Induction of Fibrosis and Autophagy in Kidney Cells by Vinyl Chloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Cell Culture Conditions
2.2. Cell Viability Assay
2.3. siRNA Knockdown
2.4. Western Blot Analysis
2.5. Animal Model
2.6. Biochemical Evaluation
2.7. Histological Analysis
2.8. Immunohistochemical (IHC) Staining Analysis
2.9. Statistical Analysis
3. Results
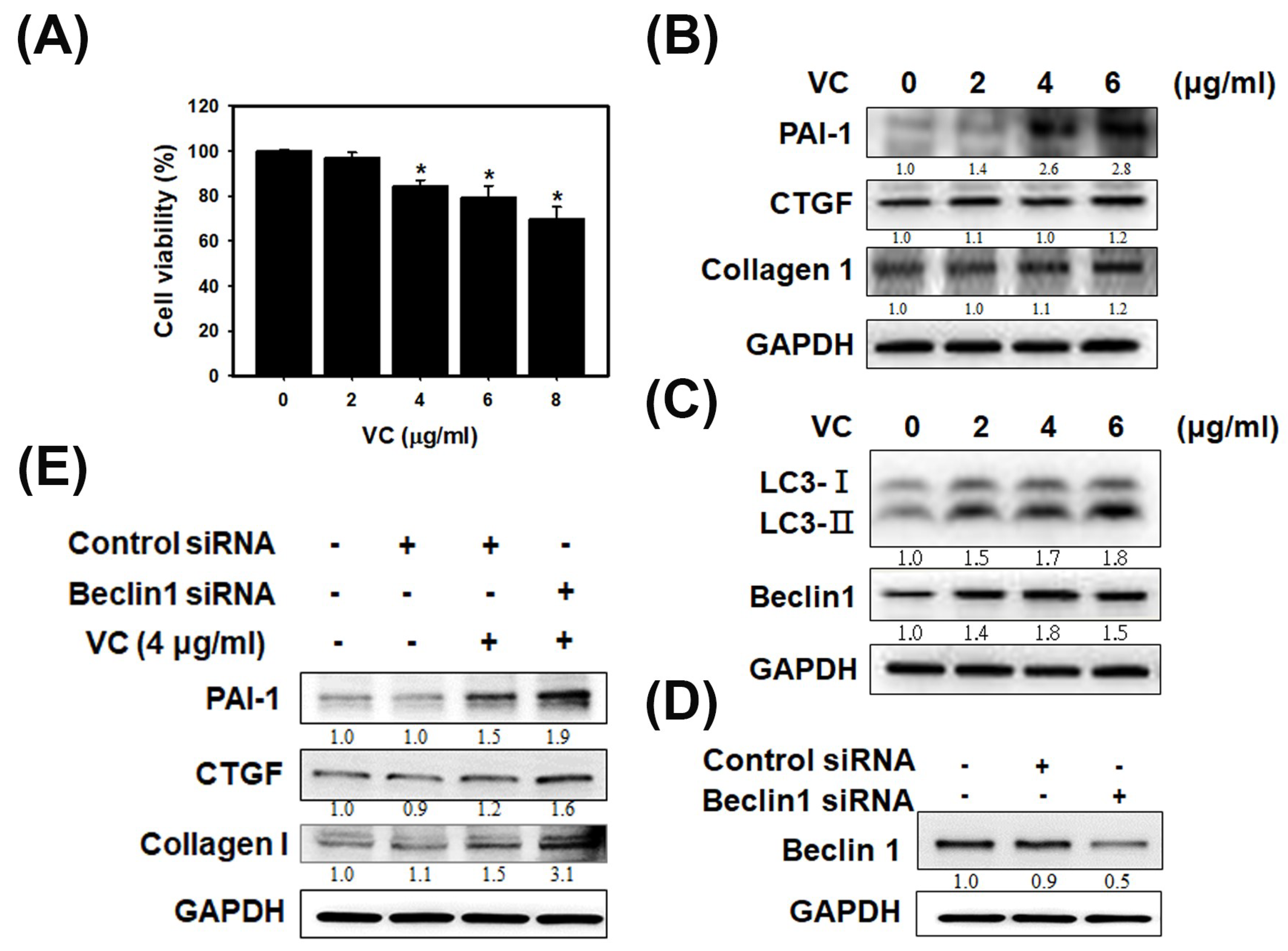
3.1. VC Affected Cell Viability and Induced Fibrosis and Autophagy Reactions in HK-2 Cells
3.2. VC Increased BUN and Creatinine Levels in An In Vivo Model
3.3. VC Increased Glomerulosclerosis and Tubular Injury in Mouse Kidney Tissues
3.4. VC Increased Fibrosis and Autophagy Markers in Mouse Kidney Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Agency for Research on Cancer. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans: Some monomers, plastics and synthetic elastomers, and acrolein. IARC Monogr Eval. Carcinog. Risk Chem. Hum. 1979, 19, 1–513. [Google Scholar]
- Matthews, G. Toxic gases. Postgrad. Med. J. 1989, 65, 224–232. [Google Scholar] [CrossRef]
- Rorison, D.G.; McPherson, S.J. Acute toxic inhalations. Emerg. Med. Clin. N. Am. 1992, 10, 409–435. [Google Scholar]
- Kistemann, T.; Hundhausen, J.; Herbst, S.; Classen, T.; Farber, H. Assessment of a groundwater contamination with vinyl chloride (VC) and precursor volatile organic compounds (VOC) by use of a geographical information system (GIS). Int. J. Hyg. Environ. Health 2008, 211, 308–317. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Kowalczyk, T.; Sitarek, P.; Skala, E.; Pawliczak, R. Analysis of Short-Term Smoking Effects in PBMC of Healthy Subjects-Preliminary Study. Int. J. Environ. Res. Public Health 2018, 15, 1021. [Google Scholar] [CrossRef]
- Ledda, C.; Loreto, C.; Zammit, C.; Marconi, A.; Fago, L.; Matera, S.; Costanzo, V.; Fuccio Sanza, G.; Palmucci, S.; Ferrante, M.; et al. Noninfective occupational risk factors for hepatocellular carcinoma: A review (Review). Mol. Med. Rep. 2017, 15, 511–533. [Google Scholar] [CrossRef]
- Hsiao, T.J.; Wang, J.D.; Yang, P.M.; Yang, P.C.; Cheng, T.J. Liver fibrosis in asymptomatic polyvinyl chloride workers. J. Occup. Environ. Med. 2004, 46, 962–966. [Google Scholar] [CrossRef]
- Jones, D.B.; Smith, P.M. Progression of vinyl chloride induced hepatic fibrosis to angiosarcoma of the liver. Br. J. Ind. Med. 1982, 39, 306–307. [Google Scholar] [CrossRef] [Green Version]
- Maroni, M.; Mocci, F.; Visentin, S.; Preti, G.; Fanetti, A.C. Periportal fibrosis and other liver ultrasonography findings in vinyl chloride workers. Occup. Environ. Med. 2003, 60, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.C.; Wann, S.R.; Chang, H.T.; Lin, S.L.; Wang, J.S.; Guo, H.R. Arsenic, vinyl chloride, viral hepatitis, and hepatic angiosarcoma: A hospital-based study and review of literature in Taiwan. BMC Gastroenterol. 2011, 11, 142. [Google Scholar] [CrossRef]
- Vianna, N.J.; Brady, J.; Harper, P. Angiosarcoma of the liver: A signal lesion of vinyl chloride exposure. Environ. Health Perspect. 1981, 41, 207–210. [Google Scholar] [CrossRef]
- Elliott, P.; Kleinschmidt, I. Angiosarcoma of the liver in Great Britain in proximity to vinyl chloride sites. Occup. Environ. Med. 1997, 54, 14–18. [Google Scholar] [CrossRef]
- Boffetta, P.; Matisane, L.; Mundt, K.A.; Dell, L.D. Meta-analysis of studies of occupational exposure to vinyl chloride in relation to cancer mortality. Scand. J. Work Environ. Health 2003, 29, 220–229. [Google Scholar] [CrossRef]
- Criscuolo, M.; Valerio, J.; Gianicolo, M.E.; Gianicolo, E.A.; Portaluri, M. A vinyl chloride-exposed worker with an adrenal gland angiosarcoma: A case report. Ind. Health 2014, 52, 66–70. [Google Scholar] [CrossRef]
- Wagoner, J.K. Toxicity of vinyl chloride and poly(vinyl chloride): A critical review. Environ. Health Perspect. 1983, 52, 61–66. [Google Scholar] [CrossRef]
- Carreon, T.; Hein, M.J.; Hanley, K.W.; Viet, S.M.; Ruder, A.M. Coronary artery disease and cancer mortality in a cohort of workers exposed to vinyl chloride, carbon disulfide, rotating shift work, and o-toluidine at a chemical manufacturing plant. Am. J. Ind. Med. 2014, 57, 398–411. [Google Scholar] [CrossRef]
- Mundt, K.A.; Dell, L.D.; Crawford, L.; Gallagher, A.E. Quantitative estimated exposure to vinyl chloride and risk of angiosarcoma of the liver and hepatocellular cancer in the US industry-wide vinyl chloride cohort: Mortality update through 2013. Occup. Environ. Med. 2017, 74, 709–716. [Google Scholar] [CrossRef]
- Wang, C.W.; Liao, K.W.; Chan, C.C.; Yu, M.L.; Chuang, H.Y.; Chiang, H.C.; Huang, P.C. Association between urinary thiodiglycolic acid level and hepatic function or fibrosis index in school-aged children living near a petrochemical complex. Environ. Pollut. 2019, 244, 648–656. [Google Scholar] [CrossRef]
- Sirit, Y.; Acero, C.; Bellorin, M.; Portillo, R. Metabolic syndrome and other factors cardiovascular risk in workers of a plant of vinyl polychloride. Rev. Salud. Publica. (Bogota) 2008, 10, 239–249. [Google Scholar] [CrossRef]
- Feron, V.J.; Kroes, R. One-year time-sequence inhalation toxicity study of vinyl chloride in rats. II. Morphological changes in the respiratory tract, ceruminous glands, brain, kidneys, heart and spleen. Toxicology 1979, 13, 131–141. [Google Scholar]
- Whysner, J.; Conaway, C.C.; Verna, L.; Williams, G.M. Vinyl chloride mechanistic data and risk assessment: DNA reactivity and cross-species quantitative risk extrapolation. Pharmacol. Ther. 1996, 71, 7–28. [Google Scholar] [CrossRef]
- Kielhorn, J.; Melber, C.; Wahnschaffe, U.; Aitio, A.; Mangelsdorf, I. Vinyl chloride: Still a cause for concern. Environ. Health Perspect. 2000, 108, 579–588. [Google Scholar] [CrossRef]
- Winell, M.; Holmberg, B.; Kronevi, T. Biological effects of vinyl chloride: An experimental study. Environ. Health Perspect. 1976, 17, 211–216. [Google Scholar] [CrossRef]
- Quan, H.; Ma, T.; Zhao, X.; Zhao, B.; Liu, Y.; Li, H. Vinyl chloride monomer (VCM) induces high occurrence of neural tube defects in embryonic mouse brain during neurulation. Cell. Mol. Neurobiol. 2014, 34, 619–630. [Google Scholar] [CrossRef]
- Viola, P.L.; Bigotti, A.; Caputo, A. Oncogenic response of rat skin, lungs, and bones to vinyl chloride. Cancer Res. 1971, 31, 516–522. [Google Scholar]
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Yan, Q.; Hu, X.; Eisner, C.; Huang, Y.; Chen, R.; Mizel, D.; Zhou, H.; Wright, E.C.; Kopp, J.B.; et al. Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int. 2010, 78, 1136–1153. [Google Scholar] [CrossRef] [Green Version]
- Stallons, L.J.; Whitaker, R.M.; Schnellmann, R.G. Suppressed mitochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis. Toxicol. Lett. 2014, 224, 326–332. [Google Scholar] [CrossRef]
- Lo, L.J.; Go, A.S.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordonez, J.D.; Hsu, C.Y. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009, 76, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Coca, S.G.; Yusuf, B.; Shlipak, M.G.; Garg, A.X.; Parikh, C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 53, 961–973. [Google Scholar] [CrossRef]
- Lee, C.C.; Bhandari, J.C.; Winston, J.M.; House, W.B.; Peters, P.J.; Dixon, R.L.; Woods, J.S. Inhalation toxicity of vinyl chloride and vinylidene chloride. Environ. Health Perspect. 1977, 21, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kawaoka, K.; Doi, S.; Nakashima, A.; Yamada, K.; Ueno, T.; Doi, T.; Masaki, T. Valproic acid attenuates renal fibrosis through the induction of autophagy. Clin. Exp. Nephrol. 2017, 21, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Kaimori, J.Y.; Matsui, I.; Namba, T.; Kitamura, H.; Niimura, F.; Matsusaka, T.; Soga, T.; et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 2011, 22, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kimura, T.; Takabatake, Y.; Namba, T.; Kaimori, J.; Kitamura, H.; Matsui, I.; Niimura, F.; Matsusaka, T.; Fujita, N.; et al. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 2012, 180, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Traylor, A.M.; Kim, J.; Joseph, R.; Ricart, K.; Landar, A.; Agarwal, A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J. Am. Soc. Nephrol. 2010, 21, 1702–1712. [Google Scholar] [CrossRef]
- Hartleben, B.; Godel, M.; Meyer-Schwesinger, C.; Liu, S.; Ulrich, T.; Kobler, S.; Wiech, T.; Grahammer, F.; Arnold, S.J.; Lindenmeyer, M.T.; et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Investig. 2010, 120, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Hartleben, B.; Kretz, O.; Wiech, T.; Igarashi, P.; Mizushima, N.; Walz, G.; Huber, T.B. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 2012, 8, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Belibi, F.; Zafar, I.; Ravichandran, K.; Segvic, A.B.; Jani, A.; Ljubanovic, D.G.; Edelstein, C.L. Hypoxia-inducible factor-1alpha (HIF-1alpha) and autophagy in polycystic kidney disease (PKD). Am. J. Physiol. Renal Physiol. 2011, 300, F1235–F1243. [Google Scholar] [CrossRef]
- Flaquer, M.; Lloberas, N.; Franquesa, M.; Torras, J.; Vidal, A.; Rosa, J.L.; Herrero-Fresneda, I.; Grinyo, J.M.; Cruzado, J.M. The combination of sirolimus and rosiglitazone produces a renoprotective effect on diabetic kidney disease in rats. Life Sci. 2010, 87, 147–153. [Google Scholar] [CrossRef]
- Forbes, J.M.; Coughlan, M.T.; Cooper, M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.L.; Poster, D.; Kistler, A.D.; Krauer, F.; Raina, S.; Young, J.; Rentsch, K.M.; Spanaus, K.S.; Senn, O.; Kristanto, P.; et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2010, 363, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Riediger, F.; Quack, I.; Qadri, F.; Hartleben, B.; Park, J.K.; Potthoff, S.A.; Sohn, D.; Sihn, G.; Rousselle, A.; Fokuhl, V.; et al. Prorenin receptor is essential for podocyte autophagy and survival. J. Am. Soc. Nephrol. 2011, 22, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.T.; Shyue, S.K.; Lai, M.K. Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation 2007, 84, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Ryter, S.W.; Xu, J.F.; Nakahira, K.; Kim, H.P.; Choi, A.M.; Kim, Y.S. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am. J. Respir Cell Mol. Biol. 2011, 45, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Wang, X.; Chen, Z.H.; Lee, S.J.; Huang, M.H.; Wang, Y.; Ryter, S.W.; Choi, A.M. Autophagic proteins regulate cigarette smoke-induced apoptosis: Protective role of heme oxygenase-1. Autophagy 2008, 4, 887–895. [Google Scholar] [CrossRef]
- Lodder, J.; Denaes, T.; Chobert, M.N.; Wan, J.; El-Benna, J.; Pawlotsky, J.M.; Lotersztajn, S.; Teixeira-Clerc, F. Macrophage autophagy protects against liver fibrosis in mice. Autophagy 2015, 11, 1280–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.C.; Liu, L.H.; Shie, R.H.; Tsai, C.H.; Liang, W.Y.; Wang, C.W.; Tsai, C.H.; Chiang, H.C.; Chan, C.C. Assessment of urinary thiodiglycolic acid exposure in school-aged children in the vicinity of a petrochemical complex in central Taiwan. Environ. Res. 2016, 150, 566–572. [Google Scholar] [CrossRef]
- Nassour, J.; Radford, R.; Correia, A.; Fuste, J.M.; Schoell, B.; Jauch, A.; Shaw, R.J.; Karlseder, J. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 2019, 565, 659–663. [Google Scholar] [CrossRef]
- Boivin-Angele, S.; Lefrancois, L.; Froment, O.; Spiethoff, A.; Bogdanffy, M.S.; Wegener, K.; Wesch, H.; Barbin, A.; Bancel, B.; Trepo, C.; et al. Ras gene mutations in vinyl chloride-induced liver tumours are carcinogen-specific but vary with cell type and species. Int. J. Cancer 2000, 85, 223–227. [Google Scholar] [CrossRef]
- Weihrauch, M.; Benick, M.; Lehner, G.; Wittekind, M.; Bader, M.; Wrbitzk, R.; Tannapfel, A. High prevalence of K-ras-2 mutations in hepatocellular carcinomas in workers exposed to vinyl chloride. Int. Arch. Occup. Environ. Health 2001, 74, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Barbin, A.; Froment, O.; Boivin, S.; Marion, M.J.; Belpoggi, F.; Maltoni, C.; Montesano, R. p53 gene mutation pattern in rat liver tumors induced by vinyl chloride. Cancer Res. 1997, 57, 1695–1698. [Google Scholar] [PubMed]
- Hollstein, M.; Marion, M.J.; Lehman, T.; Welsh, J.; Harris, C.C.; Martel-Planche, G.; Kusters, I.; Montesano, R. p53 mutations at A:T base pairs in angiosarcomas of vinyl chloride-exposed factory workers. Carcinogenesis 1994, 15, 1–3. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, I.; Marion, M.J.; Smith, S.J.; Carney, W.P.; Brandt-Rauf, P.W. Mutant c-Ki-ras p21 protein in chemical carcinogenesis in humans exposed to vinyl chloride. Cancer Causes Control 1994, 5, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Noda, T.; Yoshimori, T.; Rubinsztein, D.C. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat. Chem. Biol. 2011, 7, 9–17. [Google Scholar] [CrossRef]
- Mori, H.; Inoki, K.; Masutani, K.; Wakabayashi, Y.; Komai, K.; Nakagawa, R.; Guan, K.L.; Yoshimura, A. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem. Biophys. Res. Commun. 2009, 384, 471–475. [Google Scholar] [CrossRef]
- Fan, Y.; Xiao, W.; Lee, K.; Salem, F.; Wen, J.; He, L.; Zhang, J.; Fei, Y.; Cheng, D.; Bao, H.; et al. Inhibition of Reticulon-1A-Mediated Endoplasmic Reticulum Stress in Early AKI Attenuates Renal Fibrosis Development. J. Am. Soc. Nephrol. 2017, 28, 2007–2021. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-H.; Chuang, H.-C.; Lee, Y.-H.; Lin, Y.-F.; Chiu, Y.-J.; Wang, Y.-L.; Wu, M.-S.; Chiu, H.-W. Induction of Fibrosis and Autophagy in Kidney Cells by Vinyl Chloride. Cells 2019, 8, 601. https://doi.org/10.3390/cells8060601
Hsu Y-H, Chuang H-C, Lee Y-H, Lin Y-F, Chiu Y-J, Wang Y-L, Wu M-S, Chiu H-W. Induction of Fibrosis and Autophagy in Kidney Cells by Vinyl Chloride. Cells. 2019; 8(6):601. https://doi.org/10.3390/cells8060601
Chicago/Turabian StyleHsu, Yung-Ho, Hsiao-Chi Chuang, Yu-Hsuan Lee, Yuh-Feng Lin, Yu-Jhe Chiu, Yung-Li Wang, Mai-Szu Wu, and Hui-Wen Chiu. 2019. "Induction of Fibrosis and Autophagy in Kidney Cells by Vinyl Chloride" Cells 8, no. 6: 601. https://doi.org/10.3390/cells8060601




