Wound Healing Fluid Reflects the Inflammatory Nature and Aggressiveness of Breast Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Drainage
2.2. Analysis of Small Molecules
2.3. Cell Lines, Cultures, and Treatments
2.4. In Vitro Growth and Migration Assays
2.5. Statistical Analysis
3. Results
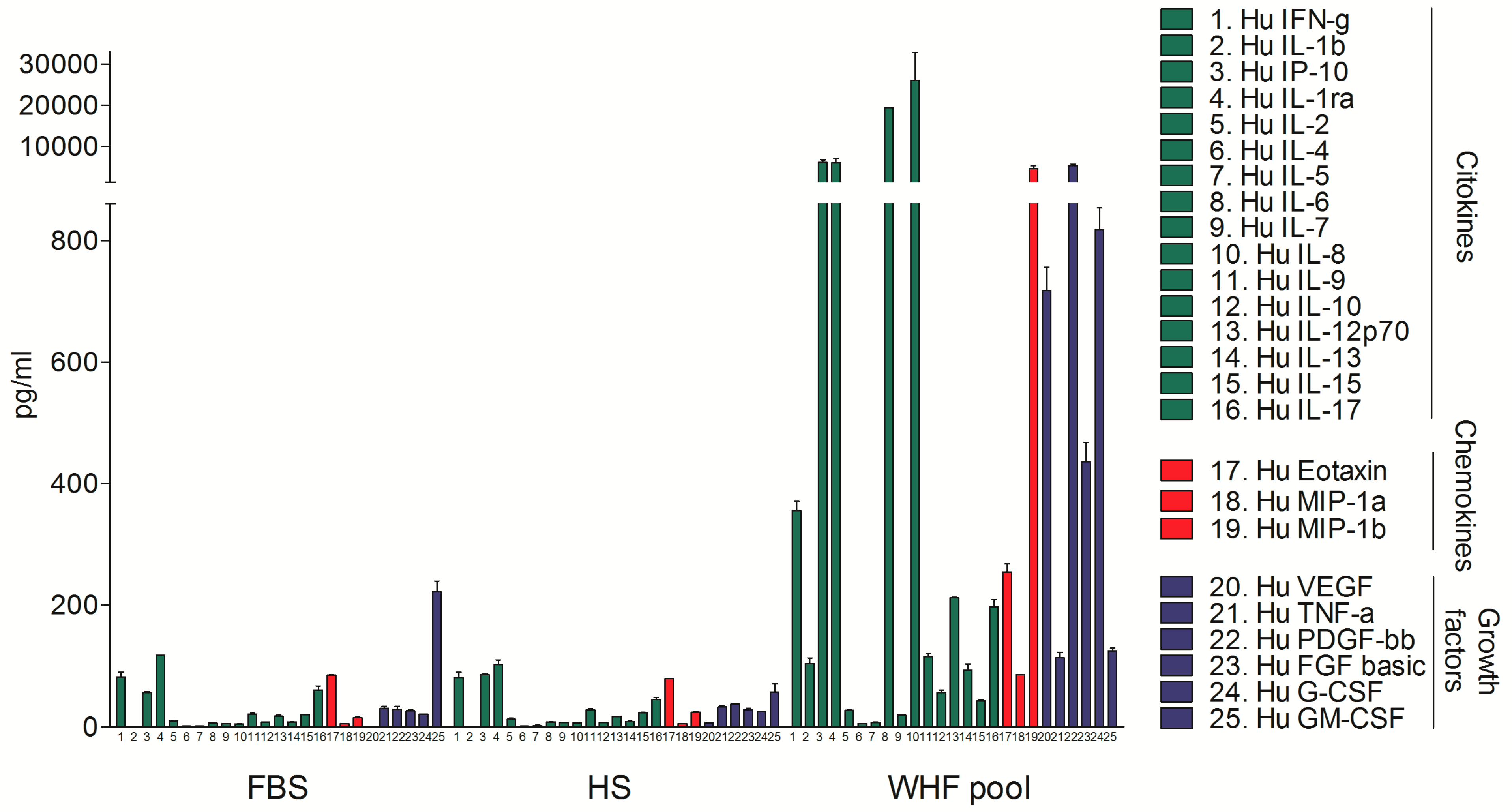
3.1. Analysis of the Composition of WHF in Breast Cancer Patients
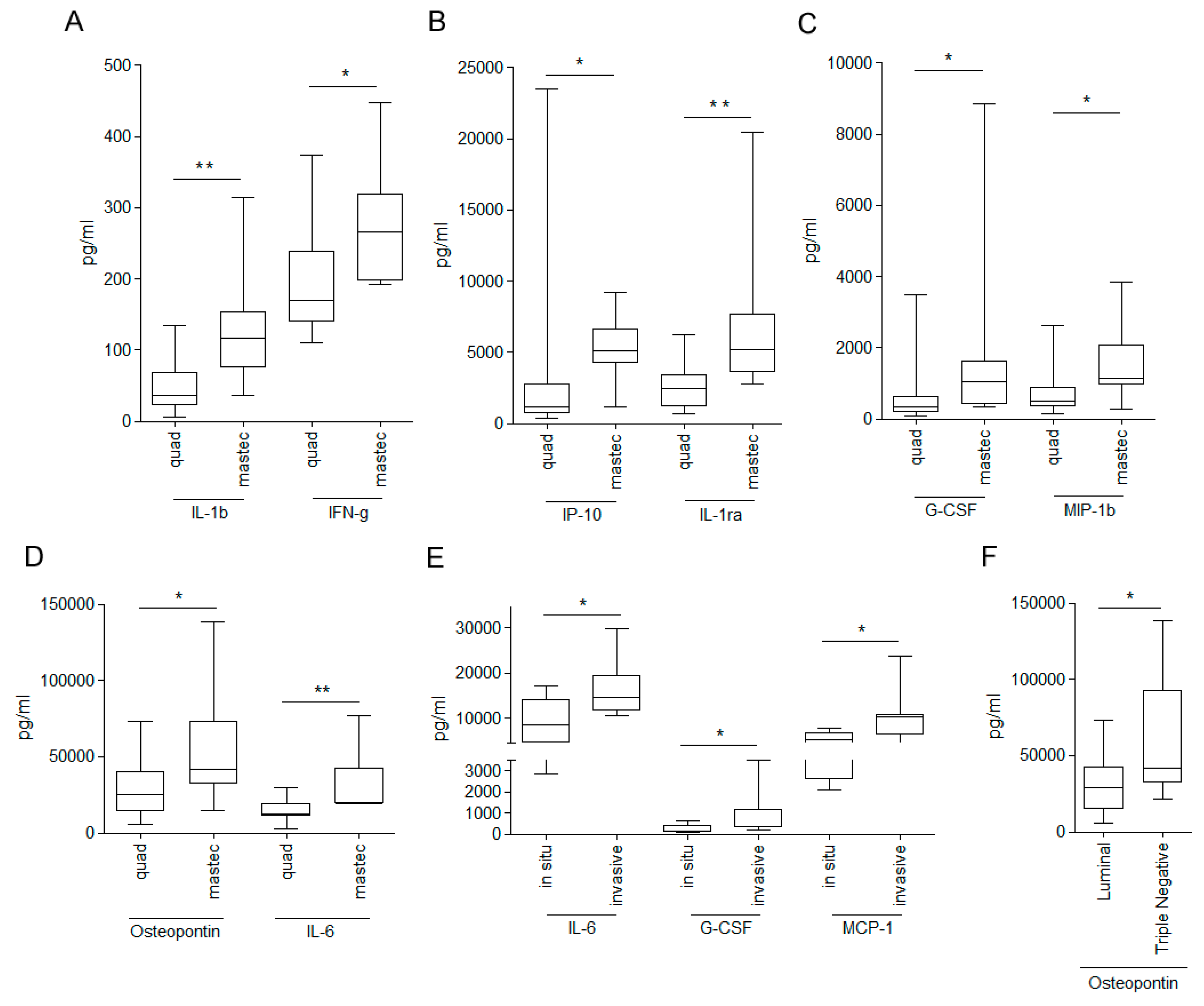
3.2. Relationship between WHF and Cancer Cell Aggressiveness
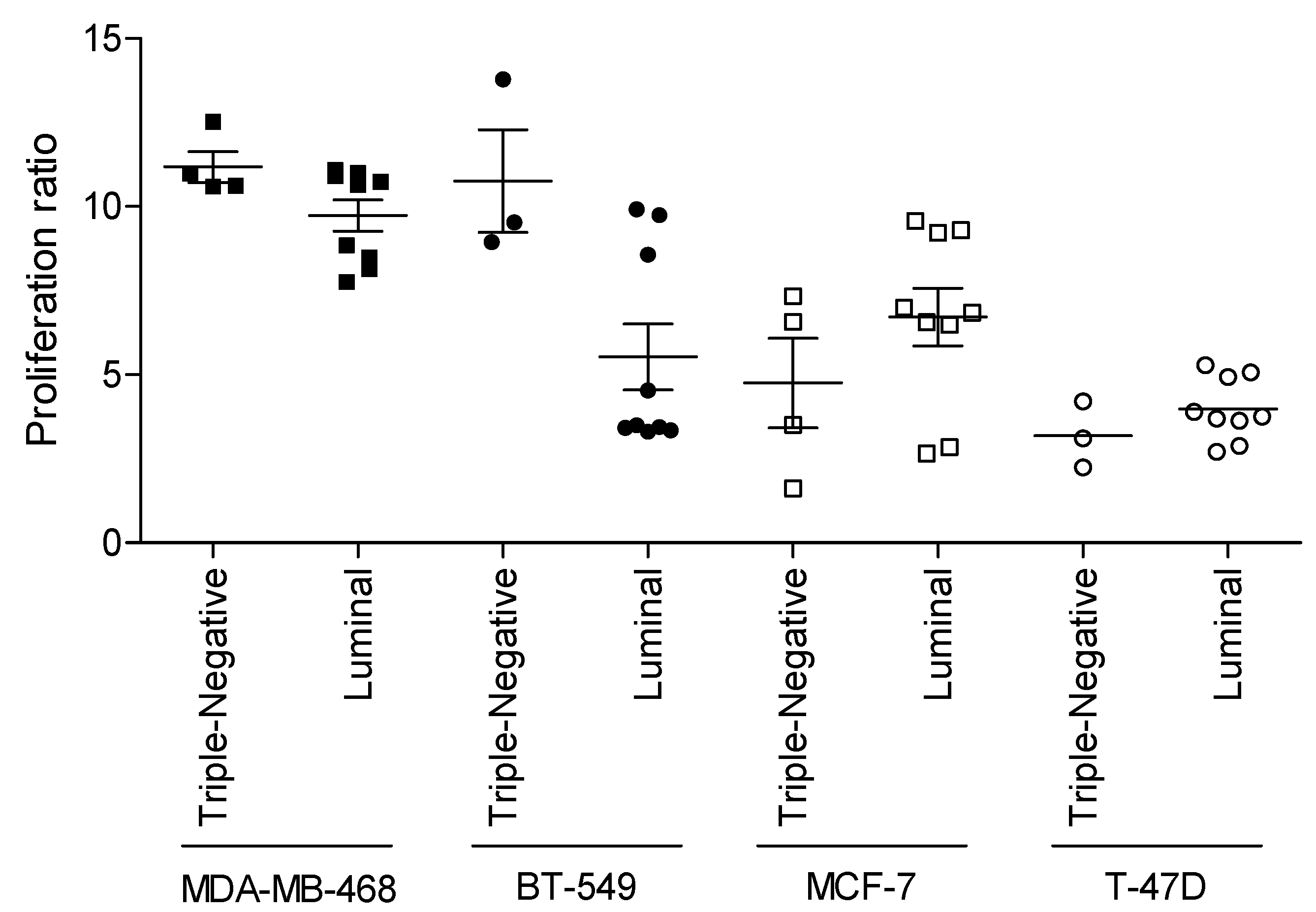
3.3. Relevance of Breast Cancer Subtype in WHF-Driven Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Retsky, M.; Demicheli, R.; Hrushesky, W.J.; Forget, P.; De, K.M.; Gukas, I.; Rogers, R.A.; Baum, M.; Sukhatme, V.; Vaidya, J.S. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: New findings and a review. Curr. Med. Chem. 2013, 20, 4163–4176. [Google Scholar] [CrossRef] [PubMed]
- Eschwege, P.; Dumas, F.; Blanchet, P.; Le, V.M.; Benoit, G.; Jardin, A.; Lacour, B.; Loric, S. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 1995, 346, 1528–1530. [Google Scholar] [CrossRef]
- Forget, P.; Vandenhende, J.; Berliere, M.; Machiels, J.P.; Nussbaum, B.; Legrand, C.; De, K.M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth. Analg. 2010, 110, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, C.; Demicheli, R.; Fornili, M.; Bachir, I.; Duca, M.; Viglietti, G.; Berliere, M.; Piccart, M.; Sotiriou, C.; Sosnowski, M.; et al. Potential Benefit of Intra-operative Administration of Ketorolac on Breast Cancer Recurrence According to the Patient’s Body Mass Index. J. Natl. Cancer Inst. 2018, 110, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.; Pattyn, P.; Mareel, M. Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit. Rev. Oncol. Hematol. 2014, 89, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.M.; Opdenaker, L.M.; Flynn, D.; Sims-Mourtada, J. Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis 2015, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Sinno, H.; Prakash, S. Complements and the wound healing cascade: An updated review. Plast. Surg. Int. 2013, 2013, 146764. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Hamm, R.L. Factors that impair wound healing. J. Am. Coll. Clin. Wound Spec. 2014, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Carlini, M.J.; De Lorenzo, M.S.; Puricelli, L. Cross-talk between tumor cells and the microenvironment at the metastatic niche. Curr. Pharm. Biotechnol. 2011, 12, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Abbattista, A.; Miceli, R.; Valagussa, P.; Bonadonna, G. Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: Further support about the concept of tumor dormancy. Breast Cancer Res. Treat. 1996, 41, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.; Baum, M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: Learning from failures. Nat. Clin. Pr. Oncol. 2007, 4, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10, eaan3464. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Agresti, R.; Carcangiu, M.L.; Ghirelli, C.; Morelli, D.; Campiglio, M.; Martel, M.; Giovanazzi, R.; Greco, M.; Balsari, A.; et al. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet 2003, 362, 527–533. [Google Scholar] [CrossRef]
- Tagliabue, E.; Agresti, R.; Casalini, P.; Mariani, L.; Carcangiu, M.L.; Balsari, A.; Veronesi, U.; Ménard, S. Linking survival of HER2-positive breast carcinoma patients with surgical invasiveness. Eur. J. Cancer 2006, 42, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Turdo, F.; Bianchi, F.; Gasparini, P.; Sandri, M.; Sasso, M.; De Cecco, L.; Forte, L.; Casalini, P.; Aiello, P.; Sfondrini, L.; et al. CDCP1 is a novel marker of the most aggressiveness human triple-negative breast cancer. Oncotarget 2016, 7, 69649–69665. [Google Scholar] [CrossRef] [PubMed]
- Forte, L.; Turdo, F.; Ghirelli, C.; Aiello, P.; Casalini, P.; Iorio, M.V.; D’Ippolito, E.; Gasparini, P.; Agresti, R.; Belmonte, B.; et al. The PDGFRbeta/ERK1/2 pathway regulates CDCP1 expression in triple-negative breast cancer. BMC Cancer 2018, 18, 586. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, M.; Sfondrini, L.; Campiglio, M.; Sommariva, M.; Bianchi, F.; Perego, P.; van Rooijen, N.; Supino, R.; Rumio, C.; Zunino, F.; et al. Ascites regression and survival increase in mice bearing advanced-stage human ovarian carcinomas and repeatedly treated intraperitoneally with CpG-ODN. J. Immunother. 2010, 33, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Martinetti, A.; Miceli, R.; Sottotetti, E.; Di Bartolomeo, M.; de, B.F.; Gevorgyan, A.; Dotti, K.F.; Bajetta, E.; Campiglio, M.; Bianchi, F.; et al. Circulating biomarkers in advanced colorectal cancer patients randomly assigned to three bevacizumab-based regimens. Cancers (Basel) 2014, 6, 1753–1768. [Google Scholar] [CrossRef] [PubMed]
- Plantamura, I.; Casalini, P.; Dugnani, E.; Sasso, M.; D’Ippolito, E.; Tortoreto, M.; Cacciatore, M.; Guarnotta, C.; Ghirelli, C.; Barajon, I.; et al. PDGFRβ and FGFR2 mediate endothelial cell differentiation capability of triple negative breast carcinoma cells. Mol. Oncol. 2014, 8, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Sasso, M.; Turdo, F.; Beretta, G.L.; Casalini, P.; Ghirelli, C.; Sfondrini, L.; Menard, S.; Tagliabue, E.; Campiglio, M. Fhit nuclear import following EGF stimulation sustains proliferation of Breast Cancer cells. J. Cell Physiol. 2015, 230, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, A.; Sfondrini, L.; Pennati, M.; De Marco, C.; Motta, V.; Tagliabue, E.; Deraco, M.; Balsari, A.; Zaffaroni, N. CpG-oligodeoxynucleotides exert remarkable antitumor activity against diffuse malignant peritoneal mesothelioma orthotopic xenografts. J. Transl. Med. 2016, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Le Noci, V.; Sommariva, M.; Tortoreto, M.; Zaffaroni, N.; Campiglio, M.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Reprogramming the lung microenvironment by inhaled immunotherapt forsters immune destruction of tumor. OncoImmunology 2016, 5, e1234571. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. OncoImmunology 2016, 5, e1093722. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Nieto-Jimenez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Antonio, N.; Bonnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, S.; Stiles, J.K. The emerging role of CXCL10 in cancer (Review). Oncol. Lett. 2011, 2, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Hikita, A.; Inoue, Y. The roles of TGF-beta signaling in carcinogenesis and breast cancer metastasis. Breast Cancer 2012, 19, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The role of osteopontin in inflammatory processes. J. Cell Commun. Signal. 2009, 3, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chao, L.; Ma, G.; Chen, L.; Tian, B.; Zang, Y.; Sun, J. Increased expression of osteopontin in patients with triple-negative breast cancer. Eur. J. Clin. Investig. 2008, 38, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Thorat, D.; Sahu, A.; Behera, R.; Lohite, K.; Deshmukh, S.; Mane, A.; Karnik, S.; Doke, S.; Kundu, G.C. Association of osteopontin and cyclooxygenase-2 expression with breast cancer subtypes and their use as potential biomarkers. Oncol. Lett. 2013, 6, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, S.; Tripodo, C.; Sandri, S.; Torselli, I.; Vitali, C.; Ratti, C.; Botti, L.; Burocchi, A.; Porcasi, R.; Tomirotti, A.; et al. Osteopontin shapes immunosuppression in the metastatic niche. Cancer Res. 2014, 74, 4706–4719. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, K.; Gao, N.; Zhang, H.; Jiang, Y.; Liu, C.; Wang, S.; Zhao, Z. High throughput screening of cytokines, chemokines and matrix metalloproteinases in wound fluid induced by mammary surgery. Oncotarget 2015, 6, 29296–29310. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.E.; McCain, N.L.; Walter, J.; Schubert, C. Cytokine comparisons between women with breast cancer and women with a negative breast biopsy. Nurs. Res. 2008, 57, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Compte, D.; Jacquemin, B.; Robles-Vidal, C.; Volkow, P. Surgical site infections in breast surgery: Case-control study. World J. Surg. 2004, 28, 242–246. [Google Scholar] [PubMed]
- Woodworth, P.A.; McBoyle, M.F.; Helmer, S.D.; Beamer, R.L. Seroma formation after breast cancer surgery: Incidence and predicting factors. Am. Surg. 2000, 66, 444–450. [Google Scholar] [PubMed]
- Hofer, S.O.; Shrayer, D.; Reichner, J.S.; Hoekstra, H.J.; Wanebo, H.J. Wound-induced tumor progression: A probable role in recurrence after tumor resection. Arch. Surg. 1998, 133, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Bogden, A.E.; Moreau, J.P.; Eden, P.A. Proliferative response of human and animal tumours to surgical wounding of normal tissues: Onset, duration and inhibition. Br. J. Cancer 1997, 75, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Kuraishy, A.; Karin, M.; Grivennikov, S.I. Tumor promotion via injury- and death-induced inflammation. Immunity 2011, 35, 467–477. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.P.; Wang, J.H.; Cotter, T.G.; Redmond, H.P. Less stress, more success? Oncological implications of surgery-induced oxidative stress. Gut 2013, 62, 461–470. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agresti, R.; Triulzi, T.; Sasso, M.; Ghirelli, C.; Aiello, P.; Rybinska, I.; Campiglio, M.; Sfondrini, L.; Tagliabue, E.; Bianchi, F. Wound Healing Fluid Reflects the Inflammatory Nature and Aggressiveness of Breast Tumors. Cells 2019, 8, 181. https://doi.org/10.3390/cells8020181
Agresti R, Triulzi T, Sasso M, Ghirelli C, Aiello P, Rybinska I, Campiglio M, Sfondrini L, Tagliabue E, Bianchi F. Wound Healing Fluid Reflects the Inflammatory Nature and Aggressiveness of Breast Tumors. Cells. 2019; 8(2):181. https://doi.org/10.3390/cells8020181
Chicago/Turabian StyleAgresti, Roberto, Tiziana Triulzi, Marianna Sasso, Cristina Ghirelli, Piera Aiello, Ilona Rybinska, Manuela Campiglio, Lucia Sfondrini, Elda Tagliabue, and Francesca Bianchi. 2019. "Wound Healing Fluid Reflects the Inflammatory Nature and Aggressiveness of Breast Tumors" Cells 8, no. 2: 181. https://doi.org/10.3390/cells8020181
APA StyleAgresti, R., Triulzi, T., Sasso, M., Ghirelli, C., Aiello, P., Rybinska, I., Campiglio, M., Sfondrini, L., Tagliabue, E., & Bianchi, F. (2019). Wound Healing Fluid Reflects the Inflammatory Nature and Aggressiveness of Breast Tumors. Cells, 8(2), 181. https://doi.org/10.3390/cells8020181








