes and their role in arabidopsis organelle genome repair. Plant Cell 2010, 22, 1849–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappadocia, L.; Parent, J.S.; Zampini, E.; Lepage, E.; Sygusch, J.; Brisson, N. A conserved lysine residue of plant whirly proteins is necessary for higher order protein assembly and protection against DNA damage. Nucleic Acids Res. 2012, 40, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Lepage, E.; Zampini, E.; Brisson, N. Plastid genome instability leads to reactive oxygen species production and plastid-to-nucleus retrograde signaling in arabidopsis. Plant Physiol. 2013, 163, 867–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Karpinska, B.; Krupinska, K. The functions of whirly1 and redox-responsive transcription factor 1 in cross tolerance responses in plants: A hypothesis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130226. [Google Scholar] [CrossRef] [Green Version]
- Krause, K.; Krupinska, K. Nuclear regulators with a second home in organelles. Trends Plant Sci. 2009, 14, 194–199. [Google Scholar] [CrossRef]
- Comadira, G.; Rasool, B.; Kaprinska, B.; Garcia, B.M.; Morris, J.; Verrall, S.R.; Bayer, M.; Hedley, P.E.; Hancock, R.D.; Foyer, C.H. Whirly1 functions in the control of responses to nitrogen deficiency but not aphid infestation in barley. Plant Physiol. 2015, 168, 1140–1151. [Google Scholar] [CrossRef]
- Huang, D.; Lin, W.; Deng, B.; Ren, Y.; Miao, Y. Dual-located whirly1 interacting with lhca1 alters photochemical activities of photosystem i and is involved in light adaptation in arabidopsis. Int. J. Mol. Sci. 2017, 18, 2352. [Google Scholar] [CrossRef] [Green Version]
- Hinderhofer, K.; Zentgraf, U. Identification of a transcription factor specifically expressed at the onset of leaf senescence. Planta 2001, 213, 469–473. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; He, J.; Qin, Y.; Hua, D.; Duan, Y.; Chen, Z.; Gong, Z. Aba-mediated ros in mitochondria regulate root meristem activity by controlling plethora expression in arabidopsis. PLoS Genet. 2014, 10, e1004791. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yu, L.J.; Zhang, X.; Fan, B.; Wang, F.Z.; Dai, Y.S.; Qi, H.; Zhou, Y.; Xie, L.J.; Xiao, S. Autophagy regulates glucose-mediated root meristem activity by modulating ros production in arabidopsis. Autophagy 2019, 15, 407–422. [Google Scholar] [CrossRef] [Green Version]
- Antreich, S.; Sassmann, S.; Lang, I. Limited accumulation of copper in heavy metal adapted mosses. Plant Physiol. Biochem. 2016, 101, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lee, H.J.; Xiong, L.M.; Zhu, J.K. A mitochondrial complex i defect impairs cold-regulated nuclear gene expression. Plant Cell 2002, 14, 1235–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Zentgraf, U. The antagonist function of arabidopsis wrky53 and esr/esp in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 2007, 19, 819–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallon, O.; Høyer-Hansen, G.; Simpson, D.J. Photosystem ii and cytochromeb-559 in the stroma lamellae of barley chloroplasts. Carlsberg Res. Commun. 1987, 52, 405. [Google Scholar] [CrossRef] [Green Version]
- Buet, A.; Costa, M.L.; Martinez, D.E.; Guiamet, J.J. Chloroplast protein degradation in senescing leaves: Proteases and lytic compartments. Front. Plant Sci. 2019, 10, 747. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Porcheron, B.; Hennion, N.; Maurousset, L.; Lemoine, R.; Pourtau, N. Water deficit enhances c export to the roots in arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol. 2016, 170, 1460–1479. [Google Scholar] [CrossRef] [Green Version]
- James, M.; Masclaux-Daubresse, C.; Marmagne, A.; Azzopardi, M.; Laine, P.; Goux, D.; Etienne, P.; Trouverie, J. A new role for sag12 cysteine protease in roots of arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1998. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class iii peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. Plantcare, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Zentgraf, U.; Laun, T.; Miao, Y. The complex regulation of wrky53 during leaf senescence of arabidopsis thaliana. Eur. J. Cell Biol. 2010, 89, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016, 171, 1535–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, P.; Li, Z.; Huang, P.; Li, B.; Fang, S.; Chu, J.; Guo, H. A tripartite amplification loop involving the transcription factor wrky75, salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell 2017, 29, 2854–2870. [Google Scholar] [CrossRef] [PubMed]
- Brusslan, J.A.; Bonora, G.; Rus-Canterbury, A.M.; Tariq, F.; Jaroszewicz, A.; Pellegrini, M. A genome-wide chronological study of gene expression and two histone modifications, h3k4me3 and h3k9ac, during developmental leaf senescence. Plant Physiol. 2015, 168, 1246–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ay, N.; Irmler, K.; Fischer, A.; Uhlemann, R.; Reuter, G.; Humbeck, K. Epigenetic programming via histone methylation at wrky53 controls leaf senescence in arabidopsis thaliana. Plant J. 2009, 58, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Sharma, R.; Handa, N.; Kaur, H.; Rattan, A.; Yadav, P.; Gautam, V.; Kaur, R.; Bhardwaj, R. Redox homeostasis in plants under abiotic stress: Role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front. Environ. Sci. 2015, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Janku, M.; Luhova, L.; Petrivalsky, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. Nadph oxidase atrbohd and atrbohf genes function in ros-dependent aba signaling in arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Khokon, A.R.; Okuma, E.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in arabidopsis. Plant Cell Environ. 2011, 34, 434–443. [Google Scholar] [CrossRef]
- Yuan, H.M.; Liu, W.C.; Lu, Y.T. Catalase2 coordinates sa-mediated repression of both auxin accumulation and ja biosynthesis in plant defenses. Cell Host Microbe 2017, 21, 143–155. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Z.; Wang, W.; Yu, X.; Lin, W.; Miao, Y. Comparative proteomic analysis of coregulation of cipk14 and whirly1/3 mediated pale yellowing of leaves in arabidopsis. Int. J. Mol. Sci. 2018, 19, 2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dat, J.; Vandenabeele, S.; Vranova, E.; Van Montagu, M.; Inze, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Huang, D.; Shi, X.; Deng, B.; Ren, Y.; Lin, W.; Miao, Y. H2O2 as a Feedback Signal on Dual-Located WHIRLY1 Associates with Leaf Senescence in Arabidopsis. Cells 2019, 8, 1585. https://doi.org/10.3390/cells8121585
Lin W, Huang D, Shi X, Deng B, Ren Y, Lin W, Miao Y. H2O2 as a Feedback Signal on Dual-Located WHIRLY1 Associates with Leaf Senescence in Arabidopsis. Cells. 2019; 8(12):1585. https://doi.org/10.3390/cells8121585
Chicago/Turabian StyleLin, Wenfang, Dongmei Huang, Ximiao Shi, Ban Deng, Yujun Ren, Wenxiong Lin, and Ying Miao. 2019. "H2O2 as a Feedback Signal on Dual-Located WHIRLY1 Associates with Leaf Senescence in Arabidopsis" Cells 8, no. 12: 1585. https://doi.org/10.3390/cells8121585




