NLRP3 Inflammasome and Mineralocorticoid Receptors Are Associated with Vascular Dysfunction in Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vascular Reactivity
2.3. IL-1β and Aldosterone Measurement
2.4. Western Blotting
2.5. Caspase-1 Activity by Flow Cytometry Analysis
2.6. Lucigenin
2.7. Drugs
2.8. Data Analysis and Statistical Procedures
3. Results
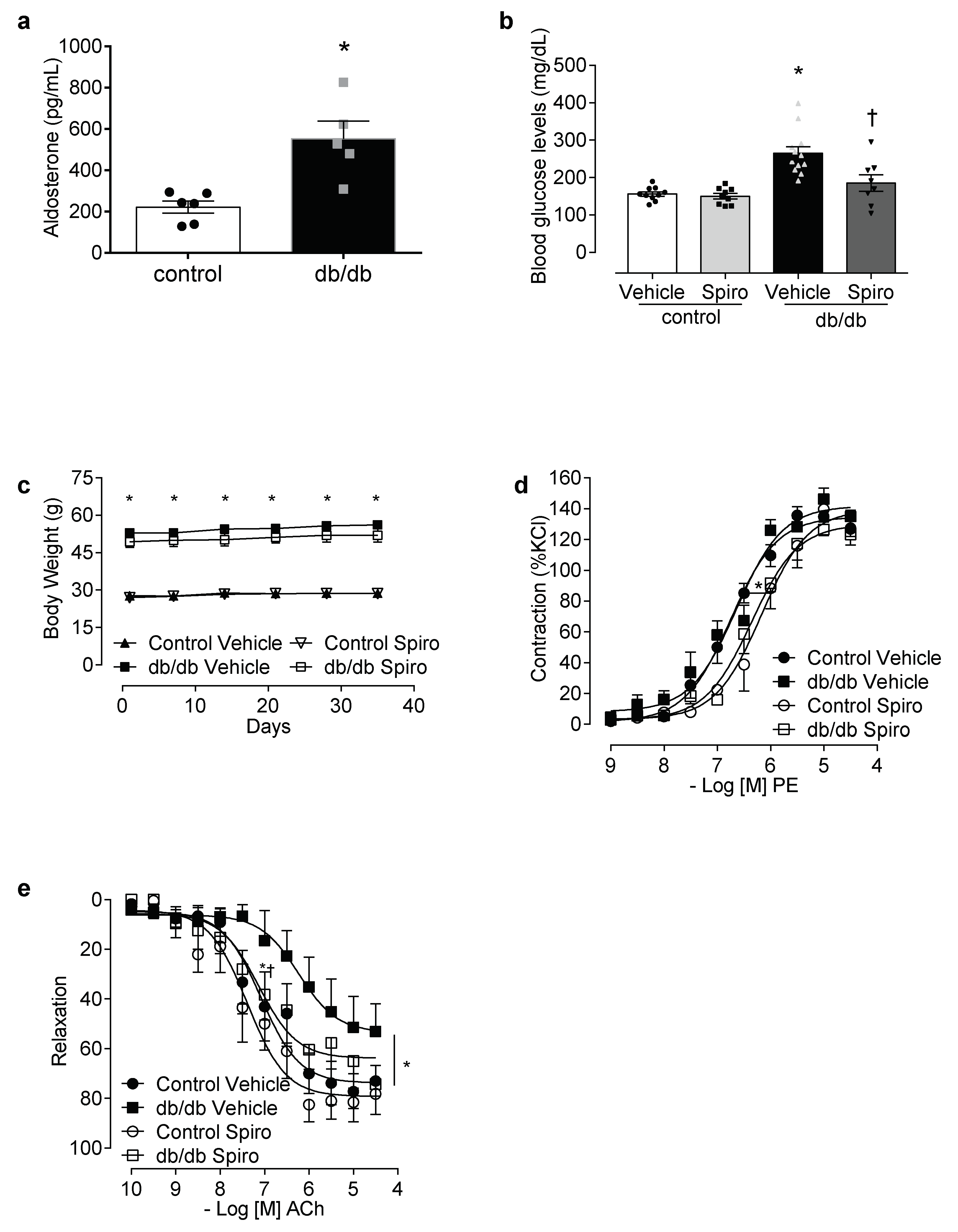
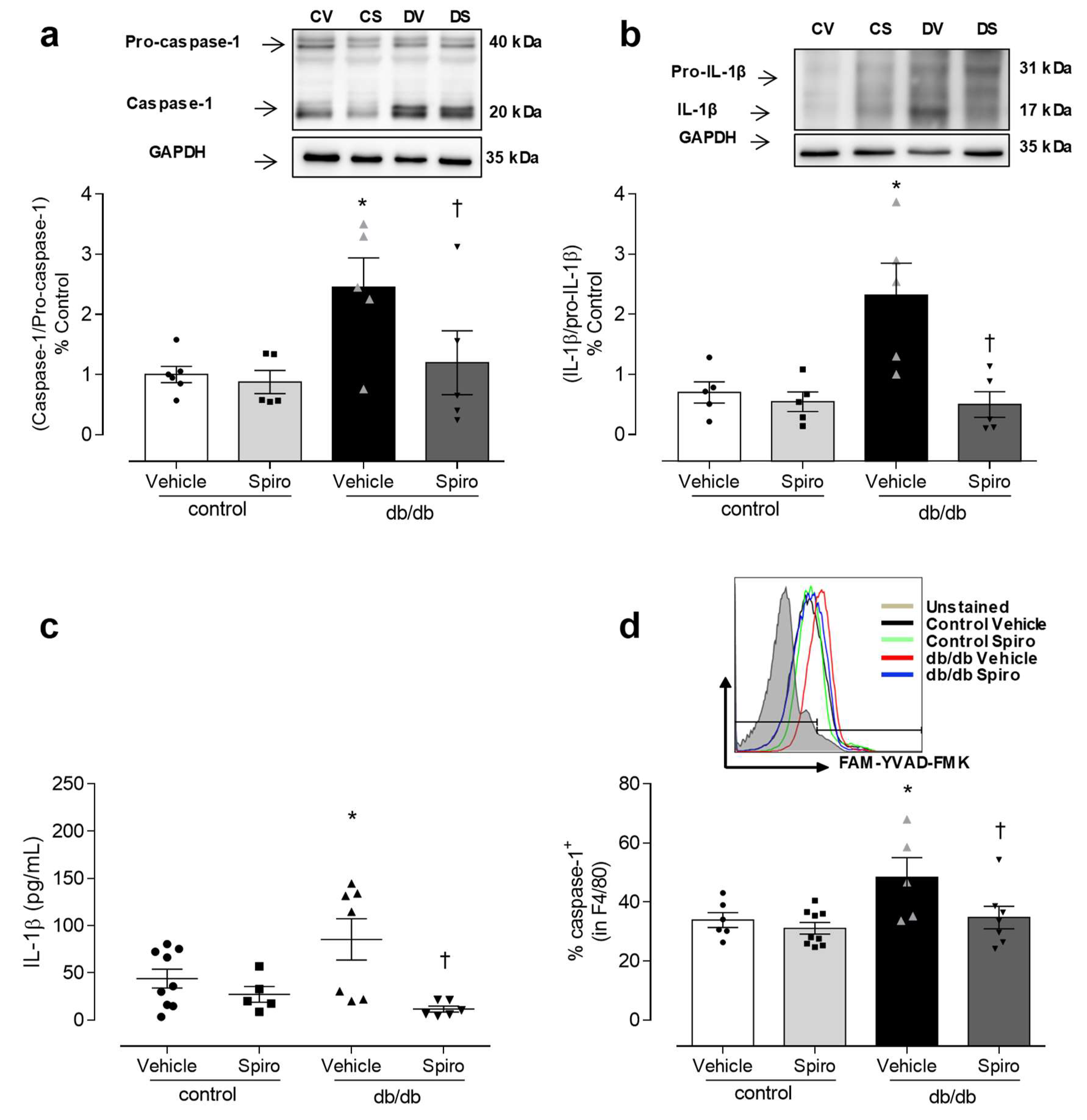
3.1. Spironolactone Treatment Reduces Vascular Dysfunction and Inflammasome Activation in db/db Mice
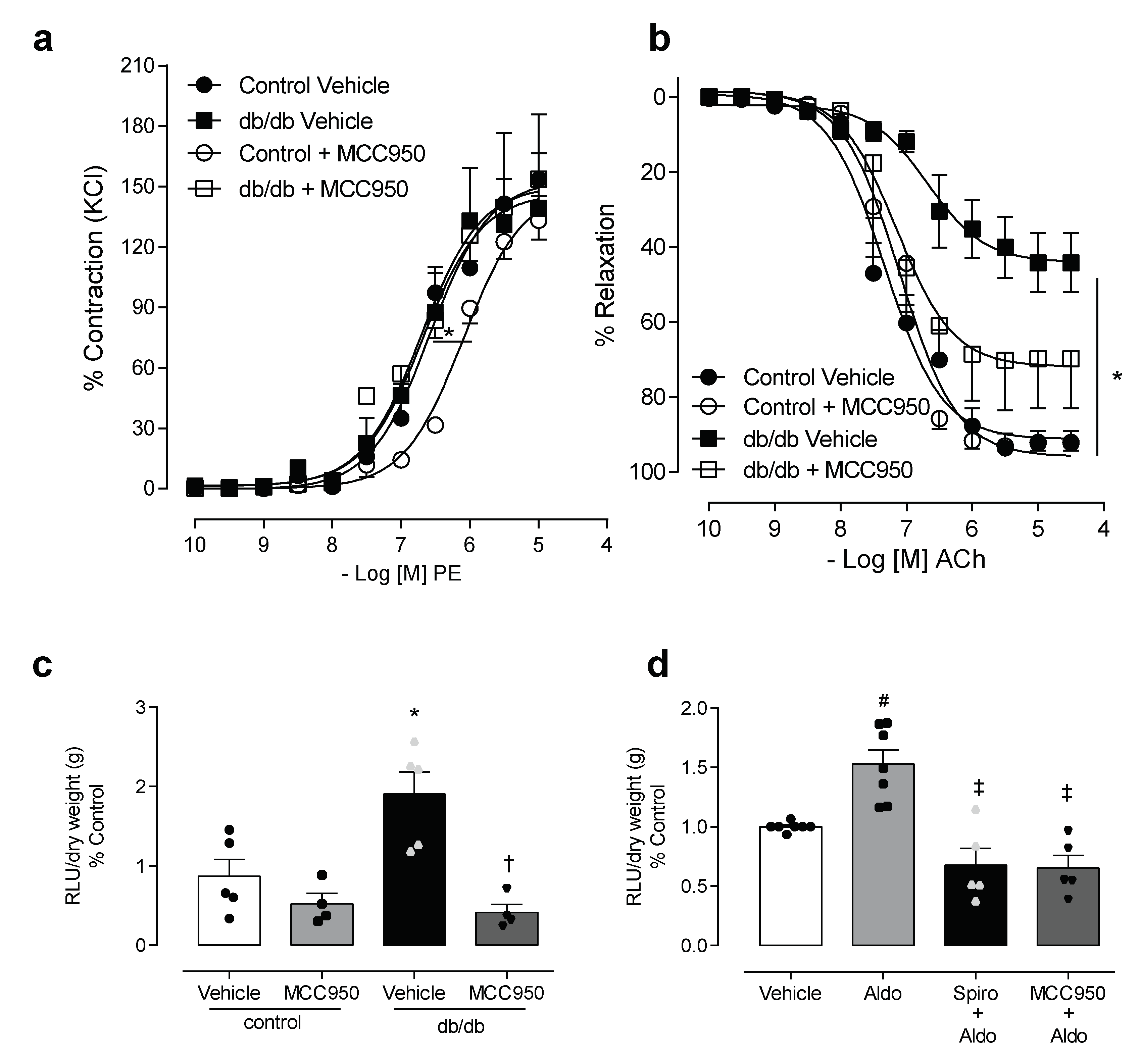
3.2. NLRP3 Inhibition Attenuates Vascular Dysfunction and Decreases Reactive Oxygen Species Generation in Mesenteric Arteries of db/db Mice
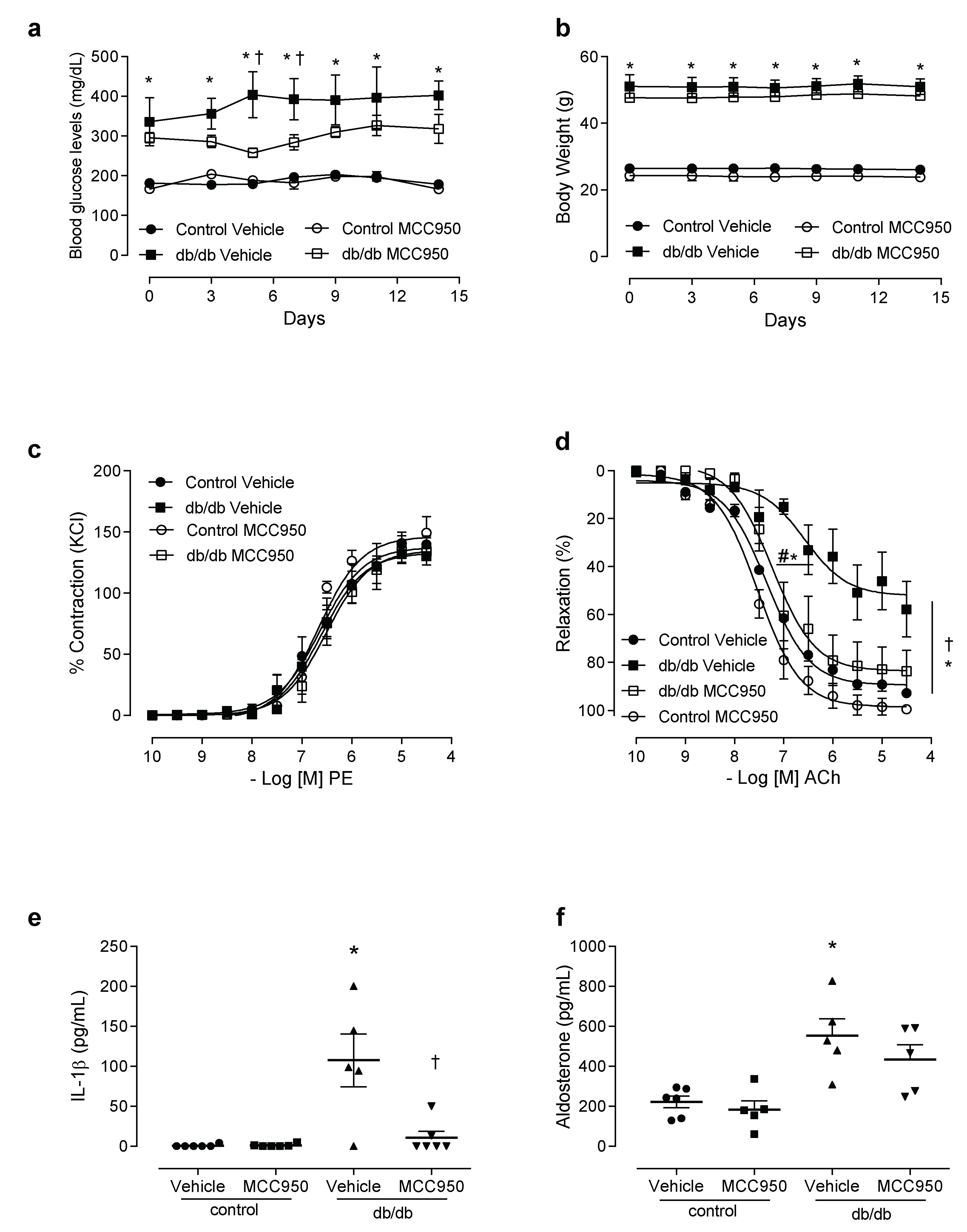
3.3. In Vivo Treatment with NLRP3 Inhibitor Reduces Vascular Dysfunction and Decreases IL-1β Levels in db/db Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- I.D.F. International Diabetes Federation Diabetes Atlas. Available online: http://feel4diabetes-study.eu/idf-diabetes-atlas-8th-edition/ (accessed on 15 November 2019).
- De Boer, I.H.; Bangalore, S.; Benetos, A.; Davis, A.M.; Michos, E.D.; Muntner, P.; Rossing, P.; Zoungas, S.; Bakris, G. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 1273–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, S.A.; Whaley-Connell, A.; Habibi, J.; Wei, Y.; Lastra, G.; Manrique, C.; Stas, S.; Sowers, J.R. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 2009–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Paneni, F.; Cosentino, F.; Creager, M.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Heart J. 2013, 34, 2444–2452. [Google Scholar] [CrossRef] [Green Version]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Davies, J.; Gavin, A.; Band, M.; Morris, A.; Struthers, A. Spironolactone reduces brachial pulse wave velocity and PIIINP levels in hypertensive diabetic patients. Br. J. Clin. Pharm. 2005, 59, 520–523. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.; Struthers, A. The potential benefits of aldosterone antagonism in Type 2 diabetes mellitus. J. Renin Angiotensin Aldosterone Syst. 2002, 3, 150–155. [Google Scholar] [CrossRef]
- Swaminathan, K.; Davies, J.; George, J.; Rajendra, N.S.; Morris, A.D.; Struthers, A.D. Spironolactone for poorly controlled hypertension in type 2 diabetes: Conflicting effects on blood pressure, endothelial function, glycaemic control and hormonal profiles. Diabetologia 2008, 51, 762–768. [Google Scholar] [CrossRef] [Green Version]
- Briet, M.; Schiffrin, E.L. Vascular Actions of Aldosterone. J. Vasc. Res. 2012, 50, 89–99. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Effects of aldosterone on the vasculature. Hypertension 2006, 47, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Bruder-Nascimento, T.; da Silva, M.A.; Tostes, R.C. The involvement of aldosterone on vascular insulin resistance: Implications in obesity and type 2 diabetes. Diabetol. Metab. Syndr. 2014, 6, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.A.; Bruder-Nascimento, T.; Cau, S.B.; Lopes, R.A.; Mestriner, F.L.; Fais, R.S.; Touyz, R.M.; Tostes, R.C. Spironolactone treatment attenuates vascular dysfunction in type 2 diabetic mice by decreasing oxidative stress and restoring NO/GC signaling. Front. Physiol. 2015, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Cau, S.B.; Lopes, R.A.; Manzato, C.P.; Neves, K.B.; Bruder-Nascimento, T.; Mestriner, F.L.; Montezano, A.C.; Nguyen Dinh Cat, A.; Touyz, R.M.; et al. Mineralocorticoid receptor blockade prevents vascular remodelling in a rodent model of type 2 diabetes mellitus. Clin. Sci. 2015, 129, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.S.; Cau, S.B.A.; Silva, M.A.B.; Manzato, C.P.; Mestriner, F.L.A.C.; Matsumoto, T.; Carneiro, F.S.; Tostes, R.C. Diabetes impairs the vascular effects of aldosterone mediated by G protein-coupled estrogen receptor activation. Front. Pharmacol. 2015, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, J.; Lu, L.; Chen, S.S.; Quinn, M.T.; Weber, K.T. Aldosterone-induced inflammation in the rat heart: Role of oxidative stress. Am. J. Pathol. 2002, 161, 1773–1781. [Google Scholar] [CrossRef]
- Brilla, C.G.; Matsubara, L.S.; Weber, K.T. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J. Mol. Cell. Cardiol. 1993, 25, 563–575. [Google Scholar] [CrossRef]
- Bruder-Nascimento, T.; Ferreira, N.S.; Zanotto, C.Z.; Ramalho, F.N.; Pequeno, I.O.; Olivon, V.C.; Neves, K.B.; Lopes, R.A.; Campos, E.; Aguiar Silva, C.A.; et al. NLRP3 Inflammasome Mediates Aldosterone-Induced Vascular Damage. Circulation 2016, 134, 1866–1880. [Google Scholar] [CrossRef]
- Brown, N.J. Aldosterone and vascular inflammation. Hypertension 2008, 51, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Kasal, D.A.; Barhoumi, T.; Li, M.W.; Yamamoto, N.; Zdanovich, E.; Rehman, A.; Neves, M.F.; Laurant, P.; Paradis, P.; Schiffrin, E.L. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 2012, 59, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef]
- Amador, C.A.; Barrientos, V.; Pena, J.; Herrada, A.A.; Gonzalez, M.; Valdes, S.; Carrasco, L.; Alzamora, R.; Figueroa, F.; Kalergis, A.M.; et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 2014, 63, 797–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrada, A.A.; Contreras, F.J.; Marini, N.P.; Amador, C.A.; Gonzalez, P.A.; Cortes, C.M.; Riedel, C.A.; Carvajal, C.A.; Figueroa, F.; Michea, L.F.; et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J. Immunol. 2010, 184, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, I.Z.; Mendelsohn, M.E. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ. Res. 2005, 96, 643–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terada, Y.; Ueda, S.; Hamada, K.; Shimamura, Y.; Ogata, K.; Inoue, K.; Taniguchi, Y.; Kagawa, T.; Horino, T.; Takao, T. Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoid-inducible protein kinase-1. Clin. Exp. Nephrol. 2012, 16, 81–88. [Google Scholar] [CrossRef]
- Sanz-Rosa, D.; Cediel, E.; de las Heras, N.; Miana, M.; Balfagon, G.; Lahera, V.; Cachofeiro, V. Participation of aldosterone in the vascular inflammatory response of spontaneously hypertensive rats: Role of the NFkappaB/IkappaB system. J. Hypertens. 2005, 23, 1167–1172. [Google Scholar] [CrossRef]
- Tschopp, J. Mitochondria: Sovereign of inflammation? J. Immunol. 2011, 41, 1196–1202. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Dowling, J.K.; Ling, Y.H.; Diep, H.; Chan, C.T.; Ferens, D.; Kett, M.M.; Pinar, A.; Samuel, C.S.; Vinh, A.; et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br. J. Pharm. 2016, 173, 752–765. [Google Scholar] [CrossRef]
- Ling, Y.H.; Krishnan, S.M.; Chan, C.T.; Diep, H.; Ferens, D.; Chin-Dusting, J.; Kemp-Harper, B.K.; Samuel, C.S.; Hewitson, T.D.; Latz, E.; et al. Anakinra reduces blood pressure and renal fibrosis in one kidney/DOCA/salt-induced hypertension. Pharmacol. Res. 2016, 116, 77–86. [Google Scholar] [CrossRef]
- Guo, C.; Martinez-Vasquez, D.; Mendez, G.P.; Toniolo, M.F.; Yao, T.M.; Oestreicher, E.M.; Kikuchi, T.; Lapointe, N.; Pojoga, L.; Williams, G.H.; et al. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology 2006, 147, 5363–5373. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Su, W.; Allen, S.; Pang, H.; Daugherty, A.; Smart, E.; Gong, M.C. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc. Res. 2005, 67, 723–735. [Google Scholar] [CrossRef] [Green Version]
- Hollenberg, N.K. Aldosterone in the development and progression of renal injury. Kidney Int. 2004, 66, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollenberg, N.K.; Stevanovic, R.; Agarwal, A.; Lansang, M.C.; Price, D.A.; Laffel, L.M.; Williams, G.H.; Fisher, N.D. Plasma aldosterone concentration in the patient with diabetes mellitus. Kidney Int. 2004, 65, 1435–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briones, A.M.; Nguyen Dinh Cat, A.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Correa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Callera, G.E.; Touyz, R.M.; Teixeira, S.A.; Muscara, M.N.; Carvalho, M.H.; Fortes, Z.B.; Nigro, D.; Schiffrin, E.L.; Tostes, R.C. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension 2003, 42, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Rickard, A.J.; Morgan, J.; Tesch, G.; Funder, J.W.; Fuller, P.J.; Young, M.J. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension 2009, 54, 537–543. [Google Scholar] [CrossRef]
- Usher, M.G.; Duan, S.Z.; Ivaschenko, C.Y.; Frieler, R.A.; Berger, S.; Schutz, G.; Lumeng, C.N.; Mortensen, R.M. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J. Clin. Investig. 2010, 120, 3350–3364. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Doi, S.; Nakashima, A.; Ueno, T.; Yokoyama, Y.; Kohno, N.; Masaki, T. Mizoribine ameliorates renal injury and hypertension along with the attenuation of renal caspase-1 expression in aldosterone-salt-treated rats. PLoS ONE 2014, 9, e93513. [Google Scholar] [CrossRef] [Green Version]
- Dorrance, A.M. Interleukin 1-beta (IL-1beta) enhances contractile responses in endothelium-denuded aorta from hypertensive, but not normotensive, rats. Vasc. Pharm. 2007, 47, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, S.; Palacios, E.; Romacho, T.; Villalobos, L.; Peiro, C.; Sanchez-Ferrer, C.F. The interleukin-1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2014, 13, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, N.S.; Bruder-Nascimento, T.; Pereira, C.A.; Zanotto, C.Z.; Prado, D.S.; Silva, J.F.; Rassi, D.M.; Foss-Freitas, M.C.; Alves-Filho, J.C.; Carlos, D.; et al. NLRP3 Inflammasome and Mineralocorticoid Receptors Are Associated with Vascular Dysfunction in Type 2 Diabetes Mellitus. Cells 2019, 8, 1595. https://doi.org/10.3390/cells8121595
Ferreira NS, Bruder-Nascimento T, Pereira CA, Zanotto CZ, Prado DS, Silva JF, Rassi DM, Foss-Freitas MC, Alves-Filho JC, Carlos D, et al. NLRP3 Inflammasome and Mineralocorticoid Receptors Are Associated with Vascular Dysfunction in Type 2 Diabetes Mellitus. Cells. 2019; 8(12):1595. https://doi.org/10.3390/cells8121595
Chicago/Turabian StyleFerreira, Nathanne Santos, Thiago Bruder-Nascimento, Camila André Pereira, Camila Zillioto Zanotto, Douglas Silva Prado, Josiane Fernandes Silva, Diane Meyre Rassi, Maria Cristina Foss-Freitas, Jose Carlos Alves-Filho, Daniela Carlos, and et al. 2019. "NLRP3 Inflammasome and Mineralocorticoid Receptors Are Associated with Vascular Dysfunction in Type 2 Diabetes Mellitus" Cells 8, no. 12: 1595. https://doi.org/10.3390/cells8121595
APA StyleFerreira, N. S., Bruder-Nascimento, T., Pereira, C. A., Zanotto, C. Z., Prado, D. S., Silva, J. F., Rassi, D. M., Foss-Freitas, M. C., Alves-Filho, J. C., Carlos, D., & Tostes, R. d. C. (2019). NLRP3 Inflammasome and Mineralocorticoid Receptors Are Associated with Vascular Dysfunction in Type 2 Diabetes Mellitus. Cells, 8(12), 1595. https://doi.org/10.3390/cells8121595




