Cargo Sorting at the trans-Golgi Network for Shunting into Specific Transport Routes: Role of Arf Small G Proteins and Adaptor Complexes
Abstract
1. Introduction
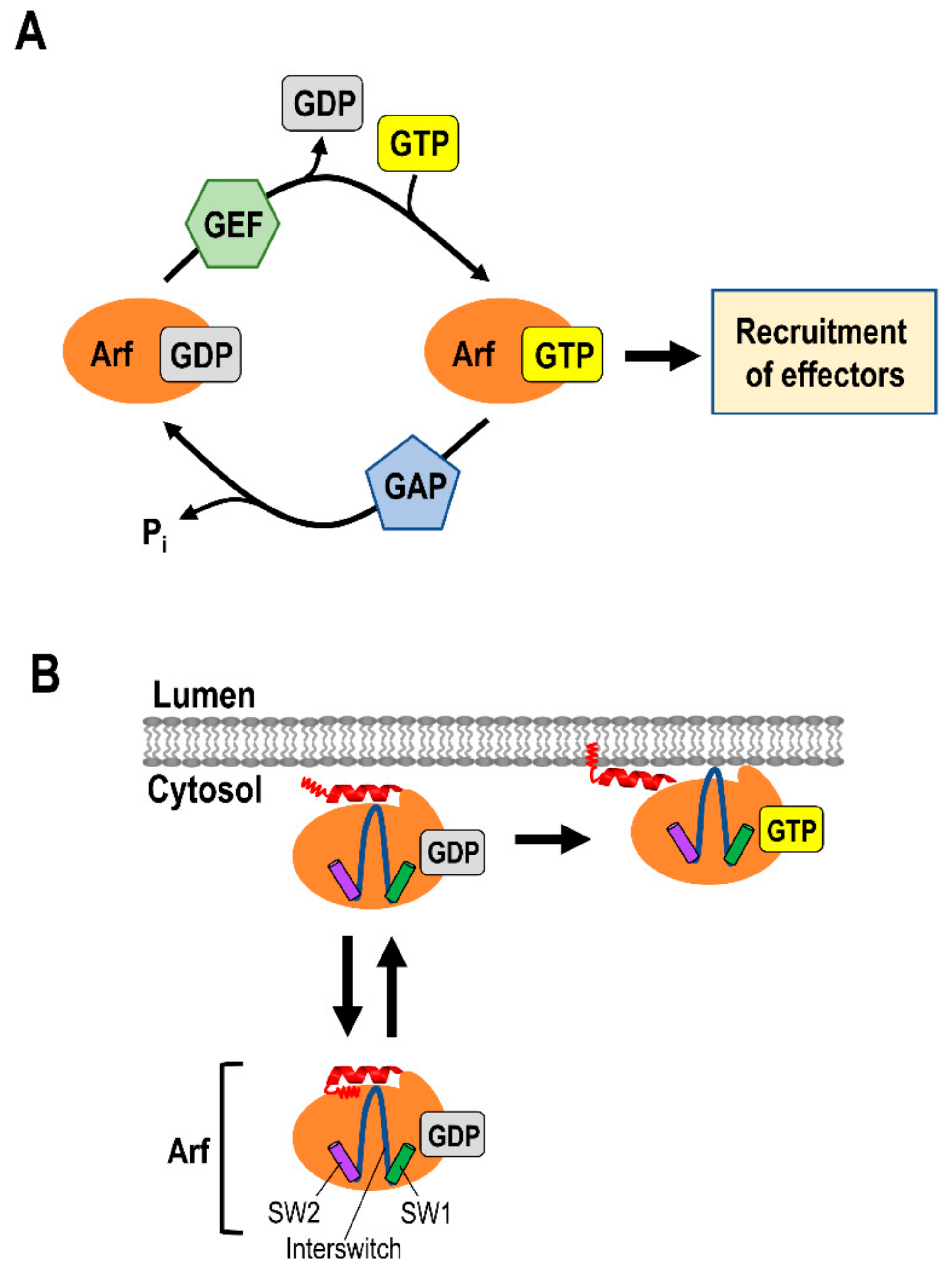
2. Small G Proteins of the ADP Ribosylation Factor (Arf) Family
3. Roles of Phospholipids in Regulating Protein Sorting
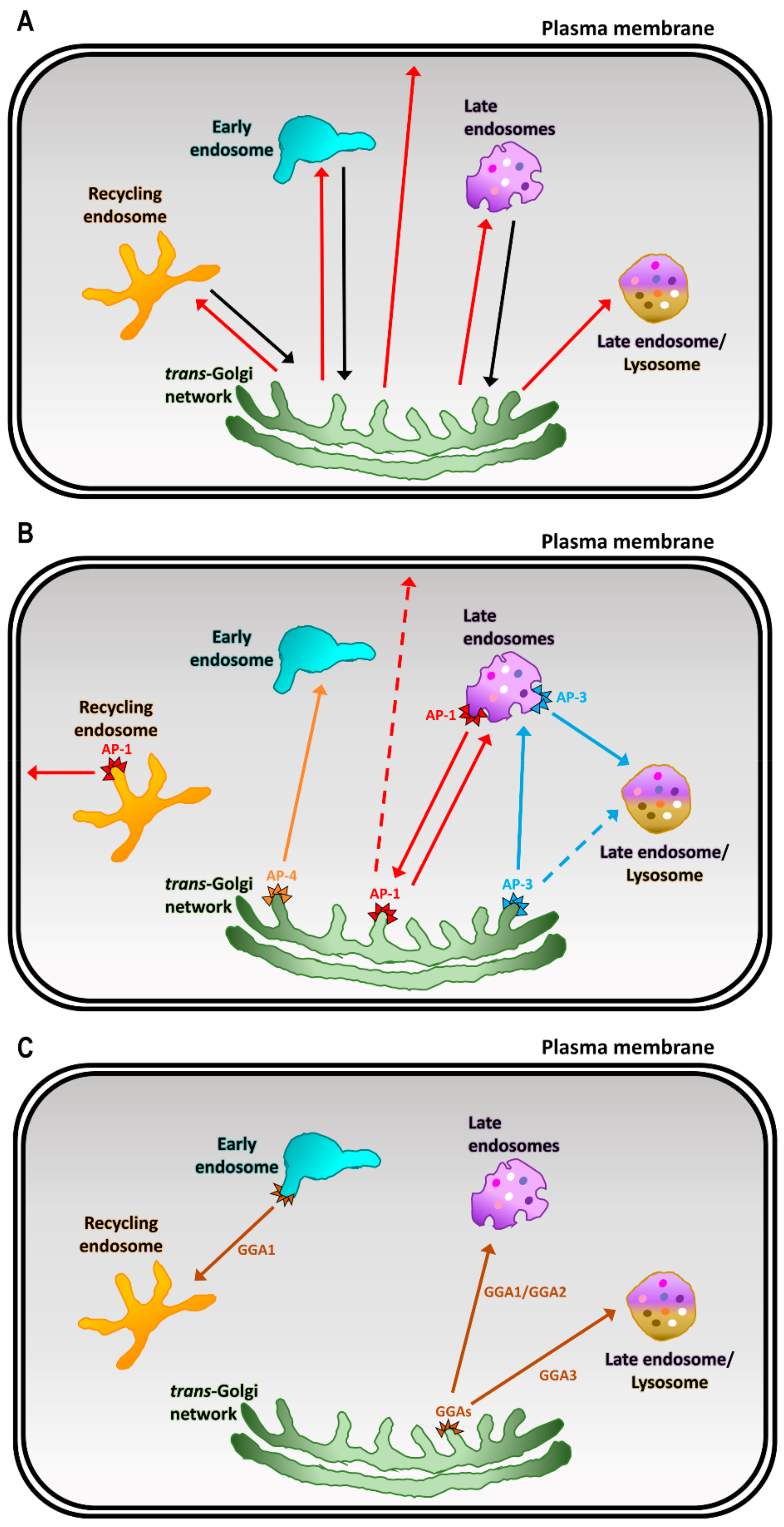
4. Cargo Adaptors at the TGN
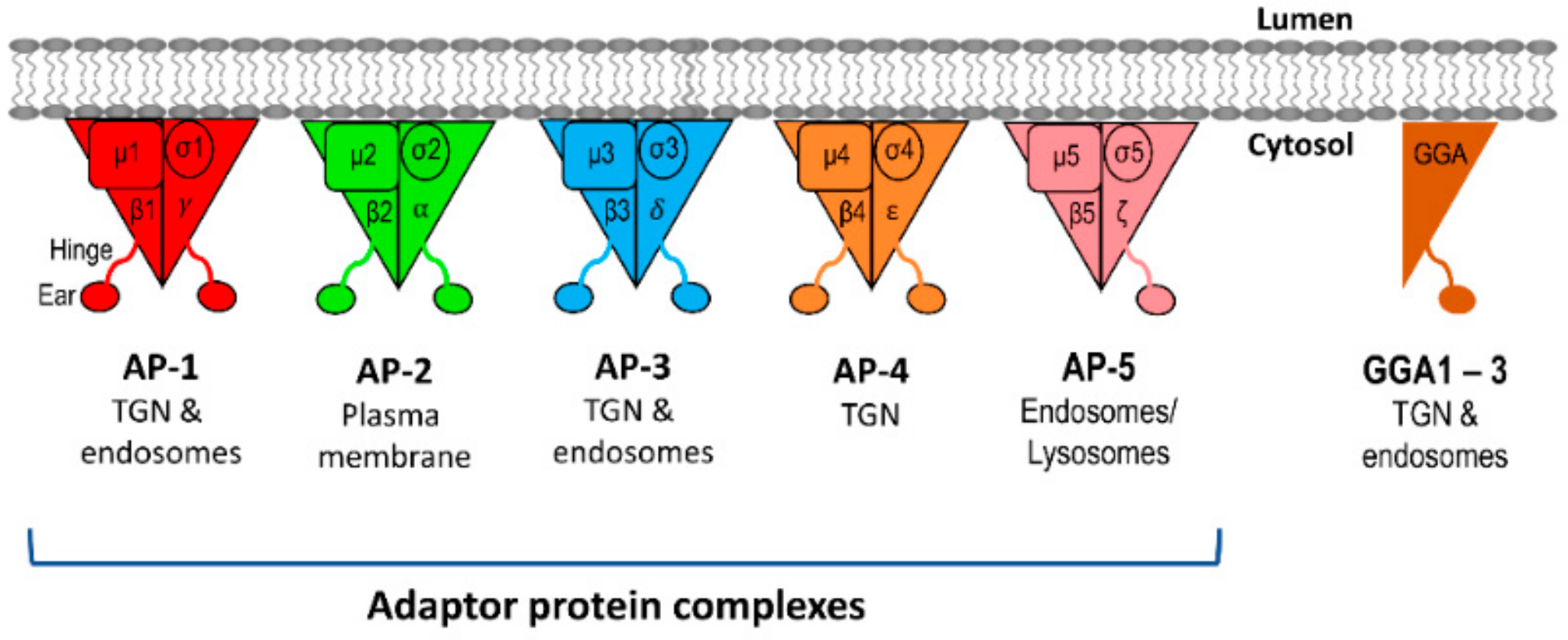
4.1. Adaptor Protein (AP) Complexes at the TGN
4.2. Golgi-Localized, γ Ear-Containing, Arf-Binding Proteins (GGAs)
5. Cargo Sorting Signals
6. Accessory Proteins of AP Complexes and the Formation of Transport Vesicles
7. Challenges and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ladinsky, M.S.; Mastronarde, D.N.; McIntosh, J.R.; Howell, K.E.; Staehelin, L.A. Golgi structure in three dimensions: Functional insights from the normal rat kidney cell. J. Cell Biol. 1999, 144, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Klumperman, J. Architecture of the mammalian golgi. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sirkis, D.W.; Schekman, R. Protein sorting at the trans-Golgi network. Annu. Rev. Cell Dev. Biol. 2014, 30, 169–206. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, M.A.; Luini, A. Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol 2008, 9, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M.; Spang, A. Intracellular parcel service: Current issues in intracellular membrane trafficking. Methods Mol. Biol 2015, 1270, 1–12. [Google Scholar] [PubMed]
- De Matteis, M.A.; Luini, A. Mendelian disorders of membrane trafficking. N. Engl. J. Med. 2011, 365, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Derby, M.C.; Gleeson, P.A. New insights into membrane trafficking and protein sorting. Int. Rev. Cytol. 2007, 261, 47–116. [Google Scholar]
- Donaldson, J.G.; Jackson, C.L. Arf family G proteins and their regulators: Roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011, 12, 362–375. [Google Scholar] [CrossRef]
- Gillingham, A.K.; Munro, S. The small G proteins of the arf family and their regulators. Annu. Rev. Cell Dev. Biol. 2007, 23, 579–611. [Google Scholar] [CrossRef]
- Gleeson, P.A.; Lock, J.G.; Luke, M.R.; Stow, J.L. Domains of the TGN: Coats, tethers and G proteins. Traffic 2004, 5, 315–326. [Google Scholar] [CrossRef]
- Kirchhausen, T.; Bonifacino, J.S.; Riezman, H. Linking cargo to vesicle formation: Receptor tail interactions with coat proteins. Curr. Opin. Cell Biol. 1997, 9, 488–495. [Google Scholar] [CrossRef]
- Bonifacino, J.S. Adaptor proteins involved in polarized sorting. J. Cell Biol. 2014, 204, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Traub, L.M.; Bonifacino, J.S. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 2013, 5, a016790. [Google Scholar] [CrossRef] [PubMed]
- Faini, M.; Beck, R.; Wieland, F.T.; Briggs, J.A. Vesicle coats: Structure, function, and general principles of assembly. Trends Cell Biol. 2013, 23, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; Edgar, J.R.; Borner, G.H.; Li, S.; Sahlender, D.A.; Antrobus, R.; Robinson, M.S. Contributions of epsinR and gadkin to clathrin-mediated intracellular trafficking. Mol. Biol. Cell 2015, 26, 3085–3103. [Google Scholar] [CrossRef]
- Boucrot, E.; Pick, A.; Camdere, G.; Liska, N.; Evergren, E.; McMahon, H.T.; Kozlov, M.M. Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent bar domains. Cell 2012, 149, 124–136. [Google Scholar] [CrossRef]
- Ford, M.G.; Mills, I.G.; Peter, B.J.; Vallis, Y.; Praefcke, G.J.; Evans, P.R.; McMahon, H.T. Curvature of clathrin-coated pits driven by epsin. Nature 2002, 419, 361–366. [Google Scholar] [CrossRef]
- Hirst, J.; Irving, C.; Borner, G.H. Adaptor protein complexes ap-4 and ap-5: New players in endosomal trafficking and progressive spastic paraplegia. Traffic 2013, 14, 153–164. [Google Scholar] [CrossRef]
- Robinson, M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004, 14, 167–174. [Google Scholar] [CrossRef]
- Robinson, M.S.; Bonifacino, J.S. Adaptor-related proteins. Curr. Opin. Cell Biol. 2001, 13, 444–453. [Google Scholar] [CrossRef]
- Goldberg, J. Structural basis for activation of Arf GTPase: Mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 1998, 95, 237–248. [Google Scholar] [CrossRef]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. Gefs and gaps: Critical elements in the control of small G proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Antonny, B.; Beraud-Dufour, S.; Chardin, P.; Chabre, M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 1997, 36, 4675–4684. [Google Scholar] [CrossRef] [PubMed]
- Chavrier, P.; Gorvel, J.P.; Stelzer, E.; Simons, K.; Gruenberg, J.; Zerial, M. Hypervariable c-terminal domain of rab proteins acts as a targeting signal. Nature 1991, 353, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.A.; Cherfils, J.; Elias, M.; Lovering, R.C.; Munro, S.; Schurmann, A. Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J. Cell Biol. 2006, 172, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Manolea, F.; Chun, J.; Chen, D.W.; Clarke, I.; Summerfeldt, N.; Dacks, J.B.; Melancon, P. Arf3 is activated uniquely at the trans-golgi network by brefeldin A-inhibited guanine nucleotide exchange factors. Mol. Biol. Cell 2010, 21, 1836–1849. [Google Scholar] [CrossRef]
- Hosaka, M.; Toda, K.; Takatsu, H.; Torii, S.; Murakami, K.; Nakayama, K. Structure and intracellular localization of mouse ADP-ribosylation factors type 1 to type 6 (Arf1-Arf6). J. Biochem. 1996, 120, 813–819. [Google Scholar] [CrossRef]
- Cavenagh, M.M.; Whitney, J.A.; Carroll, K.; Zhang, C.; Boman, A.L.; Rosenwald, A.G.; Mellman, I.; Kahn, R.A. Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane. J. Biol. Chem. 1996, 271, 21767–21774. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Li, Y.; Zhang, C.J.; Kahn, R.A. Isoform-selective effects of the depletion of ADP-ribosylation factors 1-5 on membrane traffic. Mol. Biol. Cell 2005, 16, 4495–4508. [Google Scholar] [CrossRef]
- Toh, W.H.; Tan, J.Z.; Zulkefli, K.L.; Houghton, F.J.; Gleeson, P.A. Amyloid precursor protein traffics from the Golgi directly to early endosomes in an arl5b- and ap4-dependent pathway. Traffic 2017, 18, 159–175. [Google Scholar] [CrossRef]
- Sadakata, T.; Shinoda, Y.; Sekine, Y.; Saruta, C.; Itakura, M.; Takahashi, M.; Furuichi, T. Interaction of calcium-dependent activator protein for secretion 1 (caps1) with the class ii ADP-ribosylation factor small GTPases is required for dense-core vesicle trafficking in the trans-Golgi network. J. Biol. Chem. 2010, 285, 38710–38719. [Google Scholar] [CrossRef] [PubMed]
- Nakai, W.; Kondo, Y.; Saitoh, A.; Naito, T.; Nakayama, K.; Shin, H.W. Arf1 and Arf4 regulate recycling endosomal morphology and retrograde transport from endosomes to the Golgi apparatus. Mol. Biol. Cell 2013, 24, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Sadakata, T.; Itakura, M.; Kozaki, S.; Sekine, Y.; Takahashi, M.; Furuichi, T. Differential distributions of the Ca2+ -dependent activator protein for secretion family proteins (caps2 and caps1) in the mouse brain. J. Comp. Neurol. 2006, 495, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, R.; Aguilar, R.C.; Gorshkova, I.; Crouch, R.J.; Bonifacino, J.S. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science 2001, 292, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, R.; Randazzo, P.A.; Presley, J.F.; Hartnell, L.M.; Bonifacino, J.S. The GGAs promote Arf-dependent recruitment of clathrin to TGN. Cell 2001, 105, 93–102. [Google Scholar] [CrossRef]
- Mazelova, J.; Astuto-Gribble, L.; Inoue, H.; Tam, B.M.; Schonteich, E.; Prekeris, R.; Moritz, O.L.; Randazzo, P.A.; Deretic, D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009, 28, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Farias, G.G.; Canagarajah, B.J.; Bonifacino, J.S.; Hurley, J.H. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell 2013, 152, 755–767. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Vicinanza, M.; Wilson, C.; De Matteis, M.A. Phosphoinositides in Golgi complex function. Subcell. Biochem. 2012, 59, 255–270. [Google Scholar] [PubMed]
- Vicinanza, M.; D’Angelo, G.; Di Campli, A.; De Matteis, M.A. Function and dysfunction of the PI system in membrane trafficking. EMBO J. 2008, 27, 2457–2470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, J.; Sun, H.Q.; Martinez, M.; Sun, Y.X.; Macia, E.; Kirchhausen, T.; Albanesi, J.P.; Roth, M.G.; Yin, H.L. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 2003, 114, 299–310. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.Q.; Macia, E.; Kirchhausen, T.; Watson, H.; Bonifacino, J.S.; Yin, H.L. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol. Biol. Cell 2007, 18, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Godi, A.; Pertile, P.; Meyers, R.; Marra, P.; Di Tullio, G.; Iurisci, C.; Luini, A.; Corda, D.; De Matteis, M.A. ARF mediates recruitment of Ptdins-4-OH kinase-beta and stimulates synthesis of Ptdins(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1999, 1, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Varnai, P.; Tuymetova, G.; Balla, A.; Toth, Z.E.; Oker-Blom, C.; Roder, J.; Jeromin, A.; Balla, T. Interaction of neuronal calcium sensor-1 (ncs-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in Cos-7 cells. J. Biol. Chem. 2001, 276, 40183–40189. [Google Scholar] [CrossRef] [PubMed]
- Haynes, L.P.; Thomas, G.M.; Burgoyne, R.D. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase beta and trans-Golgi network-plasma membrane traffic. J. Biol. Chem. 2005, 280, 6047–6054. [Google Scholar] [CrossRef] [PubMed]
- Blagoveshchenskaya, A.; Cheong, F.Y.; Rohde, H.M.; Glover, G.; Knodler, A.; Nicolson, T.; Boehmelt, G.; Mayinger, P. Integration of Golgi trafficking and growth factor signaling by the lipid phosphatase sac1. J. Cell Biol. 2008, 180, 803–812. [Google Scholar] [CrossRef]
- Bonifacino, J.S. The gga proteins: Adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004, 5, 23–32. [Google Scholar] [CrossRef]
- Guardia, C.M.; De Pace, R.; Mattera, R.; Bonifacino, J.S. Neuronal functions of adaptor complexes involved in protein sorting. Curr. Opin. Neurobiol. 2018, 51, 103–110. [Google Scholar] [CrossRef]
- Collins, B.M.; McCoy, A.J.; Kent, H.M.; Evans, P.R.; Owen, D.J. Molecular architecture and functional model of the endocytic AP2 complex. Cell 2002, 109, 523–535. [Google Scholar] [CrossRef]
- Heldwein, E.E.; Macia, E.; Wang, J.; Yin, H.L.; Kirchhausen, T.; Harrison, S.C. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl. Acad. Sci. USA 2004, 101, 14108–14113. [Google Scholar] [CrossRef]
- Dell’Angelica, E.C.; Puertollano, R.; Mullins, C.; Aguilar, R.C.; Vargas, J.D.; Hartnell, L.M.; Bonifacino, J.S. GGAs: A family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 2000, 149, 81–93. [Google Scholar] [CrossRef]
- Hirst, J.; Lui, W.W.Y.; Bright, N.A.; Totty, N.; Seaman, M.N.J.; Robinson, M.S. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 2000, 149, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, H.; Yoshino, K.; Nakayama, K. Adaptor gamma ear homology domain conserved in gamma-adaptin and GGA proteins that interact with gamma-synergin. Biochem. Biophys. Res. Commun. 2000, 271, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.S. Forty years of clathrin-coated vesicles. Traffic 2015, 16, 1210–1238. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Madsen, P.; Christensen, E.I.; Nykjaer, A.; Gliemann, J.; Kasper, D.; Pohlmann, R.; Petersen, C.M. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001, 20, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.M.; Watson, P.J.; Owen, D.J. The structure of the GGA1-GAT domain reveals the molecular basis for ARF binding and membrane association of GGAs. Dev. Cell 2003, 4, 321–332. [Google Scholar] [CrossRef]
- Gallusser, A.; Kirchhausen, T. The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J. 1993, 12, 5237–5244. [Google Scholar] [CrossRef] [PubMed]
- ter Haar, E.; Harrison, S.C.; Kirchhausen, T. Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc. Natl. Acad. Sci. USA 2000, 97, 1096–1100. [Google Scholar] [CrossRef]
- Simpson, F.; Peden, A.A.; Christopoulou, L.; Robinson, M.S. Characterization of the adaptor-related protein complex, AP-3. J. Cell Biol. 1997, 137, 835–845. [Google Scholar] [CrossRef]
- Dell’Angelica, E.C.; Mullins, C.; Bonifacino, J.S. AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem. 1999, 274, 7278–7285. [Google Scholar] [CrossRef]
- Hirst, J.; Bright, N.A.; Rous, B.; Robinson, M.S. Characterization of a fourth adaptor-related protein complex. Mol. Biol. Cell 1999, 10, 2787–2802. [Google Scholar] [CrossRef]
- Ohno, H.; Tomemori, T.; Nakatsu, F.; Okazaki, Y.; Aguilar, R.C.; Foelsch, H.; Mellman, I.; Saito, T.; Shirasawa, T.; Bonifacino, J.S. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999, 449, 215–220. [Google Scholar] [CrossRef]
- Guo, X.; Mattera, R.; Ren, X.; Chen, Y.; Retamal, C.; Gonzalez, A.; Bonifacino, J.S. The adaptor protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev. Cell 2013, 27, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Hille-Rehfeld, A. Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. BBA rev. Biomembr. 1995, 1241, 177–194. [Google Scholar] [CrossRef]
- Meyer, C.; Zizioli, D.; Lausmann, S.; Eskelinen, E.L.; Hamann, J.; Saftig, P.; von Figura, K.; Schu, P. Mu1a-adaptin-deficient mice: Lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000, 19, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; Borner, G.H.; Antrobus, R.; Peden, A.A.; Hodson, N.A.; Sahlender, D.A.; Robinson, M.S. Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr. Biol. 2012, 22, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Farias, G.G.; Cuitino, L.; Guo, X.; Ren, X.; Jarnik, M.; Mattera, R.; Bonifacino, J.S. Signal-mediated, AP-1/clathrin-dependent sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons. Neuron 2012, 75, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Gravotta, D.; Carvajal-Gonzalez, J.M.; Mattera, R.; Deborde, S.; Banfelder, J.R.; Bonifacino, J.S.; Rodriguez-Boulan, E. The clathrin adaptor AP-1A mediates basolateral polarity. Dev. Cell 2012, 22, 811–823. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, A.; Abraham, N.; Khan, L.A.; Hall, D.H.; Fleming, J.T.; Gobel, V. Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis. Development 2012, 139, 2071–2083. [Google Scholar] [CrossRef]
- Folsch, H.; Pypaert, M.; Schu, P.; Mellman, I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 2001, 152, 595–606. [Google Scholar] [CrossRef]
- Folsch, H.; Pypaert, M.; Maday, S.; Pelletier, L.; Mellman, I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 2003, 163, 351–362. [Google Scholar] [CrossRef]
- Zizioli, D.; Meyer, C.; Guhde, G.; Saftig, P.; von Figura, K.; Schu, P. Early embryonic death of mice deficient in gamma-adaptin. J. Biol. Chem. 1999, 274, 5385–5390. [Google Scholar] [CrossRef] [PubMed]
- Tarpey, P.S.; Stevens, C.; Teague, J.; Edkins, S.; O’Meara, S.; Avis, T.; Barthorpe, S.; Buck, G.; Butler, A.; Cole, J.; et al. Mutations in the gene encoding the Sigma 2 subunit of the adaptor protein 1 complex, AP1S2, cause x-linked mental retardation. Am. J. Hum. Genet. 2006, 79, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Saillour, Y.; Zanni, G.; Des Portes, V.; Heron, D.; Guibaud, L.; Iba-Zizen, M.T.; Pedespan, J.L.; Poirier, K.; Castelnau, L.; Julien, C.; et al. Mutations in the AP1S2 gene encoding the sigma 2 subunit of the adaptor protein 1 complex are associated with syndromic X-linked mental retardation with hydrocephalus and calcifications in basal ganglia. J. Med. Genet. 2007, 44, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, A.; Cote, S.; Brustein, E.; Drouin, C.A.; Lapointe, L.; Boudreau, M.; Meloche, C.; Drouin, R.; Hudson, T.J.; Drapeau, P.; et al. Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet. 2008, 4, e1000296. [Google Scholar] [CrossRef] [PubMed]
- Pevsner, J.; Volknandt, W.; Wong, B.R.; Scheller, R.H. Two rat homologs of clathrin-associated adaptor proteins. Gene 1994, 146, 279–283. [Google Scholar] [CrossRef]
- Newman, L.S.; McKeever, M.O.; Okano, H.J.; Darnell, R.B. Beta-nap, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell 1995, 82, 773–783. [Google Scholar] [CrossRef]
- Dell’Angelica, E.C.; Ohno, H.; Ooi, C.E.; Rabinovich, E.; Roche, K.W.; Bonifacino, J.S. AP-3: An adaptor-like protein complex with ubiquitous expression. EMBO J. 1997, 16, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Dell’Angelica, E.C.; Shotelersuk, V.; Aguilar, R.C.; Gahl, W.A.; Bonifacino, J.S. Altered trafficking of lysosomal proteins in hermansky-pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell 1999, 3, 11–21. [Google Scholar] [CrossRef]
- Kantheti, P.; Qiao, X.; Diaz, M.E.; Peden, A.A.; Meyer, G.E.; Carskadon, S.L.; Kapfhamer, D.; Sufalko, D.; Robinson, M.S.; Noebels, J.L.; et al. Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron 1998, 21, 111–122. [Google Scholar] [CrossRef]
- Peden, A.A.; Oorschot, V.; Hesser, B.A.; Austin, C.D.; Scheller, R.H.; Klumperman, J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 2004, 164, 1065–1076. [Google Scholar] [CrossRef]
- Abou Jamra, R.; Philippe, O.; Raas-Rothschild, A.; Eck, S.H.; Graf, E.; Buchert, R.; Borck, G.; Ekici, A.; Brockschmidt, F.F.; Nothen, M.M.; et al. Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. Am. J. Hum. Genet. 2011, 88, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.; Leshinsky-Silver, E.; Blumkin, L.; Schlipf, N.; Schroder, C.; Schicks, J.; Lev, D.; Riess, O.; Lerman-Sagie, T.; Schols, L. Mutation in the AP4B1 gene cause hereditary spastic paraplegia type 47 (SPG47). Neurogenetics 2012, 13, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour, H.; Alawi, M.; Kortum, F.; Beckstette, M.; Seemanova, E.; Komarek, V.; Rosenberger, G.; Kutsche, K. An AP4B1 frameshift mutation in siblings with intellectual disability and spastic tetraplegia further delineates the AP-4 deficiency syndrome. Eur. J. Hum. Genet. 2015, 23, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Tuysuz, B.; Bilguvar, K.; Kocer, N.; Yalcinkaya, C.; Caglayan, O.; Gul, E.; Sahin, S.; Comu, S.; Gunel, M. Autosomal recessive spastic tetraplegia caused by AP4M1 and AP4B1 gene mutation: Expansion of the facial and neuroimaging features. Am. J. Med Genet. PART A 2014, 164, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Accogli, A.; Hamdan, F.F.; Poulin, C.; Nassif, C.; Rouleau, G.A.; Michaud, J.L.; Srour, M. A novel homozygous AP4B1 mutation in two brothers with AP-4 deficiency syndrome and ocular anomalies. Am. J. Med. Genet. PART A 2018, 176, 985–991. [Google Scholar] [CrossRef]
- Moreno-De-Luca, A.; Helmers, S.L.; Mao, H.; Burns, T.G.; Melton, A.M.; Schmidt, K.R.; Fernhoff, P.M.; Ledbetter, D.H.; Martin, C.L. Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J. Med. Genet. 2011, 48, 141–144. [Google Scholar] [CrossRef]
- Najmabadi, H.; Hu, H.; Garshasbi, M.; Zemojtel, T.; Abedini, S.S.; Chen, W.; Hosseini, M.; Behjati, F.; Haas, S.; Jamali, P.; et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 2011, 478, 57–63. [Google Scholar] [CrossRef]
- Raza, M.H.; Mattera, R.; Morell, R.; Sainz, E.; Rahn, R.; Gutierrez, J.; Paris, E.; Root, J.; Solomon, B.; Brewer, C.; et al. Association between rare variants in AP4E1, a component of intracellular trafficking, and persistent stuttering. Am. J. Hum. Genet. 2015, 97, 715–725. [Google Scholar] [CrossRef]
- Verkerk, A.J.; Schot, R.; Dumee, B.; Schellekens, K.; Swagemakers, S.; Bertoli-Avella, A.M.; Lequin, M.H.; Dudink, J.; Govaert, P.; van Zwol, A.L.; et al. Mutation in the AP4M1 gene provides a model for neuroaxonal injury in cerebral palsy. Am. J. Hum. Genet. 2009, 85, 40–52. [Google Scholar] [CrossRef]
- Hardies, K.; May, P.; Djemie, T.; Tarta-Arsene, O.; Deconinck, T.; Craiu, D.; Helbig, I.; Suls, A.; Balling, R.; Weckhuysen, S.; et al. Recessive loss-of-function mutations in ap4s1 cause mild fever-sensitive seizures, developmental delay, and spastic paraplegia through loss of AP-4 complex assembly. Hum. Mol. Genet. 2014. [Google Scholar] [CrossRef]
- Matsuda, S.; Miura, E.; Matsuda, K.; Kakegawa, W.; Kohda, K.; Watanabe, M.; Yuzaki, M. Accumulation of ampa receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron 2008, 57, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.C.; Murate, M.; Kishigami, S.; Muto, Y.; Kishida, H.; Hashikawa, T.; Yano, R. Adaptor protein complex-4 (AP-4) is expressed in the central nervous system neurons and interacts with glutamate receptor delta2. Mol. Cell. Neurosci. 2003, 24, 283–295. [Google Scholar] [CrossRef]
- Aguilar, R.C.; Boehm, M.; Gorshkova, I.; Crouch, R.J.; Tomita, K.; Saito, T.; Ohno, H.; Bonifacino, J.S. Signal-binding specificity of the mu4 subunit of the adaptor protein complex AP-4. J. Biol. Chem. 2001, 276, 13145–13152. [Google Scholar] [CrossRef] [PubMed]
- Janvier, K.; Bonifacino, J.S. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol. Biol. Cell 2005, 16, 4231–4242. [Google Scholar] [CrossRef] [PubMed]
- Simmen, T.; Honing, S.; Icking, A.; Tikkanen, R.; Hunziker, W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial mdck cells. Nat. Cell Biol. 2002, 4, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Borner, G.H.; Antrobus, R.; Hirst, J.; Bhumbra, G.S.; Kozik, P.; Jackson, L.P.; Sahlender, D.A.; Robinson, M.S. Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J. Cell Biol. 2012, 197, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Burgos, P.V.; Mardones, G.A.; Rojas, A.L.; daSilva, L.L.; Prabhu, Y.; Hurley, J.H.; Bonifacino, J.S. Sorting of the alzheimer’s disease amyloid precursor protein mediated by the AP-4 complex. Dev. Cell 2010, 18, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Z.A.; Gleeson, P.A. The trans-Golgi network is a major site for alpha-secretase processing of amyloid precursor protein in primary neurons. J. Biol. Chem. 2019, 294, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Mattera, R.; Park, S.Y.; De Pace, R.; Guardia, C.M.; Bonifacino, J.S. AP-4 mediates export of ATG9A from the trans-Golgi network to promote autophagosome formation. Proc. Natl. Acad. Sci. USA 2017, 114, E10697–E10706. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.K.; Itzhak, D.N.; Edgar, J.R.; Archuleta, T.L.; Hirst, J.; Jackson, L.P.; Robinson, M.S.; Borner, G.H.H. AP-4 vesicles contribute to spatial control of autophagy via RUSC-dependent peripheral delivery of ATG9A. Nat. Commun. 2018, 9, 3958. [Google Scholar] [CrossRef] [PubMed]
- De Pace, R.; Skirzewski, M.; Damme, M.; Mattera, R.; Mercurio, J.; Foster, A.M.; Cuitino, L.; Jarnik, M.; Hoffmann, V.; Morris, H.D.; et al. Altered distribution of ATG9A and accumulation of axonal aggregates in neurons from a mouse model of AP-4 deficiency syndrome. PLoS Genet. 2018, 14, e1007363. [Google Scholar] [CrossRef] [PubMed]
- Govero, J.; Doray, B.; Bai, H.; Kornfeld, S. Analysis of gga null mice demonstrates a non-redundant role for mammalian GGA2 during development. PLoS ONE 2012, 7, e30184. [Google Scholar] [CrossRef] [PubMed]
- Doray, B.; Govero, J.; Kornfeld, S. Impact of genetic background on neonatal lethality of GGA2 gene-trap mice. G3 2014, 4, 885–890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doray, B.; Ghosh, P.; Griffith, J.; Geuze, H.J.; Kornfeld, S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 2002, 297, 1700–1703. [Google Scholar] [CrossRef]
- Toh, W.H.; Chia, P.Z.C.; Hossain, M.I.; Gleeson, P.A. GGA1 regulates signal-dependent sorting of BACE1 to recycling endosomes, which moderates Abeta production. Mol. Biol. Cell 2018, 29, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Kametaka, S.; Waguri, S. GGA2 interacts with EGFR cytoplasmic domain to stabilize the receptor expression and promote cell growth. Sci. Rep. 2018, 8, 1368. [Google Scholar] [CrossRef]
- Puertollano, R.; Bonifacino, J.S. Interactions of GGA3 with the ubiquitin sorting machinery. Nat. Cell Biol. 2004, 6, 244–251. [Google Scholar] [CrossRef]
- Tesco, G.; Koh, Y.H.; Kang, E.L.; Cameron, A.N.; Das, S.; Sena-Esteves, M.; Hiltunen, M.; Yang, S.H.; Zhong, Z.; Shen, Y.; et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 2007, 54, 721–737. [Google Scholar] [CrossRef]
- Kang, E.L.; Cameron, A.N.; Piazza, F.; Walker, K.R.; Tesco, G. Ubiquitin regulates GGA3-mediated degradation of BACE1. J. Biol. Chem. 2010, 285, 24108–24119. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Traub, L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003, 72, 395–447. [Google Scholar] [CrossRef]
- Park, S.Y.; Guo, X. Adaptor protein complexes and intracellular transport. Biosci. Rep. 2014, 34, 00123. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.W.; Honing, S.; Rodionov, D.; Dobberstein, B.; von Figura, K.; Bakke, O. The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J. Biol. Chem. 1999, 274, 36153–36158. [Google Scholar] [CrossRef] [PubMed]
- Honing, S.; Sandoval, I.V.; von Figura, K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998, 17, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Le Borgne, R.; Alconada, A.; Bauer, U.; Hoflack, B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem. 1998, 273, 29451–29461. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.J.; Evans, P.R. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 1998, 282, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Mardones, G.A.; Burgos, P.V.; Lin, Y.; Kloer, D.P.; Magadan, J.G.; Hurley, J.H.; Bonifacino, J.S. Structural basis for the recognition of tyrosine-based sorting signals by the μ3A subunit of the AP-3 adaptor complex. J. Biol. Chem. 2013, 288, 9563–9571. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Stewart, J.; Fournier, M.C.; Bosshart, H.; Rhee, I.; Miyatake, S.; Saito, T.; Gallusser, A.; Kirchhausen, T.; Bonifacino, J.S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 1995, 269, 1872–1875. [Google Scholar] [CrossRef]
- Honing, S.; Griffith, J.; Geuze, H.J.; Hunziker, W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996, 15, 5230–5239. [Google Scholar] [CrossRef]
- Salamero, J.; Le Borgne, R.; Saudrais, C.; Goud, B.; Hoflack, B. Expression of major histocompatibility complex class II molecules in hela cells promotes the recruitment of AP-1 Golgi-specific assembly proteins on golgi membranes. J. Biol. Chem. 1996, 271, 30318–30321. [Google Scholar] [CrossRef]
- Prabhu, Y.; Burgos, P.V.; Schindler, C.; Farias, G.G.; Magadan, J.G.; Bonifacino, J.S. Adaptor protein 2-mediated endocytosis of the beta-secretase BACE1 is dispensable for amyloid precursor protein processing. Mol. Biol. Cell 2012, 23, 2339–2351. [Google Scholar] [CrossRef]
- He, X.; Chang, W.P.; Koelsch, G.; Tang, J. Memapsin 2 (beta-secretase) cytosolic domain binds to the VHS domains of GGA1 and GGA2: Implications on the endocytosis mechanism of memapsin 2. FEBS Lett. 2002, 524, 183–187. [Google Scholar] [CrossRef]
- He, X.; Li, F.; Chang, W.P.; Tang, J. GGA proteins mediate the recycling pathway of memapsin 2 (BACE). J. Biol. Chem. 2005, 280, 11696–11703. [Google Scholar] [CrossRef]
- Navarro Negredo, P.; Edgar, J.R.; Wrobel, A.G.; Zaccai, N.R.; Antrobus, R.; Owen, D.J.; Robinson, M.S. Contribution of the clathrin adaptor AP-1 subunit micro1 to acidic cluster protein sorting. J. Cell Biol. 2017, 216, 2927–2943. [Google Scholar]
- Stockli, J.; Honing, S.; Rohrer, J. The acidic cluster of the CK2 site of the cation-dependent mannose 6-phosphate receptor (CD-MPR) but not its phosphorylation is required for GGA1 and AP-1 binding. J. Biol. Chem. 2004, 279, 23542–23549. [Google Scholar] [CrossRef]
- Teuchert, M.; Schafer, W.; Berghofer, S.; Hoflack, B.; Klenk, H.D.; Garten, W. Sorting of furin at the trans-Golgi network. Interaction of the cytoplasmic tail sorting signals with AP-1 Golgi-specific assembly proteins. J. Biol. Chem. 1999, 274, 8199–8207. [Google Scholar] [CrossRef]
- Jia, X.; Singh, R.; Homann, S.; Yang, H.; Guatelli, J.; Xiong, Y. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat. Struct. Mol. Biol. 2012, 19, 701–706. [Google Scholar] [CrossRef]
- Aguilar, R.C.; Ohno, H.; Roche, K.W.; Bonifacino, J.S. Functional domain mapping of the clathrin-associated adaptor medium chains mu1 and mu2. J. Biol. Chem. 1997, 272, 27160–22716. [Google Scholar] [CrossRef]
- Ross, B.H.; Lin, Y.; Corales, E.A.; Burgos, P.V.; Mardones, G.A. Structural and functional characterization of cargo-binding sites on the mu4-subunit of adaptor protein complex 4. PLoS ONE 2014, 9, e88147. [Google Scholar] [CrossRef]
- Chernomordik, L.V.; Kozlov, M.M. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003, 72, 175–207. [Google Scholar] [CrossRef]
- Frolov, V.A.; Escalada, A.; Akimov, S.A.; Shnyrova, A.V. Geometry of membrane fission. Chem. Phys. Lipids 2015, 185, 129–140. [Google Scholar] [CrossRef]
- Rosenthal, J.A.; Chen, H.; Slepnev, V.I.; Pellegrini, L.; Salcini, A.E.; Di Fiore, P.P.; De Camilli, P. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J. Biol. Chem. 1999, 274, 33959–33965. [Google Scholar] [CrossRef]
- Legendre-Guillemin, V.; Wasiak, S.; Hussain, N.K.; Angers, A.; McPherson, P.S. ENTH/ANTH proteins and clathrin-mediated membrane budding. J. Cell Sci. 2004, 117, 9–18. [Google Scholar] [CrossRef]
- Hirst, J.; Motley, A.; Harasaki, K.; Peak Chew, S.Y.; Robinson, M.S. EpsinR: An ENTH domain-containing protein that interacts with AP-1. Mol. Biol. Cell 2003, 14, 625–641. [Google Scholar] [CrossRef]
- Archuleta, T.L.; Frazier, M.N.; Monken, A.E.; Kendall, A.K.; Harp, J.; McCoy, A.J.; Creanza, N.; Jackson, L.P. Structure and evolution of ENTH and VPS/ENTH-like domains in tepsin. Traffic 2017, 18, 590–603. [Google Scholar] [CrossRef]
- Miller, S.E.; Collins, B.M.; McCoy, A.J.; Robinson, M.S.; Owen, D.J. A SNARE-adaptor interaction is a new mode of cargo recognition in clathrin-coated vesicles. Nature 2007, 450, 570–574. [Google Scholar] [CrossRef]
- Ferguson, S.M.; De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol 2012, 13, 75–88. [Google Scholar] [CrossRef]
- Owen, D.J.; Vallis, Y.; Noble, M.E.; Hunter, J.B.; Dafforn, T.R.; Evans, P.R.; McMahon, H.T. A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell 1999, 97, 805–815. [Google Scholar] [CrossRef]
- Owen, D.J.; Vallis, Y.; Pearse, B.M.; McMahon, H.T.; Evans, P.R. The structure and function of the beta 2-adaptin appendage domain. EMBO J. 2000, 19, 4216–4227. [Google Scholar] [CrossRef]
- Mills, I.G.; Praefcke, G.J.; Vallis, Y.; Peter, B.J.; Olesen, L.E.; Gallop, J.L.; Butler, P.J.; Evans, P.R.; McMahon, H.T. EpsinR: An AP1/clathrin interacting protein involved in vesicle trafficking. J. Cell Biol. 2003, 160, 213–222. [Google Scholar] [CrossRef]
- Mattera, R.; Guardia, C.M.; Sidhu, S.S.; Bonifacino, J.S. Bivalent motif-ear interactions mediate the association of the accessory protein tepsin with the AP-4 adaptor complex. J. Biol. Chem. 2015, 290, 30736–43079. [Google Scholar] [CrossRef]
- Frazier, M.N.; Davies, A.K.; Voehler, M.; Kendall, A.K.; Borner, G.H.; Chazin, W.J.; Robinson, M.S.; Jackson, L.P. Molecular basis for the interaction between AP4 β4 and its accessory protein, Tepsin. Traffic 2016, 17, 400–415. [Google Scholar] [CrossRef]
- Itzhak, D.N.; Tyanova, S.; Cox, J.; Borner, G.H. Global, quantitative and dynamic mapping of protein subcellular localization. Elife 2016, 5, e16950. [Google Scholar] [CrossRef]
- Itzhak, D.N.; Davies, C.; Tyanova, S.; Mishra, A.; Williamson, J.; Antrobus, R.; Cox, J.; Weekes, M.P.; Borner, G.H.H. A mass spectrometry-based approach for mapping protein subcellular localization reveals the spatial proteome of mouse primary neurons. Cell Rep. 2017, 20, 2706–2718. [Google Scholar] [CrossRef]
- Hirst, J.; Itzhak, D.N.; Antrobus, R.; Borner, G.H.H.; Robinson, M.S. Role of the AP-5 adaptor protein complex in late endosome-to-Golgi retrieval. PLoS Biol. 2018, 16, e2004411. [Google Scholar] [CrossRef]
- Theos, A.C.; Tenza, D.; Martina, J.A.; Hurbain, I.; Peden, A.A.; Sviderskaya, E.V.; Stewart, A.; Robinson, M.S.; Bennett, D.C.; Cutler, D.F.; et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell 2005, 16, 5356–5372. [Google Scholar] [CrossRef]
- Boncompain, G.; Divoux, S.; Gareil, N.; de Forges, H.; Lescure, A.; Latreche, L.; Mercanti, V.; Jollivet, F.; Raposo, G.; Perez, F. Synchronization of secretory protein traffic in populations of cells. Nat. Methods 2012, 9, 493–498. [Google Scholar] [CrossRef]
- Chen, Y.; Gershlick, D.C.; Park, S.Y.; Bonifacino, J.S. Segregation in the Golgi complex precedes export of endolysosomal proteins in distinct transport carriers. J. Cell Biol. 2017, 216, 4141–4151. [Google Scholar] [CrossRef]
- Nakagawa, T.; Setou, M.; Seog, D.; Ogasawara, K.; Dohmae, N.; Takio, K.; Hirokawa, N. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with ap-1 complex. Cell 2000, 103, 569–581. [Google Scholar] [CrossRef]
- Schmidt, M.R.; Maritzen, T.; Kukhtina, V.; Higman, V.A.; Doglio, L.; Barak, N.N.; Strauss, H.; Oschkinat, H.; Dotti, C.G.; Haucke, V. Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proc. Natl. Acad. Sci. USA 2009, 106, 15344–15349. [Google Scholar] [CrossRef]
- Jia, X.; Weber, E.; Tokarev, A.; Lewinski, M.; Rizk, M.; Suarez, M.; Guatelli, J.; Xiong, Y. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. Elife 2014, 3, e02362. [Google Scholar] [CrossRef]


| Motifs | Cargoes | Functions | Cargo Adaptor Proteins | Reference |
|---|---|---|---|---|
| YXXΦ | TGN38, TfR, Furin, MPRs, EGFR, CD63, LAMP1, LAMP2 | Endocytosis, Endosomal and Lysosomal targeting | μ subunits of AP complexes | [48,79,93,110,114,115,116,117,118] |
| TfR, CAR | TGN → somatodendritic domains in neurons | μ1A (AP-1A) | [66] | |
| [DE]XXXL[LI] | Invariant chain | TGN → endosomes | Hemicomplexesof AP-1 γ–σ1 | [112,119] |
| LIMP-II, Tyrosinase | Lysosomal targeting | Hemicomplexesof AP-3 δ–σ3 | [113,114] | |
| BACE1 | Endocytosis | Hemicomplexesof AP-2 α–σ2 | [120] | |
| YXXΦE | APP family members, ATG9A | TGN → endosomes | μ4 (AP-4) | [97,99,119] |
| Noncanonical Tyrosine-based motif | LDLR | TGN → basolateral PM | μ1B (AP-1B) | [62] |
| TARP (AMPA receptor) | TGN → somatodendritic domains in neurons | μ4 (AP-4) | [91] | |
| Phenylalanine-based motif | δ2 glutamate receptor | TGN → somatodendritic domains in neurons | μ4 (AP-4) | [92] |
| Acidic clusters-DXXLL | MPRs | TGN↔endosomes | GGAs | [34] |
| BACE1 | Endosomal recycling | GGAs | [105,121,122] | |
| Acidic clusters | Nef protein of HIV-1 | Endocytosis: Downregulation of MHC-I | μ1 (AP-1) | [123] |
| Furin, CD-MPR | TGN sorting | [124,125] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.Z.A.; Gleeson, P.A. Cargo Sorting at the trans-Golgi Network for Shunting into Specific Transport Routes: Role of Arf Small G Proteins and Adaptor Complexes. Cells 2019, 8, 531. https://doi.org/10.3390/cells8060531
Tan JZA, Gleeson PA. Cargo Sorting at the trans-Golgi Network for Shunting into Specific Transport Routes: Role of Arf Small G Proteins and Adaptor Complexes. Cells. 2019; 8(6):531. https://doi.org/10.3390/cells8060531
Chicago/Turabian StyleTan, Jing Zhi Anson, and Paul Anthony Gleeson. 2019. "Cargo Sorting at the trans-Golgi Network for Shunting into Specific Transport Routes: Role of Arf Small G Proteins and Adaptor Complexes" Cells 8, no. 6: 531. https://doi.org/10.3390/cells8060531
APA StyleTan, J. Z. A., & Gleeson, P. A. (2019). Cargo Sorting at the trans-Golgi Network for Shunting into Specific Transport Routes: Role of Arf Small G Proteins and Adaptor Complexes. Cells, 8(6), 531. https://doi.org/10.3390/cells8060531






