3,5 Diiodo-l-Thyronine (T2) Promotes the Browning of White Adipose Tissue in High-Fat Diet-Induced Overweight Male Rats Housed at Thermoneutrality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Animal Care
- The first group (N) received a standard diet (total metabolizable percentage of energy: 60.4 carbohydrates, 29 proteins, 10.6 fat J J−1; 15.88 kJ gross energy g−1; Muscedola, Milan, Italy) for fourteen weeks with a daily injection intraperitoneally of vehicle (saline), for the last four weeks;
- The second group (HFD) received a high-fat diet (280 g diet supplemented with 395 g of lyophilized lamb meat (Liomellin, Milan, Italy), 120 g cellulose (Sigma-Aldrich, St. Louis, MO, USA), 20 g mineral mix (ICN Biomedical, Solon, OH, USA), 7 g vitamin mix (ICN), and 200 g low-salt butter (Lurpak, Denmark); total metabolizable percentage of energy: 21 carbohydrates, 29 proteins, 50 fat J J−1; 19.85 kJ gross energy g−1) for fourteen weeks with a daily injection intraperitoneally of vehicle (saline), for the last four weeks;
- The third group (HFD-T2) received an HFD like the second group for 10 weeks and was subsequently treated for four weeks contemporary with HFD and T2 (50 μg/100 g BW).
2.2. Histochemical Analysis
2.3. Insulin Tolerance Test
2.4. Preparation of Intrascapular BAT Lysate for Western Blotting
2.5. Preparation of Mitochondria
2.6. Preparation of Total Lysates
2.7. Western Blot Analysis
2.8. Irisin Serum Determination
2.9. miRNA and Total RNA Isolation
- rβ-ACT sense 5′-GGA GAT TAC TGC CCT GGC TCC TA-3′
- rβ-ACT Antisense 5′-GAC TCA TCG TAC TCC TGC TTG CTG-3′
- rPGC1α sense 5′-AACCAGTACAACAATGAGCCCGC-3′
- rPGC1α antisense 5′-TGAGGACCGCTAGCAAGTTTGC-3′
- rCIDEA sense 5′-TTCCTCGGCTGTCTCAATGT-3′
- rCIDEA antisense 5′- GCCCGCATAAACCAGGAAC-3′
2.10. Statistical Analysis
3. Results
3.1. Administration of T2 to Rats Pre-Fed with a High-Fat Diet Modulates Adiposity without Inducing a Thyrotoxic State
3.2. Effect of T2 Administration on BAT Morphology and on UCP1 Content
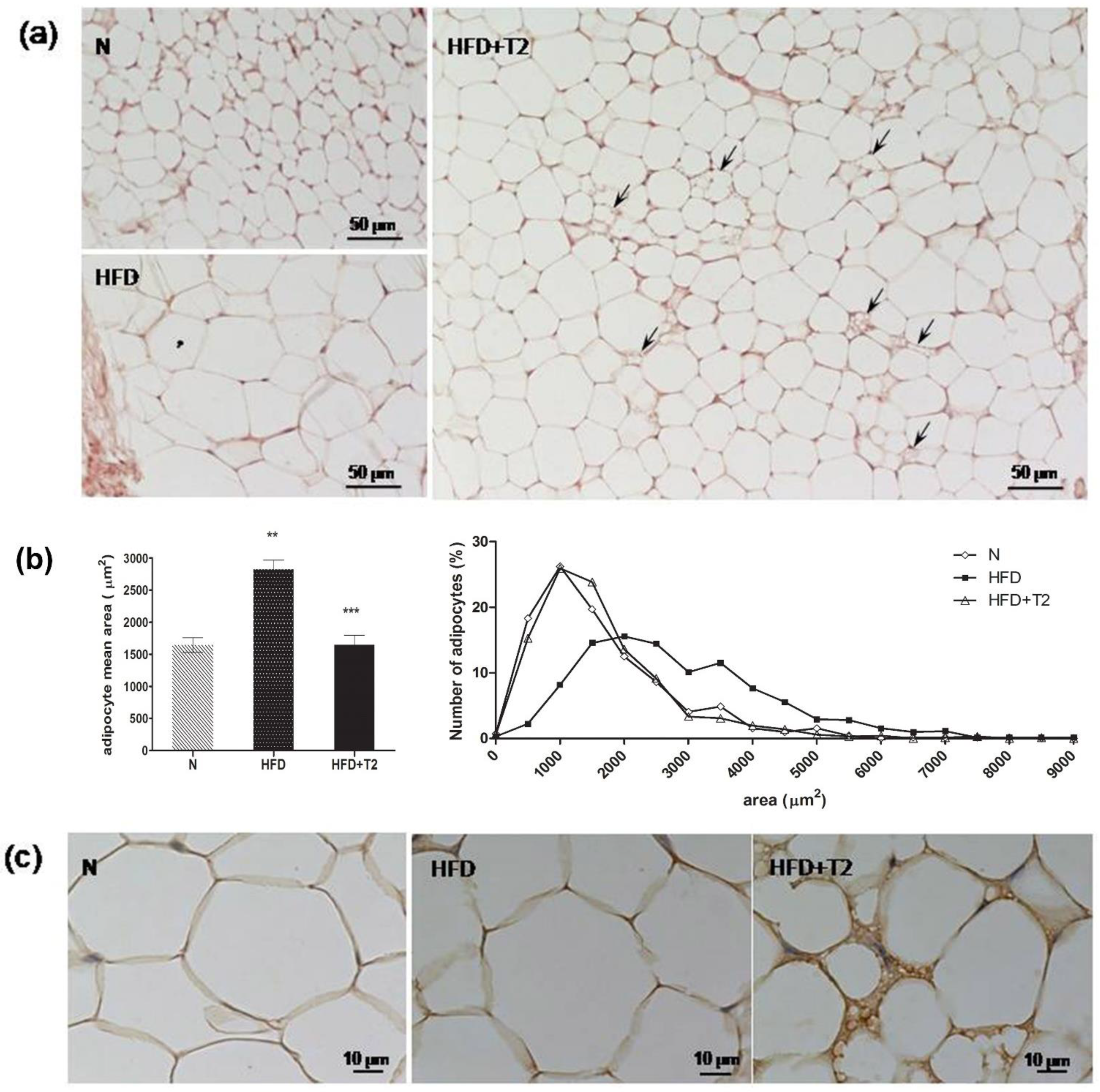
3.3. T2 Administration Induces Browning of sWAT in Rats
3.4. T2 Administration Induces Downregulation of miR-133a, Resulting in Up-Regulation of PRDM16
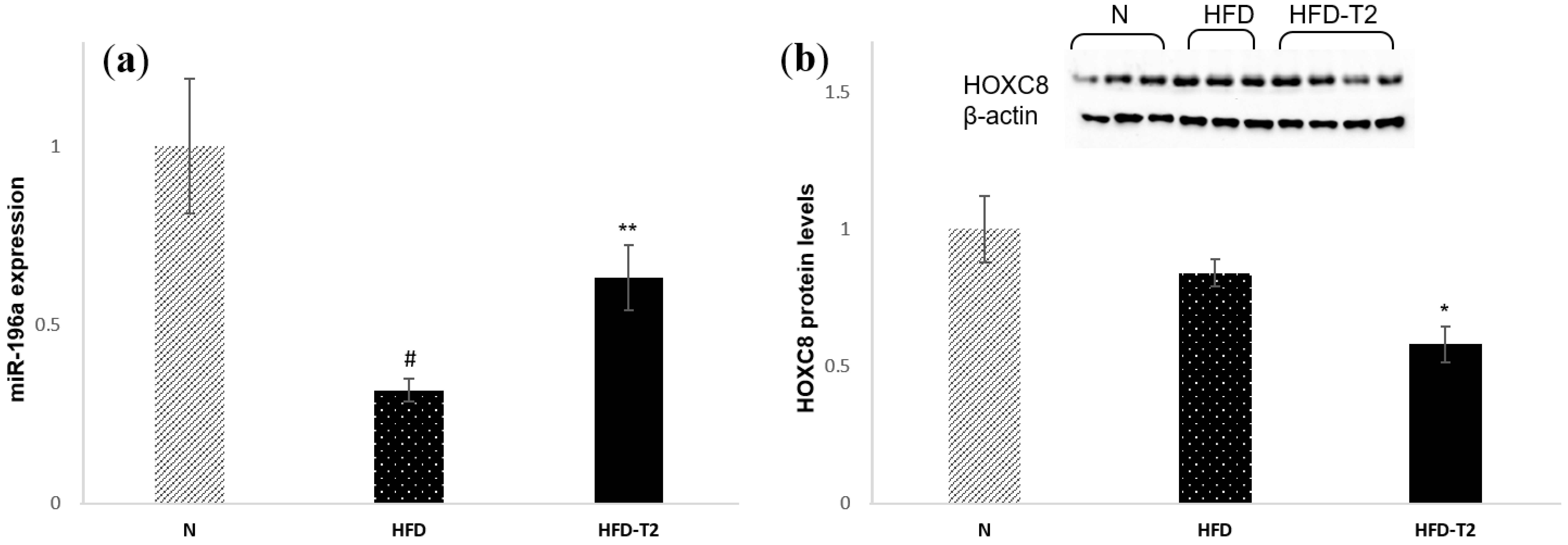
3.5. T2 Administration Induces Up-Regulation of miR-196a, Resulting in Down-Regulation of HOXC8
3.6. T2 Administration Reduces the Phosphorylation of an Important Regulator of Browning: MAPK Kinase 6 (MKK6)
3.7. T2 Administration Increased the Serum Levels of Irisin
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. The global epidemic of obesity: An overview. Epidemiol. Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int. J. Obes. 2010, 34 (Suppl. 1), S7–S16. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The changed metabolic world with human brown adipose tissue: Therapeutic visions. Cell Metab. 2010, 11, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.; Tseng, Y.H. Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte 2012, 1, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Matsushita, M.; Yoneshiro, T.; Aita, S.; Kameya, T.; Sugie, H.; Saito, M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int. J. Obes. 2014, 38, 812–817. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Okamatsu-Ogura, Y.; Kameya, T.; Kawai, Y.; Miyagawa, M.; Tsujisaki, M.; Saito, M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity 2011, 19, 1755–1760. [Google Scholar] [CrossRef]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-induced ‘browning’ of adipose tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Carriere, A.; Jeanson, Y.; Cousin, B.; Arnaud, E.; Casteilla, L. Recruitment and activation of brown and/or BRITE adipocytes: Potential therapeutic against metabolic diseases. Med. Sci. 2013, 29, 729–735. [Google Scholar] [CrossRef]
- Cousin, B.; Cinti, S.; Morroni, M.; Raimbault, S.; Ricquier, D.; Penicaud, L.; Casteilla, L. Occurrence of brown adipocytes in rat white adipose tissue: Molecular and morphological characterization. J. Cell Sci. 1992, 103 Pt 4, 931–942. [Google Scholar]
- Guerra, C.; Koza, R.A.; Yamashita, H.; Walsh, K.; Kozak, L.P. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J. Clin. Investig. 1998, 102, 412–420. [Google Scholar] [CrossRef]
- Ghorbani, M.; Himms-Hagen, J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankovic, A.; Golic, I.; Markelic, M.; Stancic, A.; Otasevic, V.; Buzadzic, B.; Korac, A.; Korac, B. Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation. J. Physiol. 2015, 593, 3267–3280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Nakhuda, A.; Josse, A.R.; Gburcik, V.; Crossland, H.; Raymond, F.; Metairon, S.; Good, L.; Atherton, P.J.; Phillips, S.M.; Timmons, J.A. Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss. Am. J. Clin. Nutr. 2016, 104, 557–565. [Google Scholar] [CrossRef]
- Dodd, G.T.; Decherf, S.; Loh, K.; Simonds, S.E.; Wiede, F.; Balland, E.; Merry, T.L.; Munzberg, H.; Zhang, Z.Y.; Kahn, B.B.; et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 2015, 160, 88–104. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Mao, L.; Ding, P.; Zhuang, X.; Zhou, Y.; Yu, L.; Liu, Y.; Nie, T.; Xu, T.; Xu, Y.; et al. 1-Benzyl-4-phenyl-1H-1,2,3-triazoles improve the transcriptional functions of estrogen-related receptor gamma and promote the browning of white adipose. Bioorg. Med. Chem. 2015, 23, 3751–3760. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.W. The significance of beige and brown fat in humans. Endocr. Connect. 2017, 6, R70–R79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiefer, F.W. Browning and thermogenic programing of adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Walden, T.B.; Hansen, I.R.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Recruited vs. nonrecruited molecular signatures of brown, “brite” and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E19–E31. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, O.; Dempersmier, J.; Sul, H.S. Genetic and epigenetic control of adipose development. Biochim. Biophys. Acta 2019, 1864, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kuang, S. miR-133 links to energy balance through targeting Prdm16. J. Mol. Cell Biol. 2013, 5, 432–434. [Google Scholar] [CrossRef] [Green Version]
- Arias, N.; Aguirre, L.; Fernandez-Quintela, A.; Gonzalez, M.; Lasa, A.; Miranda, J.; Macarulla, M.T.; Portillo, M.P. MicroRNAs involved in the browning process of adipocytes. J. Physiol. Biochem. 2016, 72, 509–521. [Google Scholar] [CrossRef]
- Trajkovski, M.; Lodish, H. MicroRNA networks regulate development of brown adipocytes. Trends Endocrinol. Metab. 2013, 24, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.; Nakagami, H.; Rodriguez-Araujo, G.; Nimura, K.; Kaneda, Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012, 10, e1001314. [Google Scholar] [CrossRef]
- Fu, T.; Seok, S.; Choi, S.; Huang, Z.; Suino-Powell, K.; Xu, H.E.; Kemper, B.; Kemper, J.K. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol. Cell. Biol. 2014, 34, 4130–4142. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Vidal-Puig, A. Beyond the sympathetic tone: The new brown fat activators. Cell Metab. 2013, 17, 638–643. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The browning of white adipose tissue: Some burning issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, N.; Moreno-Navarrete, J.M.; Contreras, C.; Rial-Pensado, E.; Ferno, J.; Nogueiras, R.; Dieguez, C.; Fernandez-Real, J.M.; Lopez, M. Thyroid hormones induce browning of white fat. J. Endocrinol. 2017, 232, 351–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Wu, W.; Dai, Y.; Maneix, L.; Huang, B.; Warner, M.; Gustafsson, J.A. Liver X receptor beta controls thyroid hormone feedback in the brain and regulates browning of subcutaneous white adipose tissue. Proc. Natl. Acad. Sci. USA 2015, 112, 14006–14011. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Yehuda-Shnaidman, E.; Medvedev, A.V.; Kumar, N.; Daniel, K.W.; Robidoux, J.; Czech, M.P.; Mangelsdorf, D.J.; Collins, S. Liver X receptor alpha is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Mol. Cell. Biol. 2008, 28, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.; Hankir, M.; Heiker, J.T.; Fenske, W.; Krause, K. Thyroid hormones and browning of adipose tissue. Mol. Cell. Endocrinol. 2017, 458, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, N.; Bernardo, E.; Acin-Perez, R.; Manieri, E.; Perez-Sieira, S.; Hernandez-Cosido, L.; Montalvo-Romeral, V.; Mora, A.; Rodriguez, E.; Leiva-Vega, L.; et al. MKK6 controls T3-mediated browning of white adipose tissue. Nat. Commun. 2017, 8, 856. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.; Lanni, A.; Lombardi, A.; Goglia, F. How the thyroid controls metabolism in the rat: Different roles for triiodothyronine and diiodothyronines. J. Physiol. 1997, 505 Pt 2, 529–538. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; de Lange, P.; Silvestri, E.; Ragni, M.; Farina, P.; Baccari, G.C.; Fallahi, P.; Antonelli, A.; et al. 3,5-diiodo-l-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J. 2005, 19, 1552–1554. [Google Scholar] [CrossRef]
- De Lange, P.; Cioffi, F.; Senese, R.; Moreno, M.; Lombardi, A.; Silvestri, E.; De Matteis, R.; Lionetti, L.; Mollica, M.P.; Goglia, F.; et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-l-thyronine in rats. Diabetes 2011, 60, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Padron, A.S.; Neto, R.A.; Pantaleao, T.U.; de Souza dos Santos, M.C.; Araujo, R.L.; de Andrade, B.M.; da Silva Leandro, M.; de Castro, J.P.; Ferreira, A.C.; de Carvalho, D.P. Administration of 3,5-diiodothyronine (3,5-T2) causes central hypothyroidism and stimulates thyroid-sensitive tissues. J. Endocrinol. 2014, 221, 415–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardi, A.; Beneduce, L.; Moreno, M.; Diano, S.; Colantuoni, V.; Ursini, M.V.; Lanni, A.; Goglia, F. 3,5-diiodo-l-thyronine regulates glucose-6-phosphate dehydrogenase activity in the rat. Endocrinology 2000, 141, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Di Domenicantonio, A.; Moreno, M.; Lanni, A.; Goglia, F. 3,5-diiodo-l-thyronine increases resting metabolic rate and reduces body weight without undesirable side effects. J. Biol. Regul. Homeost. Agents 2011, 25, 655–660. [Google Scholar] [PubMed]
- Fallahi, P.; Ferrari, S.M.; Santini, E.; Camastra, S.; Frenzilli, G.; Puccini, M.; Goglia, F.; Lanni, A.; Marchetti, P.; Antonelli, A. Both 3,5-diiodo-l-thyronine (T2) and T3 modulate glucose-induced insulin secretion. J. Biol. Regul. Homeost. Agents 2017, 31, 503–508. [Google Scholar] [PubMed]
- Fischer, A.W.; Cannon, B.; Nedergaard, J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol. Metab. 2018, 7, 161–170. [Google Scholar] [CrossRef]
- Sacripanti, G.; Nguyen, N.M.; Lorenzini, L.; Frascarelli, S.; Saba, A.; Zucchi, R.; Ghelardoni, S. 3,5-Diiodo-l-Thyronine Increases Glucose Consumption in Cardiomyoblasts Without Affecting the Contractile Performance in Rat Heart. Front. Endocrinol. 2018, 9, 282. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- McGuinness, O.P.; Ayala, J.E.; Laughlin, M.R.; Wasserman, D.H. NIH experiment in centralized mouse phenotyping: The Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E849–E855. [Google Scholar] [CrossRef]
- Mollica, M.P.; Lionetti, L.; Moreno, M.; Lombardi, A.; De Lange, P.; Antonelli, A.; Lanni, A.; Cavaliere, G.; Barletta, A.; Goglia, F. 3,5-diiodo-l-thyronine, by modulating mitochondrial functions, reverses hepatic fat accumulation in rats fed a high-fat diet. J. Hepatol. 2009, 51, 363–370. [Google Scholar] [CrossRef]
- Moreno, M.; Silvestri, E.; De Matteis, R.; de Lange, P.; Lombardi, A.; Glinni, D.; Senese, R.; Cioffi, F.; Salzano, A.M.; Scaloni, A.; et al. 3,5-Diiodo-l-thyronine prevents high-fat-diet-induced insulin resistance in rat skeletal muscle through metabolic and structural adaptations. FASEB J. 2011, 25, 3312–3324. [Google Scholar] [CrossRef]
- Kajimura, S.; Seale, P.; Tomaru, T.; Erdjument-Bromage, H.; Cooper, M.P.; Ruas, J.L.; Chin, S.; Tempst, P.; Lazar, M.A.; Spiegelman, B.M. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008, 22, 1397–1409. [Google Scholar] [CrossRef] [Green Version]
- Trajkovski, M.; Ahmed, K.; Esau, C.C.; Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 2012, 14, 1330–1335. [Google Scholar] [CrossRef]
- Liu, W.; Bi, P.; Shan, T.; Yang, X.; Yin, H.; Wang, Y.X.; Liu, N.; Rudnicki, M.A.; Kuang, S. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013, 9, e1003626. [Google Scholar] [CrossRef]
- Cao, W.; Daniel, K.W.; Robidoux, J.; Puigserver, P.; Medvedev, A.V.; Bai, X.; Floering, L.M.; Spiegelman, B.M.; Collins, S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 2004, 24, 3057–3067. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef]
- Lanna, A.; Henson, S.M.; Escors, D.; Akbar, A.N. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 2014, 15, 965–972. [Google Scholar] [CrossRef]
- Baxter, J.D.; Webb, P. Thyroid hormone mimetics: Potential applications in atherosclerosis, obesity and type 2 diabetes. Nat. Rev. Drug Discov. 2009, 8, 308–320. [Google Scholar] [CrossRef]
- Perez-Berna, A.J.; Bernabeu, A.; Moreno, M.R.; Guillen, J.; Villalain, J. The pre-transmembrane region of the HCV E1 envelope glycoprotein: Interaction with model membranes. Biochim. Biophys. Acta 2008, 1778, 2069–2080. [Google Scholar] [CrossRef]
- Ball, S.G.; Sokolov, J.; Chin, W.W. 3,5-Diiodo-l-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J. Mol. Endocrinol. 1997, 19, 137–147. [Google Scholar] [CrossRef]
- Cioffi, F.; Lanni, A.; Goglia, F. Thyroid hormones, mitochondrial bioenergetics and lipid handling. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 402–407. [Google Scholar] [CrossRef]
- Mendoza, A.; Navarrete-Ramirez, P.; Hernandez-Puga, G.; Villalobos, P.; Holzer, G.; Renaud, J.P.; Laudet, V.; Orozco, A. 3,5-T2 is an alternative ligand for the thyroid hormone receptor beta1. Endocrinology 2013, 154, 2948–2958. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.; Rother, E.; Munzberg, H.; Wunderlich, F.T.; Morgan, D.A.; Hampel, B.; Shanabrough, M.; Janoschek, R.; Konner, A.C.; Alber, J.; et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007, 6, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Tews, D.; Fischer-Posovszky, P.; Fromme, T.; Klingenspor, M.; Fischer, J.; Ruther, U.; Marienfeld, R.; Barth, T.F.; Moller, P.; Debatin, K.M.; et al. FTO deficiency induces UCP-1 expression and mitochondrial uncoupling in adipocytes. Endocrinology 2013, 154, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.D.; Frank, A.P.; Zechner, J.F.; Li, Q.; Vishvanath, L.; Palmer, B.F.; Aguirre, V.; Gupta, R.K.; Clegg, D.J. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol. Metab. 2015, 4, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Gonzalez-Garcia, I.; Seoane-Collazo, P.; Martinez-Sanchez, N.; Linares-Pose, L.; Rial-Pensado, E.; Ferno, J.; Tena-Sempere, M.; Casals, N.; Dieguez, C.; et al. Reduction of Hypothalamic Endoplasmic Reticulum Stress Activates Browning of White Fat and Ameliorates Obesity. Diabetes 2017, 66, 87–99. [Google Scholar] [CrossRef]
- Jia, R.; Luo, X.Q.; Wang, G.; Lin, C.X.; Qiao, H.; Wang, N.; Yao, T.; Barclay, J.L.; Whitehead, J.P.; Luo, X.; et al. Characterization of cold-induced remodelling reveals depot-specific differences across and within brown and white adipose tissues in mice. Acta Physiol. 2016, 217, 311–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, L.; Seoane-Collazo, P.; Contreras, C.; Gonzalez-Garcia, I.; Martinez-Sanchez, N.; Gonzalez, F.; Zalvide, J.; Gallego, R.; Dieguez, C.; Nogueiras, R.; et al. A Functional Link between AMPK and Orexin Mediates the Effect of BMP8B on Energy Balance. Cell Rep. 2016, 16, 2231–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.H.; Kim, S.N.; Kwon, H.J.; Maddipati, K.R.; Granneman, J.G. Adipogenic role of alternatively activated macrophages in beta-adrenergic remodeling of white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R55–R65. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]







| Parameters | N (14 Weeks) | HFD (14 Weeks) | HFD-T2 (10 Weeks + 4 Weeks) |
|---|---|---|---|
| BW gain (g) | 121 ± 13.65 | 192.3 ± 8.08 * | 183.25 ± 9.4 * |
| WW (g) | 10.85 ± 0.98 | 30.67 ± 1.80 * | 22.4 ± 1.76 ** |
| % adip. | 3.19 ± 0.26 | 7.24 ± 0.45 * | 5.24 ± 0.32 ** |
| BT (g) | 0.49 ± 0.068 | 0.70 ± 0.028 | 0.83 ± 0.080 * |
| HW (g) | 1.08 ± 0.097 | 1.14 ± 0.104 | 1.18 ± 0.039 |
| GW (g) | 1.83 ± 0.045 | 2.36 ± 0.088 * | 2.37 ± 0.064 * |
| TSH (μU/mL) | 0.0065 ± 0.00028 | 0.007 ± 0.0011 | 0.0068 ± 0.00048 |
| Free T3 (pg/mL) | 1.86 ± 0.2 | 1.94 ± 0.3 | 1.81 ± 0.2 |
| Free T4 (ng/mL) | 0.91 ± 0.08 | 0.88 ± 0.07 | 0.79 ± 0.09 |
| Cholesterol (mg/dL) | 40.66 ± 2.72 | 74.66 ± 2.84 * | 57.33 ± 4.63 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senese, R.; Cioffi, F.; De Matteis, R.; Petito, G.; de Lange, P.; Silvestri, E.; Lombardi, A.; Moreno, M.; Goglia, F.; Lanni, A. 3,5 Diiodo-l-Thyronine (T2) Promotes the Browning of White Adipose Tissue in High-Fat Diet-Induced Overweight Male Rats Housed at Thermoneutrality. Cells 2019, 8, 256. https://doi.org/10.3390/cells8030256
Senese R, Cioffi F, De Matteis R, Petito G, de Lange P, Silvestri E, Lombardi A, Moreno M, Goglia F, Lanni A. 3,5 Diiodo-l-Thyronine (T2) Promotes the Browning of White Adipose Tissue in High-Fat Diet-Induced Overweight Male Rats Housed at Thermoneutrality. Cells. 2019; 8(3):256. https://doi.org/10.3390/cells8030256
Chicago/Turabian StyleSenese, Rosalba, Federica Cioffi, Rita De Matteis, Giuseppe Petito, Pieter de Lange, Elena Silvestri, Assunta Lombardi, Maria Moreno, Fernando Goglia, and Antonia Lanni. 2019. "3,5 Diiodo-l-Thyronine (T2) Promotes the Browning of White Adipose Tissue in High-Fat Diet-Induced Overweight Male Rats Housed at Thermoneutrality" Cells 8, no. 3: 256. https://doi.org/10.3390/cells8030256
APA StyleSenese, R., Cioffi, F., De Matteis, R., Petito, G., de Lange, P., Silvestri, E., Lombardi, A., Moreno, M., Goglia, F., & Lanni, A. (2019). 3,5 Diiodo-l-Thyronine (T2) Promotes the Browning of White Adipose Tissue in High-Fat Diet-Induced Overweight Male Rats Housed at Thermoneutrality. Cells, 8(3), 256. https://doi.org/10.3390/cells8030256











