Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains
Abstract
:1. Introduction
2. Material and Methods
2.1. Mouse Sperm
2.2. Generation of Transgenic Mice
2.3. LAPD Activity Assay
2.4. Western Blot Analysis
2.5. Immunohistochemistry and Immunocytochemistry
2.6. Determination of Total cAMP Content
2.7. Imaging
2.8. Image Analysis
2.9. Software Availability
3. Results
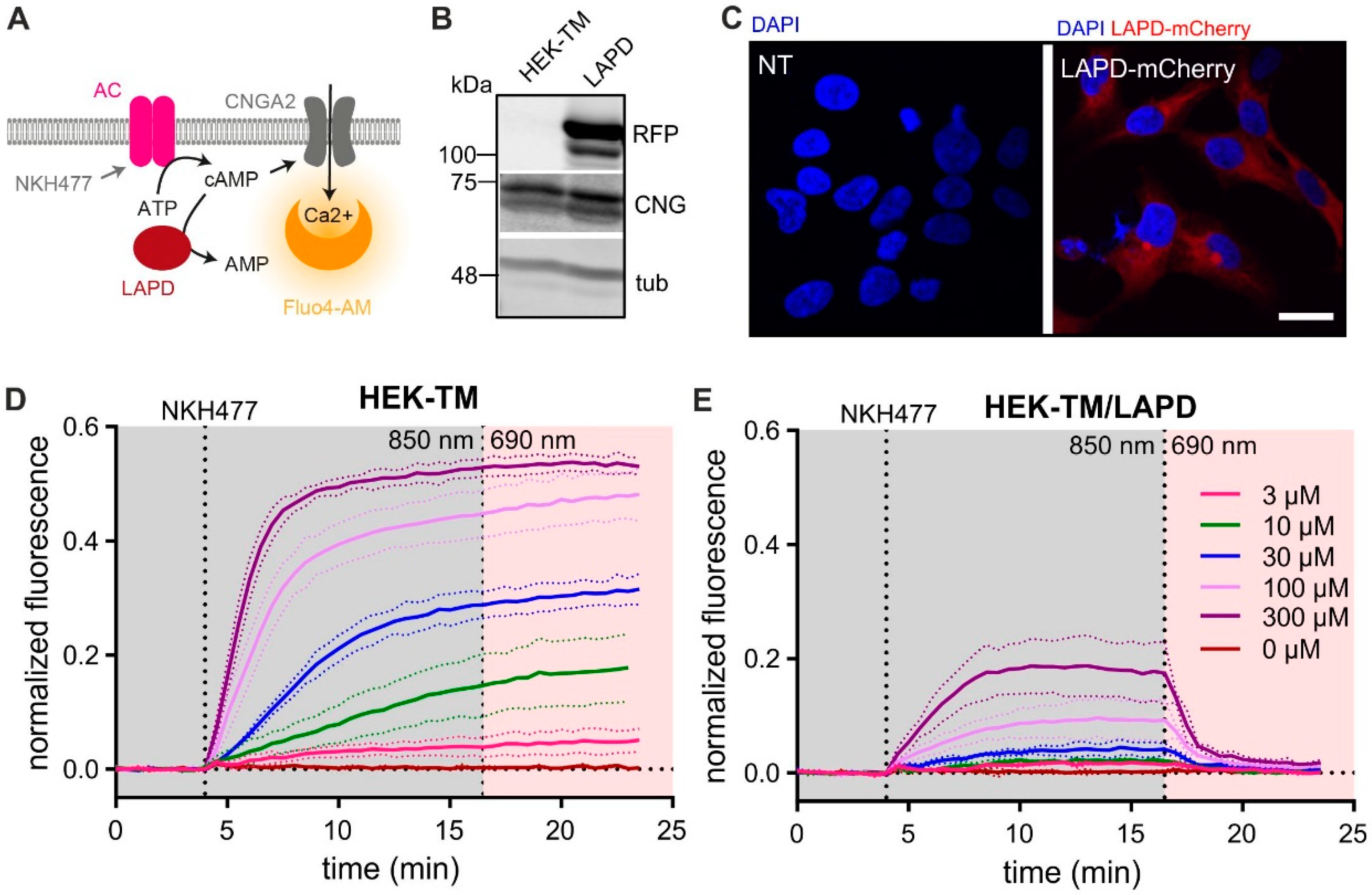
3.1. Characterization of Heterologously Expressed LAPD
3.2. Generation of Transgenic Mice
3.3. Characterization of LAPD Function in Mouse Sperm
3.4. Controlling Sperm Motility by Optogenetics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wachten, D.; Jikeli, J.F.; Kaupp, U.B. Sperm Sensory Signaling. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef]
- Alvarez, L.; Friedrich, B.M.; Gompper, G.; Kaupp, U.B. The computational sperm cell. Trends Cell. Biol. 2014, 24, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Balbach, M.; Beckert, V.; Hansen, J.N.; Wachten, D. Shedding light on the role of cAMP in mammalian sperm physiology. Mol. Cell. Endocrinol. 2018, 468, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Jaiswal, B.S.; Xie, F.; Krajnc-Franken, M.A.M.; Robben, T.J.A.A.; Strik, A.M.; Kuil, C.; Philipsen, R.L.A.; Van Duin, M.; Conti, M.; et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl. Acad. Sci. USA 2004, 101, 2993–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, K.C.; Jones, B.H.; Marquez, B.; Chen, Y.; Ord, T.S.; Kamenetsky, M.; Miyamoto, C.; Zippin, J.H.; Kopf, G.S.; Suarez, S.S.; et al. The “Soluble” Adenylyl Cyclase in Sperm Mediates Multiple Signaling Events Required for Fertilization. Dev. Cell 2005, 9, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Garcia, M.A.; Carlson, A.E.; Schuh, S.M.; Babcock, D.F.; Jaiswal, B.S.; Gossen, J.A.; Esposito, G.; Van Duin, M.; Conti, M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Boil. 2006, 296, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.E.; Hille, B.; Babcock, D.F. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev. Biol. 2007, 312, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Wennemuth, G.; Carlson, A.E.; Harper, A.J.; Babcock, D.F. Bicarbonate actions on flagellar and Ca2+-channel responses: Initial events in sperm activation. Development 2003, 130, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Lackey, B.R.; Gray, S.L. Identification of kinases, phosphatases, and phosphorylation sites in human and porcine spermatozoa. Syst. Boil. Reprod. Med. 2015, 61, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, M.A.; Babcock, D.F.; Wennemuth, G.; Brown, W.; Burton, K.A.; McKnight, G.S. Sperm-specific protein kinase A catalytic subunit Ca2+ orchestrates cAMP signaling for male fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 13483–13488. [Google Scholar] [CrossRef]
- Alvau, A.; Battistone, M.A.; Gervasi, M.G.; Navarrete, F.A.; Xu, X.; Sánchez-Cárdenas, C.; De La Vega-Beltran, J.L.; Da Ros, V.G.; Greer, P.A.; Darszon, A.; et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Develpoment 2016, 143, 2325–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod. Updat. 2008, 14, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, V.; Álvarez, L.; Balbach, M.; Strünker, T.; Hegemann, P.; Kaupp, U.B.; Wachten, D.; Clapham, D.E. Controlling fertilization and cAMP signaling in sperm by optogenetics. ELife 2015, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.; Jikeli, J.F.; Wachten, D. How to control cyclic nucleotide signaling by light. Curr. Opin. Biotechnol. 2017, 48, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Jansen, V.; Jikeli, J.F.; Hamzeh, H.; Alvarez, L.; Dombrowski, M.; Balbach, M.; Strünker, T.; Seifert, R.; Kaupp, U.B.; et al. A novel biosensor to study cAMP dynamics in cilia and flagella. ELife 2016, 5, 694. [Google Scholar] [CrossRef] [PubMed]
- Stierl, M.; Stumpf, P.; Udwari, D.; Gueta, R.; Hagedorn, R.; Losi, A.; Gärtner, W.; Petereit, L.; Efetova, M.; Schwarzel, M.; et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 2011, 286, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Gasser, C.; Taiber, S.; Hegemann, P.; Ryu, S.; Wunder, F.; Möglich, A.; Yeh, C.-M.; Wittig, C.H. Engineering of a red-light-activated human cAMP/cGMP-specific phosphodiesterase. Proc. Natl. Acad. Sci. USA 2014, 111, 8803–8808. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.N.; Rassmann, S.; Jikeli, J.F.; Wachten, D. SpermQ–A Simple Analysis Software to Comprehensively Study Flagellar Beating and Sperm Steering. Cells 2018, 8, 10. [Google Scholar] [CrossRef]
- Wachten, S.; Schlenstedt, J.; Gauss, R.; Baumann, A. Molecular identification and functional characterization of an adenylyl cyclase from the honeybee. J. Neurochem. 2006, 96, 1580–1590. [Google Scholar] [CrossRef]
- Morgan, D.J.; Weisenhaus, M.; Shum, S.; Su, T.; Zheng, R.; Zhang, C.; Shokat, K.M.; Hille, B.; Babcock, D.F.; McKnight, G.S. Tissue-specific PKA inhibition using a chemical genetic approach and its application to studies on sperm capacitation. Proc. Natl. Acad. Sci. USA 2008, 105, 20740–20745. [Google Scholar] [CrossRef] [Green Version]
- Wertheimer, E.; Krapf, D.; De La Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Navarrete, F.; Haddad, D.; Escoffier, J.; Salicioni, A.M.; Levin, L.R.; Buck, J.; et al. Compartmentalization of Distinct cAMP Signaling Pathways in Mammalian Sperm*. J. Boil. Chem. 2013, 288, 35307–35320. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Fiedler, S.E.; Huang, Z.; Vijayaraghavan, S.; Olson, G.E.; Livera, G.; Conti, M.; Carr, D.W. AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa. Biol. Reprod. 2006, 74, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Fisch, J.D.; Behr, B.; Conti, M. Enhancement of motility and acrosome reaction in human spermatozoa: Differential activation by type-specific phosphodiesterase inhibitors. Hum. Reprod. 1998, 13, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, P. Mouse sperm adenylyl cyclase: General properties and regulation by the zona pellucida. Boil. Reprod. 1995, 52, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Develpoment 1995, 121, 1139–1150. [Google Scholar]
- Hoang, H.D.; Miller, M.A. Sperm Navigation Mechanisms in the Female Reproductive Tract. Results Probl Cell. Differ. 2017, 59, 241–267. [Google Scholar]
- Teves, M.E.; Guidobaldi, H.A.; Uñates, D.R.; Sánchez, R.; Miska, W.; Publicover, S.J.; Garcia, A.A.M.; Giojalas, L.C. Molecular Mechanism for Human Sperm Chemotaxis Mediated by Progesterone. PLoS ONE 2009, 4, e8211. [Google Scholar] [CrossRef] [PubMed]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krähling, M.; Müller, A.; Kaupp, U.B.; Strünker, T. The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Boryshpolets, S.; Afanzar, O.; Brandis, A.; Nevo, R.; Kiss, V.; Eisenbach, M. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015, 5, 16146. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Giannakis, D.; Pardalidis, N.; Zikopoulos, K.; Paraskevaidis, E.; Giotitsas, N.; Kalaboki, V.; Tsounapi, P.; Baltogiannis, D.; Georgiou, I.; et al. Effects of phosphodiesterase 5 inhibitors on sperm parameters and fertilizing capacity. Asian J. Androl. 2008, 10, 115–133. [Google Scholar] [CrossRef]
- Peri, A.; Serio, M. The CREM system in human spermatogenesis. J. Endocrinol. Investig. 2000, 23, 578–583. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Setting |
|---|---|
| Thresholding Method | Triangle |
| Gauss Sigma | 1.0 |
| Repeat gauss fit after binarization | false |
| Blur only inside ROI selection | false |
| Upscaling of point list (fold) | 3 |
| Add head center-of-mass as first point | false |
| Unify start points | false |
| Filter points by gauss fits | false |
| Maximum vector length (points) | 14 |
| Normal radius for gauss fit (µm) | 5.0 |
| Exclude head from correction or deletion | true |
| Smooth normal for XY gauss fit | true |
| Accepted xy distance of points for fit-width-smoothing (µm) | 9.6 |
| # (+/−)-consecutive points for xy- and fit-width-smoothing | 15 |
| Distance of point to first point to form the reference vector (µm) | 10.0 |
| Curvature: reference point distance | 10.0 |
| FFT: Grouped consecutive time-steps | 400 |
| FFT: Do not analyze (frequency results of flagellar parameters for the) initial … µm from head | 0.0 |
| Head rotation matrix radius | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, D.N.; Hansen, J.N.; Rassmann, S.; Stüven, B.; Jikeli, J.F.; Strünker, T.; Körschen, H.G.; Möglich, A.; Wachten, D. Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains. Cells 2019, 8, 648. https://doi.org/10.3390/cells8070648
Raju DN, Hansen JN, Rassmann S, Stüven B, Jikeli JF, Strünker T, Körschen HG, Möglich A, Wachten D. Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains. Cells. 2019; 8(7):648. https://doi.org/10.3390/cells8070648
Chicago/Turabian StyleRaju, Diana N., Jan N. Hansen, Sebastian Rassmann, Birthe Stüven, Jan F. Jikeli, Timo Strünker, Heinz G. Körschen, Andreas Möglich, and Dagmar Wachten. 2019. "Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains" Cells 8, no. 7: 648. https://doi.org/10.3390/cells8070648
APA StyleRaju, D. N., Hansen, J. N., Rassmann, S., Stüven, B., Jikeli, J. F., Strünker, T., Körschen, H. G., Möglich, A., & Wachten, D. (2019). Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains. Cells, 8(7), 648. https://doi.org/10.3390/cells8070648





