Diverse Role of SNARE Protein Sec22 in Vesicle Trafficking, Membrane Fusion, and Autophagy
Abstract
1. Introduction
2. Protein Structure of Sec22
3. Role of Sec22 in Yeast
4. Filamentous Fungi
5. Plants
6. Mammals
7. Other Organisms
7.1. Drosophila Melanogaster
7.2. Caenorhabditis Elegans
7.3. Plasmodium Falciparum
8. Role of Sec22 in Autophagy
9. Future Prospects
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Palade, G. Intracellular aspects of the process of protein secretion. Science 1975. [Google Scholar] [CrossRef]
- Rothman, J.E. The Principle of Membrane Fusion in the Cell (Nobel Lecture). Angew. Chemie Int. Ed. 2014. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Denecke, J. The Endoplasmic Reticulum: Gateway of the Secretory Pathway. Plant Cell 1999. [Google Scholar] [CrossRef]
- Viotti, C. ER to golgi-dependent protein secretion: The conventional pathway. Methods Mol. Biol. 2016, 1459, 3–29. [Google Scholar] [PubMed]
- Lee, J.G.; Takahama, S.; Zhang, G.; Tomarev, S.I.; Ye, Y. Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells. Nat. Cell Biol. 2016. [Google Scholar] [CrossRef]
- Robinson, D.G.; Ding, Y.; Jiang, L. Unconventional protein secretion in plants: A critical assessment. Protoplasma 2016. [Google Scholar] [CrossRef]
- Pompa, A.; De Marchis, F.; Pallotta, M.T.; Benitez-Alfonso, Y.; Jones, A.; Schipper, K.; Moreau, K.; Žárský, V.; Di Sansebastiano, G.P.; Bellucci, M. Unconventional transport routes of soluble and membrane proteins and their role in developmental biology. Int. J. Mol. Sci. 2017, 18, 4. [Google Scholar]
- Söllner, T.; Whiteheart, S.W.; Brunner, M.; Erdjument-Bromage, H.; Geromanos, S.; Tempst, P.; Rothman, J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature 1993. [Google Scholar] [CrossRef]
- Hanson, P.I.; Heuser, J.E.; Jahn, R. Neurotransmitter release—four years of SNARE complexes. Curr. Opin. Neurobiol. 1997. [Google Scholar] [CrossRef]
- Söllner, T.; Bennett, M.K.; Whiteheart, S.W.; Scheller, R.H.; Rothman, J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 1993, 75, 409–418. [Google Scholar]
- Li, F.; Tiwari, N.; Rothman, J.E.; Pincet, F. Kinetic barriers to SNAREpin assembly in the regulation of membrane docking/priming and fusion. Proc. Natl. Acad. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Tamm, L.K. Solution NMR of SNAREs, complexin and α-synuclein in association with membrane-mimetics. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 105, 41–53. [Google Scholar]
- Bas, L.; Papinski, D.; Licheva, M.; Torggler, R.; Rohringer, S.; Schuschnig, M.; Kraft, C. Reconstitution reveals Ykt6 as the autophagosomal SNARE in autophagosome-vacuole fusion. J. Cell Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, S.; James, D.J.; Serrano, M.Q.; Esquibel, J.; Woo, S.S.; Kielar-Grevstad, E.; Crummy, E.; Qurashi, R.; Kowalchyk, J.A.; Martin, T.F.J. Small molecules that inhibit the late stage of Munc13-4–dependent secretory granule exocytosis in mast cells. J. Biol. Chem. 2018, 293, 8217–8229. [Google Scholar]
- Han, J.; Pluhackova, K.; Böckmann, R.A. The multifaceted role of SNARE proteins in membrane fusion. Front. Physiol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Tao-Cheng, J.H.; Pham, A.; Yang, Y.; Winters, C.A.; Gallant, P.E.; Reese, T.S. Syntaxin 4 is concentrated on plasma membrane of astrocytes. Neuroscience 2015. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Liu, W.; Duan, X.; Shang, W.; Xia, D.; Tong, C. Sec22 regulates endoplasmic reticulum morphology but not autophagy and is required for eye development in Drosophila. J. Biol. Chem. 2015, 290, 7943–7951. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Shen, C.; Belenkaya, T.Y.; Ray, L.; Lin, X. Drosophila p24 and Sec22 regulate Wingless trafficking in the early secretory pathway. Biochem. Biophys. Res. Commun. 2015, 463, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.J.; Mukherjee, I.; Barlowe, C. Examination of Sec22 homodimer formation and role in SNARE-dependent membrane fusion. J. Biol. Chem. 2015, 290, 10657–10666. [Google Scholar] [CrossRef]
- Lee, H.; Noh, H.; Mun, J.; Gu, C.; Sever, S.; Park, S. Anks1a regulates COPII-mediated anterograde transport of receptor tyrosine kinases critical for tumorigenesis. Nat. Commun. 2016, 7, 12799. [Google Scholar] [CrossRef]
- Hanna, M.G.; Yuan, L.; Frankel, E.B.; Knight, G.; Johnson, A.; Audhya, A.; Hou, F.; Schekman, R.; Block, S.; Ashton, R.; et al. TFG facilitates outer coat disassembly on COPII transport carriers to promote tethering and fusion with ER–Golgi intermediate compartments. Proc. Natl. Acad. Sci. 2017. [Google Scholar] [CrossRef]
- Zheng, H.; Miao, P.; Lin, X.; Li, L.; Wu, C.; Chen, X.; Abubakar, Y.S.; Norvienyeku, J.; Li, G.; Zhou, J. Small GTPase Rab7-mediated FgAtg9 trafficking is essential for autophagy-dependent development and pathogenicity in Fusarium graminearum. PLoS Genet. 2018, 14, e1007546. [Google Scholar] [CrossRef]
- Kurokawa, K.; Okamoto, M.; Nakano, A. Contact of cis-Golgi with ER exit sites executes cargo capture and delivery from the ER. Nat. Commun. 2014, 5, 3653. [Google Scholar] [CrossRef]
- Brandizzi, F.; Barlowe, C. Organization of the ER-Golgi interface for membrane traffic control. Nat. Rev. Mol. Cell Biol. 2013. [Google Scholar] [CrossRef]
- Spang, A. Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013391. [Google Scholar] [CrossRef]
- Dodonova, S.O.; Diestelkoetter-Bachert, P.; Von Appen, A.; Hagen, W.J.H.; Beck, R.; Beck, M.; Wieland, F.; Briggs, J.A.G. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science 2015. [Google Scholar] [CrossRef]
- Yu, X.; Breitman, M.; Goldberg, J. A Structure-based mechanism for Arf1-dependent recruitment of coatomer to membranes. Cell 2012. [Google Scholar] [CrossRef]
- Daste, F.; Galli, T.; Tareste, D. Structure and function of longin SNAREs. J. Cell Sci. 2015. [Google Scholar] [CrossRef]
- Davis, S.; Wang, J.; Ferro-Novick, S. Crosstalk between the secretory and autophagy pathways regulates autophagosome formation. Dev. Cell 2017, 41, 23–32. [Google Scholar] [CrossRef]
- Kimura, T.; Jia, J.; Claude-Taupin, A.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; et al. Cellular and molecular mechanism for secretory autophagy. Autophagy 2017. [Google Scholar] [CrossRef]
- Feyder, S.; De Craene, J.-O.; Bär, S.; Bertazzi, D.; Friant, S. Membrane trafficking in the yeast Saccharomyces cerevisiae model. Int. J. Mol. Sci. 2015, 16, 1509–1525. [Google Scholar]
- Tsui, M.M.K.; Tai, W.C.S.; Banfield, D.K. Selective Formation of Sed5p-containing SNARE Complexes Is Mediated by Combinatorial Binding Interactions. Mol. Biol. Cell 2001. [Google Scholar] [CrossRef]
- Meiringer, C.T.A.; Rethmeier, R.; Auffarth, K.; Wilson, J.; Perz, A.; Barlowe, C.; Schmitt, H.D.; Ungermann, C. The Dsl1 protein tethering complex is a resident endoplasmic reticulum complex, which interacts with five soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptors (SNAREs): Implications for fusion and fusion regulation. J. Biol. Chem. 2011, 286, 25039–25046. [Google Scholar]
- Furukawa, N.; Mima, J. Multiple and distinct strategies of yeast SNAREs to confer the specificity of membrane fusion. Sci. Rep. 2014, 4, 4277. [Google Scholar] [CrossRef]
- Lemus, L.; Ribas, J.L.; Sikorska, N.; Goder, V. An ER-localized SNARE protein is exported in specific COPII vesicles for autophagosome biogenesis. Cell Rep. 2016, 14, 1710–1722. [Google Scholar] [CrossRef]
- Khurana, G.K.; Vishwakarma, P.; Puri, N.; Lynn, A.M. Phylogenetic Analysis of the vesicular fusion SNARE machinery revealing its functional divergence across Eukaryotes. Bioinformation 2018, 14, 361. [Google Scholar] [CrossRef]
- Liu, Y.; Flanagan, J.J.; Barlowe, C. Sec22p export from the endoplasmic reticulum is independent of SNARE pairing. J. Biol. Chem. 2004, 279, 27225–27232. [Google Scholar] [CrossRef]
- Yang, B.; Gonzalez, L.; Prekeris, R.; Steegmaier, M.; Advani, R.J.; Scheller, R.H. SNARE interactions are not selective implications for membrane fusion specificity. J. Biol. Chem. 1999, 274, 5649–5653. [Google Scholar] [CrossRef]
- Grote, E.; Vlacich, G.; Pypaert, M.; Novick, P.J. A snc1 Endocytosis Mutant: Phenotypic Analysis and Suppression by Overproduction of Dihydrosphingosine Phosphate Lyase. Mol. Biol. Cell 2000. [Google Scholar] [CrossRef]
- Frigerio, G. The Saccharomyces cerevisiae early secretion mutant tip20 is synthetic lethal with mutants in yeast coatomer and the SNARE proteins Sec22p and Ufe1p. Yeast 1998, 14, 633–646. [Google Scholar] [CrossRef]
- Letourneur, F.; Gaynor, E.C.; Hennecke, S.; Demolliere, C.; Duden, R.; Emr, S.D.; Riezman, H.; Cosson, P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 1994, 79, 1199–1207. [Google Scholar] [CrossRef]
- Ossipov, D.; Schroder-Kohne, S.; Schmitt, H.D. Yeast ER-Golgi v-SNAREs Bos1p and Bet1p differ in steady-state localization and targeting. J. Cell Sci. 1999, 112, 4135–4142. [Google Scholar]
- Mancias, J.D.; Goldberg, J. The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol. Cell 2007, 26, 403–414. [Google Scholar] [CrossRef]
- Ossig, R.; Dascher, C.; Trepte, H.H.; Schmitt, H.D.; Gallwitz, D. The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol. Cell. Biol. 1991, 11, 2980–2993. [Google Scholar] [CrossRef]
- Kaiser, C.A.; Schekman, R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 1990, 61, 723–733. [Google Scholar] [CrossRef]
- Liu, Y.; Barlowe, C. Analysis of Sec22p in Endoplasmic Reticulum/Golgi Transport Reveals Cellular Redundancy in SNARE Protein Function. Mol. Biol. Cell 2002. [Google Scholar] [CrossRef]
- Parlati, F.; Varlamov, O.; Paz, K.; McNew, J.A.; Hurtado, D.; Söllner, T.H.; Rothman, J.E. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. 2002. [Google Scholar] [CrossRef]
- Burri, L.; Lithgow, T.; Varlamov, O.; Doege, C.A.; Rothman, J.E.; Sollner, T.H.; Hofmann, K.; Beilharz, T. A SNARE required for retrograde transport to the endoplasmic reticulum. Proc. Natl. Acad. Sci. 2003. [Google Scholar] [CrossRef]
- Grabski, R.; Hay, J.; Sztul, E. Tethering factor P115: A new model for tether-SNARE interactions. Bioarchitecture 2012. [Google Scholar] [CrossRef]
- Brunger, A.T. Structure and function of SNARE and SNARE-interacting proteins. Q. Rev. Biophys. 2005. [Google Scholar] [CrossRef]
- Dascher, C.; Ossig, R.; Gallwitz, D.; Schmitt, H.D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell. Biol. 1991, 11, 872–885. [Google Scholar]
- Hu, J.; Shibata, Y.; Zhu, P.-P.; Voss, C.; Rismanchi, N.; Prinz, W.A.; Rapoport, T.A.; Blackstone, C. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell 2009, 138, 549–561. [Google Scholar] [CrossRef]
- Anwar, K.; Klemm, R.W.; Condon, A.; Severin, K.N.; Zhang, M.; Ghirlando, R.; Hu, J.; Rapoport, T.A.; Prinz, W.A. The dynamin-like GTPase Sey1p mediates homotypic ER fusion in S. cerevisiae. J. Cell Biol. 2012, 197, 209–217. [Google Scholar] [CrossRef]
- Lee, M.; Ko, Y.J.; Moon, Y.; Han, M.; Kim, H.W.; Lee, S.H.; Kang, K.J.; Jun, Y. SNAREs support atlastin-mediated homotypic ER fusion in Saccharomyces cerevisiae. J. Cell Biol. 2015. [Google Scholar] [CrossRef]
- Newman, A.P.; Shim, J.; Ferro-Novick, S. BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol. Cell. Biol. 1990, 10, 3405–3414. [Google Scholar] [CrossRef]
- Hong, W.J.; Lev, S. Tethering the assembly of SNARE complexes. Trends Cell Biol. 2014. [Google Scholar] [CrossRef]
- Chi, M.H.; Park, S.Y.; Kim, S.; Lee, Y.H. A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathog. 2009. [Google Scholar] [CrossRef]
- Song, W.; Dou, X.; Qi, Z.; Wang, Q.; Zhang, X.; Zhang, H.; Guo, M.; Dong, S.; Zhang, Z.; Wang, P. R-SNARE homolog MoSec22 is required for conidiogenesis, cell wall integrity, and pathogenesis of Magnaporthe oryzae. PLoS ONE 2010, 5, e13193. [Google Scholar] [CrossRef]
- Kikuma, T.; Arioka, M.; Kitamoto, K. Autophagy during conidiation and conidial germination in filamentous fungi. Autophagy 2007, 3, 128–129. [Google Scholar]
- Garzia, A.; Etxebeste, O.; Herrero-Garcia, E.; Fischer, R.; Espeso, E.A.; Ugalde, U. Aspergillus nidulans FlbE is an upstream developmental activator of conidiation functionally associated with the putative transcription factor FlbB. Mol. Microbiol. 2009, 71, 172–184. [Google Scholar] [CrossRef]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar]
- Yukioka, H.; Inagaki, S.; Tanaka, R.; Katoh, K.; Miki, N.; Mizutani, A.; Masuko, M. Transcriptional activation of the alternative oxidase gene of the fungus Magnaporthe grisea by a respiratory-inhibiting fungicide and hydrogen peroxide. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1998, 1442, 161–169. [Google Scholar]
- Boenisch, M.J.; Broz, K.L.; Purvine, S.O.; Chrisler, W.B.; Nicora, C.D.; Connolly, L.R.; Freitag, M.; Baker, S.E.; Kistler, H.C. Structural reorganization of the fungal endoplasmic reticulum upon induction of mycotoxin biosynthesis. Sci. Rep. 2017, 7, 44296. [Google Scholar] [CrossRef]
- Irieda, H.; Maeda, H.; Akiyama, K.; Hagiwara, A.; Saitoh, H.; Uemura, A.; Terauchi, R.; Takano, Y. Colletotrichum orbiculare secretes virulence effectors to a biotrophic interface at the primary hyphal neck via exocytosis coupled with SEC22-mediated traffic. Plant Cell 2014, 26, 2265–2281. [Google Scholar] [CrossRef]
- Traeger, S.; Nowrousian, M. Functional Analysis of Developmentally Regulated Genes chs7 and sec22 in the Ascomycete Sordaria macrospora. G3 2015, 5, 1233–1245. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Zhang, D.-D.; Short, D.P.G.; Zhou, L.; Song, S.-S.; Liu, Y.; Wang, D.; Kong, Z.-Q.; Cui, W.-Y. SNARE-Encoding Genes VdSec22 and VdSso1 Mediate Protein Secretion Required for Full Virulence in Verticillium dahliae. Mol. Plant-Microbe Interact. 2018, 31, 651–664. [Google Scholar] [CrossRef]
- Wang, D.; Hong, J. Expression of Cellulolytic Enzymes in Yeast. In Fungal Cellulolytic Enzymes; Springer: Singapore, 2018; pp. 201–221. [Google Scholar]
- El-Kasmi, F.; Pacher, T.; Strompen, G.; Stierhof, Y.D.; Müller, L.M.; Koncz, C.; Mayer, U.; Jürgens, G. Arabidopsis SNARE protein SEC22 is essential for gametophyte development and maintenance of Golgi-stack integrity. Plant J. 2011. [Google Scholar] [CrossRef]
- Chatre, L. Sec22 and Memb11 Are v-SNAREs of the Anterograde Endoplasmic Reticulum-Golgi Pathway in Tobacco Leaf Epidermal Cells. PLANT Physiol. 2005. [Google Scholar] [CrossRef]
- Vedovato, M.; Rossi, V.; Dacks, J.B.; Filippini, F. Comparative analysis of plant genomes allows the definition of the“Phytolongins”: A novel non-SNARE longin domain protein family. BMC Genomics 2009, 10, 510. [Google Scholar]
- de Marcos Lousa, C.; Soubeyrand, E.; Bolognese, P.; Wattelet-Boyer, V.; Bouyssou, G.; Marais, C.; Boutté, Y.; Filippini, F.; Moreau, P. Subcellular localization and trafficking of phytolongins (non-SNARE longins) in the plant secretory pathway. J. Exp. Bot. 2016, 67, 2627–2639. [Google Scholar] [CrossRef]
- Malsam, J.; Söllner, T.H. Organization of SNAREs within the Golgi stack. Cold Spring Harb. Perspect. Biol. 2011, 3, a005249. [Google Scholar]
- Diefenbacher, M.; Thorsteinsdottir, H.; Spang, A. The Dsl1 tethering complex actively participates in soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor (SNARE) complex assembly at the endoplasmic reticulum in Saccharomyces cerevisiae. J. Biol. Chem. 2011, 286, 25027–25038. [Google Scholar]
- Tagaya, M.; Arasaki, K.; Inoue, H.; Kimura, H. Moonlighting functions of the NRZ (mammalian Dsl1) complex. Front. Cell Dev. Biol. 2014, 2, 25. [Google Scholar] [CrossRef]
- Chia, P.Z.C.; Gleeson, P.A. Membrane tethering. F1000Prime Rep. 2014, 6. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yurugi, C.; Sakisaka, T. The number of the C-terminal transmembrane domains has the potency to specify subcellular localization of Sec22c. Biochem. Biophys. Res. Commun. 2017, 487, 388–395. [Google Scholar] [CrossRef]
- Rossi, V.; Banfield, D.K.; Vacca, M.; Dietrich, L.E.P.; Ungermann, C.; D’Esposito, M.; Galli, T.; Filippini, F. Longins and their longin domains: Regulated SNAREs and multifunctional SNARE regulators. Trends Biochem. Sci. 2004, 29, 682–688. [Google Scholar]
- Hay, J.C.; Hirling, H.; Scheller, R.H. Mammalian vesicle trafficking proteins of the endoplasmic reticulum and Golgi apparatus. J. Biol. Chem. 1996, 271, 5671–5679. [Google Scholar] [CrossRef]
- Hay, J.C.; Chao, D.S.; Kuo, C.S.; Scheller, R.H. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell 1997, 89, 149–158. [Google Scholar]
- Paek, I.; Orci, L.; Ravazzola, M.; Erdjument-Bromage, H.; Amherdt, M.; Tempst, P.; Söllner, T.H.; Rothman, J.E. ERS-24, a mammalian v-SNARE implicated in vesicle traffic between the ER and the Golgi. J. Cell Biol. 1997, 137, 1017–1028. [Google Scholar] [CrossRef]
- Tang, B.L.; Low, D.Y.H.; Hong, W. Hsec22c: A homolog of yeast Sec22p and mammalian rsec22a and msec22b/ERS-24. Biochem. Biophys. Res. Commun. 1998, 243, 885–891. [Google Scholar] [CrossRef]
- Burri, L.; Lithgow, T. A complete set of SNAREs in yeast. Traffic 2004, 5, 45–52. [Google Scholar]
- Siddiqi, S.; Mani, A.M.; Siddiqi, S.A. The identification of the SNARE complex required for the fusion of VLDL-transport vesicle with hepatic cis -Golgi. Biochem. J. 2010. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, W.; Sheng, W.; Li, X. Identification of Biomarkers Associated with Alzheimer’s Disease by Bioinformatics Analysis. Am. J. Alzheimers. Dis. Other Demen. 2016. [Google Scholar] [CrossRef]
- Chagoyen, M.; Carmona-Saez, P.; Shatkay, H.; Carazo, J.M.; Pascual-Montano, A. Discovering semantic features in the literature: A foundation for building functional associations. BMC Bioinformatics 2006, 7, 41. [Google Scholar]
- Berchtold, N.C.; Coleman, P.D.; Cribbs, D.H.; Rogers, J.; Gillen, D.L.; Cotman, C.W. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1653–1661. [Google Scholar] [CrossRef]
- Petkovic, M.; Jemaiel, A.; Daste, F.; Specht, C.G.; Izeddin, I.; Vorkel, D.; Verbavatz, J.M.; Darzacq, X.; Triller, A.; Pfenninger, K.H.; et al. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat. Cell Biol. 2014. [Google Scholar] [CrossRef]
- Lin, S.; Sun, S.; Hu, J. Molecular basis for sculpting the endoplasmic reticulum membrane. Int. J. Biochem. Cell Biol. 2012, 44, 1436–1443. [Google Scholar]
- Chen, S.; Novick, P.; Ferro-Novick, S. ER structure and function. Curr. Opin. Cell Biol. 2013, 25, 428–433. [Google Scholar] [CrossRef]
- Zhao, Y.; Holmgren, B.T.; Hinas, A. The conserved SNARE SEC-22 localizes to late endosomes and negatively regulates RNA interference in Caenorhabditis elegans. RNA 2017, 23, 297–307. [Google Scholar] [PubMed]
- Ayong, L.; Raghavan, A.; Schneider, T.G.; Taraschi, T.F.; Fidock, D.A.; Chakrabarti, D. The longin domain regulates the steady-state dynamics of Sec22 in Plasmodium falciparum. Eukaryot. Cell 2009, 8, 1330–1340. [Google Scholar] [CrossRef]
- Dräxl, S.; Müller, J.; Li, W.B.; Michalke, B.; Scherb, H.; Hense, B.A.; Tschiersch, J.; Kanter, U.; Schäffner, A.R. Caesium accumulation in yeast and plants is selectively repressed by loss of the SNARE Sec22p/SEC22. Nat. Commun. 2013, 4, 2092. [Google Scholar]
- Van Zyl, J.H.D.; Den Haan, R.; Van Zyl, W.H. Overexpression of native Saccharomyces cerevisiae ER-to-Golgi SNARE genes increased heterologous cellulase secretion. Appl. Microbiol. Biotechnol. 2016, 100, 505–518. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the Pathogenesis of Disease. Cell 2008. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Mol. Cell Biol. 2012. [Google Scholar] [CrossRef]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011. [Google Scholar] [CrossRef]
- Stolz, A.; Ernst, A.; Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 2014, 16, 495–501. [Google Scholar] [CrossRef]
- Hayashi-Nishino, M.; Fujita, N.; Noda, T.; Yamaguchi, A.; Yoshimori, T.; Yamamoto, A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 2009. [Google Scholar] [CrossRef]
- Ylä-Anttila, P.; Vihinen, H.; Jokitalo, E.; Eskelinen, E.L. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy 2009. [Google Scholar] [CrossRef]
- Ravikumar, B.; Moreau, K.; Jahreiss, L.; Puri, C.; Rubinsztein, D.C. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat. Cell Biol. 2010. [Google Scholar] [CrossRef]
- van der Vaart, A.; Griffith, J.; Reggiori, F. Exit from the Golgi Is Required for the Expansion of the Autophagosomal Phagophore in Yeast Saccharomyces cerevisiae. Mol. Biol. Cell 2010. [Google Scholar] [CrossRef]
- Yen, W.L.; Shintani, T.; Nair, U.; Cao, Y.; Richardson, B.C.; Li, Z.; Hughson, F.M.; Baba, M.; Klionsky, D.J. The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J. Cell Biol. 2010. [Google Scholar] [CrossRef]
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria Supply Membranes for Autophagosome Biogenesis during Starvation. Cell 2010. [Google Scholar] [CrossRef]
- Weber, T.; Zemelman, B.V.; McNew, J.A.; Westermann, B.; Gmachl, M.; Parlati, F.; Söllner, T.H.; Rothman, J.E. SNAREpins: Minimal machinery for membrane fusion. Cell 1998. [Google Scholar] [CrossRef]
- Moreau, K.; Renna, M.; Rubinsztein, D.C. Connections between SNAREs and autophagy. Trends Biochem. Sci. 2013, 38, 57–63. [Google Scholar]
- Moreau, K.; Ravikumar, B.; Renna, M.; Puri, C.; Rubinsztein, D.C. Autophagosome precursor maturation requires homotypic fusion. Cell 2011, 146, 303–317. [Google Scholar] [CrossRef]
- Nair, U.; Klionsky, D.J. Autophagosome biogenesis requires SNAREs. Autophagy 2011. [Google Scholar] [CrossRef]
- Moreau, K.; Rubinsztein, D.C. The plasma membrane as a control center for autophagy. Autophagy 2012, 8, 861–863. [Google Scholar] [CrossRef]
- Nair, U.; Jotwani, A.; Geng, J.; Gammoh, N.; Richerson, D.; Yen, W.L.; Griffith, J.; Nag, S.; Wang, K.; Moss, T.; et al. SNARE proteins are required for macroautophagy. Cell 2011. [Google Scholar] [CrossRef]
- Rajput, S.S.; Chinchwadkar, S.; Aher, A.; Matheshwaran, S.; Manjithaya, R. Exocyst subcomplex functions in autophagosome biogenesis by regulating Atg9 trafficking. bioRxiv 2018, 306969. [Google Scholar]

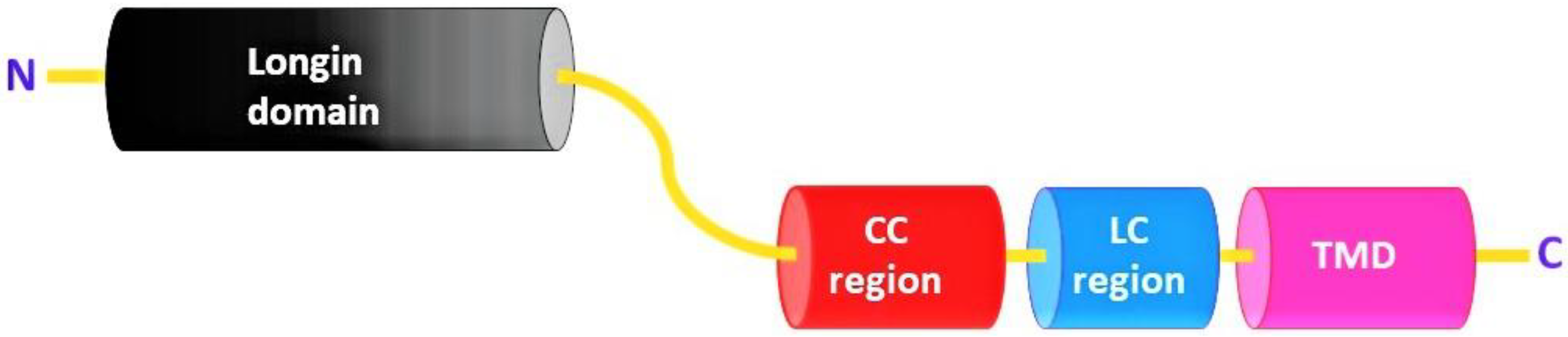
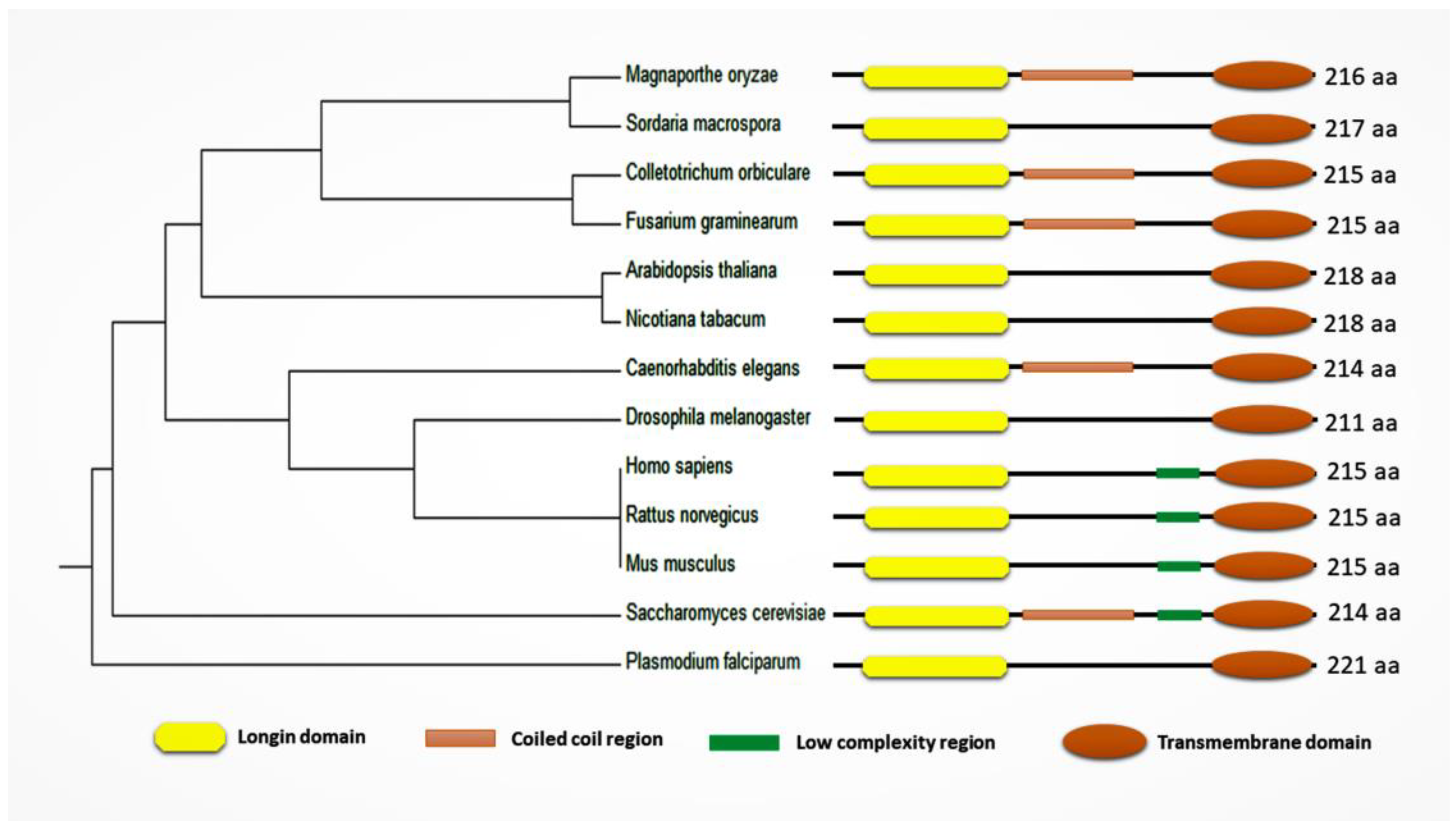
| Organism | Functions of Sec22 (Other than Anterograde and Retrograde Trafficking) | References |
|---|---|---|
| Saccharomyces cerevisiae | Uptake of caesium ions, cellulase secretion, maintenance of ER morphology and autophagy | [54,92,93] |
| Sordaria macrospora | Effects sexual, asexual reproduction, regulation of ER associated proteins, melanin biosynthesis, and development related genes | [65] |
| Colletotrichum orbiculare | Transport of virulence related effectors | [64] |
| Fusarium graminearum | Effects sexual, asexual reproduction and pathogenicity | (unpublished data) |
| Magnaporthe oryzae | Effects cell wall integrity, growth, reproduction, pathogenicity, chitin deposition, regulation of reactive oxygen species (ROS) level, endocytosis, expression of extracellular enzymes | [58] |
| Verticilium dahliae | Regulates enzymatic cell wall degradation and pathogenicity | [66] |
| Arabidopsis thaliana | Gametophyte development and uptake of caesium | [92] |
| Nicotiana tabacum | Overexpression causes collapse of Golgi membrane proteins into ER | [71] |
| Drosophila melanogaster | Eye morphogenesis, wingless signaling pathway, Sec22 mutation causes abnormal ER, and Golgi morphology | [17,18] |
| Mammals (Homo sapiens, Rattusnorvegicus, Mus musculus) | Autophagy, regulate cell motion, protein trafficking, translocation, and downregulation in the hippocampus of aging and Alzheimer’s disease brains | [72,84,86] |
| Caenorebditis elegans | Regulates RNAi | [90] |
| Pasmodium falciparum | Encompasses signals for ER/Golgi recycling and fractional export beyond the ER/Golgi interface | [91] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, M.; Islam, W.; Zhang, J.; Zheng, W.; Lu, G.-D. Diverse Role of SNARE Protein Sec22 in Vesicle Trafficking, Membrane Fusion, and Autophagy. Cells 2019, 8, 337. https://doi.org/10.3390/cells8040337
Adnan M, Islam W, Zhang J, Zheng W, Lu G-D. Diverse Role of SNARE Protein Sec22 in Vesicle Trafficking, Membrane Fusion, and Autophagy. Cells. 2019; 8(4):337. https://doi.org/10.3390/cells8040337
Chicago/Turabian StyleAdnan, Muhammad, Waqar Islam, Jing Zhang, Wenhui Zheng, and Guo-Dong Lu. 2019. "Diverse Role of SNARE Protein Sec22 in Vesicle Trafficking, Membrane Fusion, and Autophagy" Cells 8, no. 4: 337. https://doi.org/10.3390/cells8040337
APA StyleAdnan, M., Islam, W., Zhang, J., Zheng, W., & Lu, G.-D. (2019). Diverse Role of SNARE Protein Sec22 in Vesicle Trafficking, Membrane Fusion, and Autophagy. Cells, 8(4), 337. https://doi.org/10.3390/cells8040337






