RETRACTED: Circular RNA circ-FoxO3 Inhibits Myoblast Cells Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Tissue Sample Preparation
2.3. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (RT-qPCR)
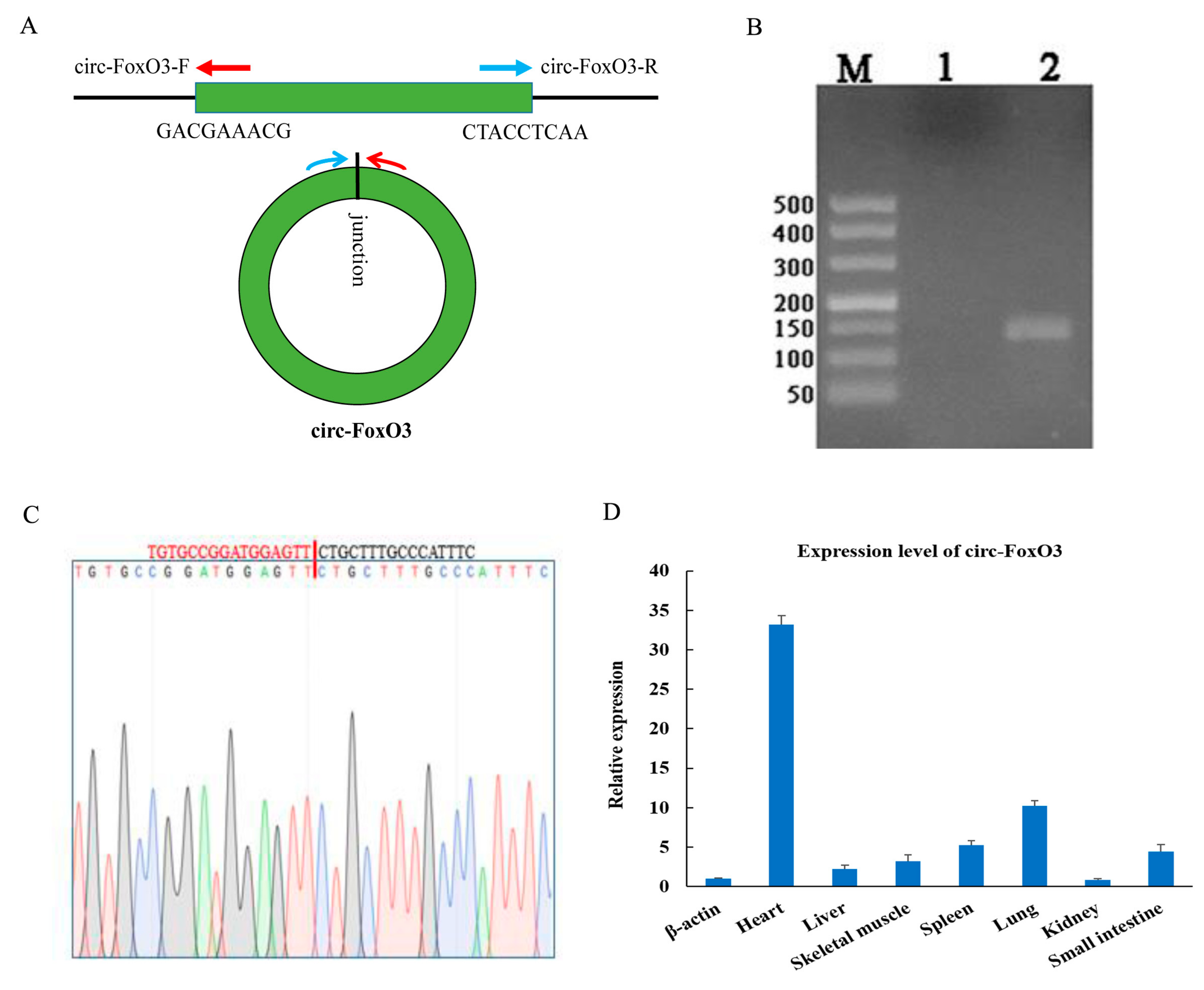
2.4. Validation of Circ-FoxO3
2.5. Vector Construction
2.6. Cell Culture and Transfection
2.7. Cell Proliferation and Differentiation Assay
2.8. Dual Luciferase Assay
2.9. Western Blotting
2.10. Statistical Analyses
3. Results
3.1. Expression Pattern of Circ-FoxO3
3.2. Effect of Circ-FoxO3 on C2C12 Myoblast Cells Proliferation
3.3. Effect of Circ-FoxO3 on C2C12 Myoblast Cells Differentiation
3.4. Prediction and Verification of the Interaction Between Circ-FoxO3 and miRNA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, J.U.; Agarwal, V.; Guo, H.; Bartel, D.P. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, X.; Chen, D.; Yu, B.; Huang, Z. FoxO1: A novel insight into its molecular mechanisms in the regulation of skeletal muscle differentiation and fiber type specification. Oncotarget 2017, 8, 10662–10674. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yu, J.; Guo, L.; Byers, M.S.; Wang, Z.; Chen, X.; Xu, H.; Nie, Q. Circular RNA circHIPK3 Promotes the Proliferation and Differentiation of Chicken Myoblast Cells by Sponging miR-30a-3p. Cells 2019, 177. [Google Scholar] [CrossRef] [PubMed]
- Güller, I.; Russell, A.P. MicroRNAs in skeletal muscle: Their role and regulation in development, disease and function. J. Physiol. 2010, 588, 4075–4087. [Google Scholar] [CrossRef] [PubMed]
- Neguembor, M.V.; Jothi, M.; Gabellini, D. Long noncoding RNAs, emerging players in muscle differentiation and disease. Skelet. Muscl. 2014. [Google Scholar] [CrossRef] [PubMed]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Keren, A.; Tamir, Y.; Bengal, E. The p38 MAPK signaling pathway: A major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 2006, 252, 224–230. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Perry, R.L.; Rudnick, M.A. Molecular mechanisms regulating myogenic determination and differentiation. Front. Biosci. 2000. [Google Scholar] [CrossRef]
- Guo, L.; Huang, W.; Chen, B.; Bekele, E.J.; Chen, X.; Cai, B.; Nie, Q. gga-mir-133a-3p regulates myoblasts proliferation and differentiation by targeting PRRX1. Front. Genet 2018. [Google Scholar] [CrossRef]
- Greco, S.; Cardinali, B.; Falcone, G.; Martelli, F. Circular RNAs in muscle function and disease. Int. J. Mol. Sci. 2018, 19, 3454. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Panda, A.C.; De, S.; Grammatikakis, I.; Kim, J.; Ding, J.; Noh, J.H.; Kim, K.M.; Mattison, J.A.; de Cabo, R.; et al. Circular RNAs in monkey muscle: Age-dependent changes. Aging 2015, 7, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; You, S.; Yao, Y.; Liu, Z.J.; Hazi, W.; Li, C.Y.; Zhang, X.Y.; Hou, X.X.; Wei, J.C.; Li, X.Y.; et al. Expression profiles of circular RNAs in sheep skeletal muscle. Asian-Australas. J. Anim. Sci. 2018, 31, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, Q.; Lv, D.; Wei, Y.; Raza, M.A.; Chen, Y.; Li, P.; Xi, X.; Xu, H.; Wen, A.; et al. Comprehensive transcriptional landscape of porcine cardiac and skeletal muscles reveals differences of aging. Oncotarget 2018, 9, 1524–1541. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Yao, Y.; Ma, Q.; Ni, W.; Zhang, X.; Cao, Y.; Hazi, W.; Wang, D.; Quan, R.; et al. Genome-wide analysis of circular RNAs in prenatal and postnatal muscle of sheep. Oncotarget 2017, 8, 97165–97177. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Chen, X.; Li, W.; Li, Z.; Nie, Q.; Zhang, X. Circular RNA circSVIL Promotes Myoblast Proliferation and Differentiation by Sponging miR-203 in Chicken. Front. Genet. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, H.; Yang, J.; Hao, D.; Dong, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Lin, F.; et al. Circular RNA profiling reveals an abundant circLMO7 that regulates myoblasts differentiation and survival by sponging miR-378a-3p. Cell Death Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, H.; Li, R.; Wu, W.; Chao, Z.; Li, C.; Xia, W.; Wang, L.; Yang, J.; Xu, Y. Assessment of myoblast circular RNA dynamics and its correlation with miRNA during myogenic differentiation. Int. J. Biochem. Cell Biol. 2018, 99, 211–218. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 2017, 66, 22–37. [Google Scholar] [CrossRef]
- Holdt, L.M.; Kohlmaier, A.; Teupser, D. Molecular roles and function of circular RNAs in eukaryotic cells. Cell. Mol. Life Sci. 2018, 75, 1071–1098. [Google Scholar] [CrossRef]
- Harland, R.; Misher, L. Stability of RNA in developing Xenopus embryos and identification of a destabilizing sequence in TFIIIA messenger RNA. Development 1988, 102, 837–852. [Google Scholar] [PubMed]
- Chen, I.; Chen, C.Y.; Chuang, T.J. Biogenesis, identification, and function of exonic circular RNAs. Wiley Interdiscip. Rev. RNA 2015, 6, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Kolakofsky, D. Isolation and characterization of Sendai virus DI-RNAs. Cell 1976, 8, 547–555. [Google Scholar] [CrossRef]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Lytton, J. A circularized sodium-calcium exchanger exon 2 transcript. J. Biol. Chem. 1999, 274, 8153–8160. [Google Scholar] [CrossRef] [PubMed]
- Cocquerelle, C.; Mascrez, B.; Hetuin, D.; Bailleul, B. Mis-splicing yields circular RNA molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef]
- Surono, A.; Takeshima, Y.; Wibawa, T.; Ikezawa, M.; Nonaka, I.; Matsuo, M. Circular dystrophin RNAs consisting of exons that were skipped by alternative splicing. Hum. Mol. Genet. 1999, 8, 493–500. [Google Scholar] [CrossRef]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015, 365, 141–148. [Google Scholar] [CrossRef]
- Chen, L.L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016, 17, 205–211. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Wang, Y.; Liu, J.; Zhang, M.; Fang, X.; Chen, H.; Zhang, C. A Zfp609 circular RNA regulates myoblast differentiation by sponging miR-194-5p. Int. J. Biol. Macromol. 2019, 121, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, W.; Liu, E.; Yang, Z.; Dhaliwal, P.; Yang, B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016, 44, 2846–2858. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, W.; Chen, Y.; Wu, Z.K.; Foster, F.S.; Yang, Z.; Li, X.; Yang, B.B. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 2016, 38, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, K.H.; Knöchel, W.; Martínez, D.E. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000, 14, 142–146. [Google Scholar] [PubMed]
- Stefanetti, R.J.; Voisin, S.; Russell, A.; Lamon, S. Recent advances in understanding the role of FOXO3. F1000Research 2018. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Candau, R.B.; Bernardi, H. FoxO transcription factors: Their roles in the maintenance of skeletal muscle homeostasis. Cell. Mol. Life Sci. 2014, 71, 1657–1671. [Google Scholar] [CrossRef]
- Anderson, M.J.; Viars, C.S.; Czekay, S.; Cavenee, W.K.; Arden, K.C. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 1998, 47, 187–199. [Google Scholar] [CrossRef]
- Chen, B.; Xu, J.; He, X.; Xu, H.; Li, G.; Du, H.; Nie, Q.; Zhang, X. A genome-wide mRNA screen and functional analysis reveal FOXO3 as a candidate gene for chicken growth. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Fortini, P.; Iorio, E.; Dogliotti, E.; Isidoro, C. Coordinated metabolic changes and modulation of autophagy during myogenesis. Front. Physiol. 2016. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.W.; Kang, K.S.; Park, T.S. Forkhead Box O3 promotes cell proliferation and inhibits myotube differentiation in chicken myoblast cells. Br. Poult. Sci. 2019, 60, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Piccolo, P.D.; Burden, S.I.; Lisi, R.D.; Sandri, C.; Zhao, J.; et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.; Squaglia, N.; Boge, A.; Fung, P.A. The Simple Western™: A gel-free, blot-free, hands-free Western blotting reinvention. Nat. Methods 2011. [Google Scholar] [CrossRef]
- Kleaveland, B.; Shi, C.Y.; Stefano, J.; Bartel, D.P. A network of noncoding regulatory RNAs acts in the mammalian brain. Cell 2018, 174, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, J.; Wang, H.; Su, X.; Hou, J.; Gu, Y.; Qian, C.; Lin, Y.; Liu, X.; Huang, M.; et al. RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology 2017, 66, 1151–1164. [Google Scholar] [CrossRef]
- Piwecka, M.; Glažar, P.; Hernandez-Miranda, L.R.; Memczak, S.; Wolf, S.A.; Rybak-Wolf, A.; Filipchyk, A.; Klironomos, F.; Cerda Jara, C.A.; Fenske, P.; et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 2017. [Google Scholar] [CrossRef] [PubMed]
- Veno, M.T.; Hansen, T.B.; Veno, S.T.; Clausen, B.H.; Grebing, M.; Finsen, B.; Holm, I.E.; Kjems, J. Spatio-temporal regulation of circular RNA expression during porcine embryonic brain development. Genome Biol. 2015. [Google Scholar] [CrossRef]
- Guarnerio, J.; Bezzi, M.; Jeong, J.C.; Paffenholz, S.V.; Berry, K.; Naldini, M.M.; Lo-Coco, F.; Tay, Y.; Beck, A.H.; Pandolfi, P.P. Oncogenic role of fusion-circRNAs derived from Cancer-associated chromosomal translocations. Cell 2016, 165, 289–302. [Google Scholar] [CrossRef]
- Clavel, S.; Siffroi-Fernandez, S.; Coldefy, A.S.; Boulukos, K.; Pisani, D.F.; Dérijard, B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol. Cell. Biol. 2010, 30, 470–480. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Wei, X.; Song, C.; Dong, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Ma, Y.; et al. CircFUT10 reduces proliferation and facilitates differentiation of myoblasts by sponging miR-133a. J. Cell. Physiol. 2018, 233, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wu, H.; Ye, Y.; Li, Z.; Hao, S.; Kong, L.; Zheng, X.; Lin, S.N.; Zhang, X. The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation. Cell Death Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Gong, Y.; Dai, J.; Chen, G.; Hu, Y. Inhibition of c2c12 cell fibrosis in vitro by miRNA138. J. Shanghai Jiaotong Univ. (Med. Sci. Ed.) 2015, 35, 51–58. [Google Scholar]
Data Availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. |




| Primer | Sequences | Tm/°C |
|---|---|---|
| circ-FoxO3-F | GGCCTCATCTCAAAGCTGG | 58 |
| circ-FoxO3-R | CTTGCCCGTGCCTTCATT | |
| linear-foxO3-F | CGCTGTGTGCCCTACTTCA | 58 |
| linear-foxO3-R | CTTGCCCGTGCCTTCATT | |
| FoxO3-F | CGGACTAGTAACTCCATCCGGCACAAC | 64 |
| FoxO3-R | CCCAAGCTTCTGCTTTGCCCATTTCC | |
| miR-214-5p-F | CCCAAGCTTCTGCATGAGGGCCAGTAAC | 60 |
| miR-214-5p-R | CGCGGATCCGCAGTGAATGTCGAGAGTGTG | |
| miR-181a-5p-F | CCCAAGCTTCTCCCTGTCTTTAACAGCCTG | 62 |
| miR-181a-5p-R | CGCGGATCCGCAGAAGTTAAACCGAGAAACG | |
| miR-96-5p-F | CCCAAGCTTTGGGGGGAGTAGGTTGTAG | 64 |
| miR-96-5p-R | CGCGGATCCAACAGGGCATCACAGAAGC | |
| miR-138-5p-F | CCGGAATTCTGCTGTGGACCTGGTATCTC | 63 |
| miR-138-5p-R | CCGCTCGAGCACAGGGGAGCAGTTCAA | |
| MyoG-F | CAATGCACTGGAGTTCGGT | 58 |
| MyoG-R | CTGGGAAGGCAACAGACAT | |
| MyHC-F | CGCAAGAATGTTCTCAGGCT | 58 |
| MyHC-R | GCCAGGTTGACATTGGATTG | |
| RT-mmu-β-actin-F | ATGGTGGGAATGGGTCAGA | 58 |
| RT-mmu-β-actin-R | TCAATGGGGTACTTCAGGGTC | |
| Mut- miR-138-5p-1-R1 | AGATACAGCTGGCTGAGCCAAGGCTGCTGGAGT | 64 |
| Mut- miR-138-5p-1-F1 | CTTGGCTCAGCCAGCTGTATCTAGCAGTCTCCCGCCAGCCAGTCTAT | |
| Mut- miR-138-5p-2-R2 | CGTTGTAGAGCTCTTGGCGGTATATGGGAAGCTGG | 64 |
| Mut- miR-138-5p-2-F2 | ATATACCGCCAAGAGCTCTACAACGGGCTCCCCAACCGGCTCCTTCA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, C.; Liu, Z.; Ni, W.; Yao, R.; Xu, Y.; Quan, R.; Zhang, M.; Li, H.; Liu, L.; et al. RETRACTED: Circular RNA circ-FoxO3 Inhibits Myoblast Cells Differentiation. Cells 2019, 8, 616. https://doi.org/10.3390/cells8060616
Li X, Li C, Liu Z, Ni W, Yao R, Xu Y, Quan R, Zhang M, Li H, Liu L, et al. RETRACTED: Circular RNA circ-FoxO3 Inhibits Myoblast Cells Differentiation. Cells. 2019; 8(6):616. https://doi.org/10.3390/cells8060616
Chicago/Turabian StyleLi, Xiaoyue, Cunyuan Li, Zhijin Liu, Wei Ni, Rui Yao, Yueren Xu, Renzhe Quan, Mengdan Zhang, Huixiang Li, Li Liu, and et al. 2019. "RETRACTED: Circular RNA circ-FoxO3 Inhibits Myoblast Cells Differentiation" Cells 8, no. 6: 616. https://doi.org/10.3390/cells8060616





