Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans
Abstract
:1. Introduction
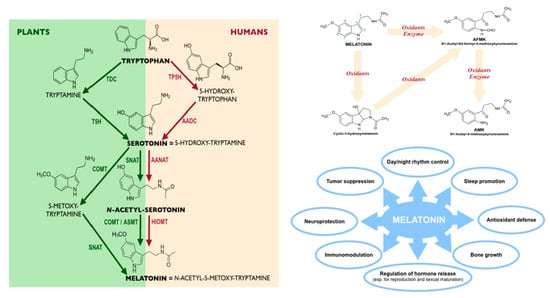
2. Melatonin in Plants
3. Melatonin in Humans: A Key Emphasis in Biological Activity
3.1. Regulation of the Circadian Rhythm, Biological Clock, and Sleep/Wake Cycle
3.2. Melatonin Receptors
3.3. Receptor-Mediated Activities
3.4. Nonreceptor-Mediated Effects
4. Melatonin Supplementation and its Health Effects for Humans
4.1. Melatonin and Inflammation
4.2. Melatonin and Wound Repair
4.3. Melatonin and Brain Injury
4.4. Melatonin and Cardio- and Neuro-Protection
5. Oral Bioavailability of Plant Melatonin
Melatonin Oral Bioavailability in Humans
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manchester, L.C.; Tan, D.-X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Conti, A.; Tettamanti, C.; Singaravel, M.; Haldar, C.; Pandi-Perumal, R.; Maestroni, G. Melatonin: An ubiquitous and evolutionary hormone. In Treatise Pineal Gland Melatonin; Science Publishers: Boca Raton, FL, USA, 2002; pp. 105–143. [Google Scholar]
- Reiter, R.J.; Fraschini, F. Endocrine Aspects of the Mammalian Pineal Gland: A Review. Neuroendocrinology 1969, 5, 219–255. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Ubiquitous melatonin-presence and effects in unicells, plants and animals. Trends Comp. Biochem. Physiol. 1996, 2, 25–45. [Google Scholar]
- Vivien-Roels, B.; Pévet, P. Melatonin: Presence and formation in invertebrates. Experientia 1993, 49, 642–647. [Google Scholar] [CrossRef]
- Poeggeler, B.; Balzer, I.; Fischer, J.; Behrmann, G.; Hardeland, R. A role of melatonin in dinoflagellates? Eur. J. Endocrinol. 1989, 120, S97. [Google Scholar] [CrossRef]
- Balzer, I.; Hardeland, R. Photoperiodism and effects of indoleamines in a unicellular alga, Gonyaulax polyedra. Science 1991, 253, 795–797. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Tan, D.-X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.; Tan, D.; Mayo, J.; Sainz, R.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Tan, D.; Rosales-Corral, S.; Galano, A.; Zhou, X.; Xu, B. Mitochondria: Central organelles for melatonin′ s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Atioxidative protection by melatonin. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef]
- Allegra, M.; Reiter, R.; Tan, D.-X.; Gentile, C.; Tesoriere, L.; Livrea, M. The chemistry of melatonin’s interaction with reactive species. J. Pineal Res. 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Bałabusta, M.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef]
- Gitto, E.; Tan, D.X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 2001, 53, 1393–1401. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate–glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Szafrańska, K.; Posmyk, M.M. Phytomelatonin physiological functions (Ch 5). In Serotonin and Melatonin: Their Functional Role in Plants, Food, Phytomedicine, and Human Health; Ravishankar, G.A., Ramakrishna, A., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 61–72. [Google Scholar]
- Kołodziejczyk, I.; Bałabusta, M.; Szewczyk, R.; Posmyk, M.M. The levels of melatonin and its metabolites in conditioned corn (Zea mays L.) and cucumber (Cucumis sativus L.) seeds during storage. Acta Physiol. Plant. 2015, 37, 105. [Google Scholar]
- Tan, D.-X.; Manchester, L.C.; Di Mascio, P.; Martinez, G.R.; Prado, F.M.; Reiter, R.J. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: Importance for phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Than, N.N.; Koch, D.; Poeggeler, B.; Laatsch, H.; Hardeland, R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006, 41, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.-X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant. Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Janas, K.M. Melatonin in plants. Acta Physiol. Plant. 2009, 31, 1. [Google Scholar] [CrossRef]
- Paredes, S.D.; Korkmaz, A.; Manchester, L.C.; Tan, D.-X.; Reiter, R.J. Phytomelatonin: A review. J. Exp. Bot. 2008, 60, 57–69. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2011, 63, 577–597. [Google Scholar] [CrossRef]
- Arnao, M.B. Phytomelatonin: Discovery, content, and role in plants. Adv. Bot. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Erland, L.A.; Murch, S.J.; Reiter, R.J.; Saxena, P.K. A new balancing act: The many roles of melatonin and serotonin in plant growth and development. Plant. Signal. Behav. 2015, 10, e1096469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Melatonin in plants–diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin promotes adventitious-and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J. Agric. Food Chem. 2008, 56, 10567–10573. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Assessment of different sample processing procedures applied to the determination of melatonin in plants. Phytochem. Anal. 2009, 20, 14–18. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.-b.; Reiter, R.J.; Wei, W.; Wang, B.-m. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef]
- Ravishankar, G.A.; Ramakrishna, A. Serotonin and Melatonin: Their Functional Role in Plants, Food, Phytomedicine, and Human Health; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef]
- Padumanonda, T.; Johns, J.; Sangkasat, A.; Tiyaworanant, S. Determination of melatonin content in traditional Thai herbal remedies used as sleeping aids. Daru J. Pharm. Sci. 2014, 22, 6. [Google Scholar] [CrossRef]
- Chen, G.; Huo, Y.; Tan, D.-X.; Liang, Z.; Zhang, W.; Zhang, Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.-X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, D.X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.t.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, S.; Tan, D.-X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Simonetti, P.; Faoro, F.; Fico, G.; Iriti, M. The presence of melatonin in grapevine (Vitis vinifera L.) berry tissues. J. Pineal Res. 2011, 51, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Stürtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar]
- Mena, P.; Gil-Izquierdo, Á.; Moreno, D.A.; Martí, N.; García-Viguera, C. Assessment of the melatonin production in pomegranate wines. LWT-Food Sci. Technol. 2012, 47, 13–18. [Google Scholar] [CrossRef]
- Murch, S.J.; Simmons, C.B.; Saxena, P.K. Melatonin in feverfew and other medicinal plants. Lancet 1997, 350, 1598–1599. [Google Scholar] [CrossRef]
- Okazaki, M.; Ezura, H. Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivar Micro-Tom. J. Pineal Res. 2009, 46, 338–343. [Google Scholar] [CrossRef]
- Stege, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silva, M.F. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.E.; Jang, H.; Byeon, Y.; Kim, Y.S.; Back, K. Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 2013, 54, 258–263. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2014, 66, 647–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Chen, K.; Wei, Y.; He, C. Fundamental issues of melatonin-mediated stress signaling in plants. Front. Plant Sci. 2016, 7, 1124. [Google Scholar] [CrossRef] [PubMed]
- Janas, K.M.; Posmyk, M.M. Melatonin, an underestimated natural substance with great potential for agricultural application. Acta Physiol. Plant. 2013, 35, 3285–3292. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejczyk, I.; Posmyk, M.M. Melatonin-a new plant biostimulator? J. Elem. 2016, 21, 1187–1198. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafrańska, K. Exogenous melatonin affects productivity of horticultural and agricultural crops (Ch 11). In Serotonin and Melatonin: Their Functional Role in Plants, Food, Phytomedicine, and Human Health; Ravishankar, G.A., Ramakrishna, A., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 61–72. [Google Scholar]
- Tan, D.-X.; Manchester, L.C.; Helton, P.; Reiter, R.J. Phytoremediative capacity of plants enriched with melatonin. Plant. Signal. Behav. 2007, 2, 514–516. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. (Lausanne) 2019, 10, 249. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in plants and other phototrophs: Advances and gaps concerning the diversity of functions. J. Exp. Bot. 2014, 66, 627–646. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Szafrańska, K.; Posmyk, M.M. Melatonin in plants (Ch 1). In Serotonin and Melatonin: Their Functional Role in Plants, Food, Phytomedicine, and Human Health; Ravishankar, G.A., Ramakrishna, A., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 61–72. [Google Scholar]
- Adams, K.L.; Palmer, J.D. Evolution of mitochondrial gene content: Gene loss and transfer to the nucleus. Mol. Phylogenet. Evol. 2003, 29, 380–395. [Google Scholar] [CrossRef]
- Lazar, D.; Murch, S.J.; Beilby, M.J.; Al Khazaaly, S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant. Signal. Behav. 2013, 8, e23279. [Google Scholar] [CrossRef] [PubMed]
- Szafrańska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin application to Pisum sativum L. seeds positively influences the function of the photosynthetic apparatus in growing seedlings during paraquat-induced oxidative stress. Front. Plant Sci. 2016, 7, 1663. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Szafrańska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front. Plant Sci. 2017, 8, 878. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Chang, C.; Feng, F.; Liang, D.; Cheng, L.; Ma, F. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 2013, 55, 424–434. [Google Scholar] [PubMed]
- Wang, P.; Sun, X.; Xie, Y.; Li, M.; Chen, W.; Zhang, S.; Liang, D.; Ma, F. Melatonin regulates proteomic changes during leaf senescence in Malus hupehensis. J. Pineal Res. 2014, 57, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2014, 66, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J. Plant Physiol. 2016, 193, 47–56. [Google Scholar]
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous melatonin expediently modifies proteome of maize (Zea mays L.) embryo during seed germination. Acta Physiol. Plant. 2016, 38, 146. [Google Scholar]
- Najeeb, S.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Therapeutic potential of melatonin in oral medicine and periodontology. Kaohsiung J. Med. Sci. 2016, 32, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Park, W.J. Melatonin as an endogenous plant regulatory signal: Debates and perspectives. J. Plant Biol. 2011, 54, 143–149. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Melatonin in traditional Mediterranean diets. J. Pineal Res. 2010, 49, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, M.; Korf, H.W.; Wicht, H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen. Comp. Endocrinol. 2018, 258, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 5th ed.; Garland Science: New York, NY, USA, 2008; p. 1616. [Google Scholar]
- Radogna, F.; Diederich, M.; Ghibelli, L. Melatonin: A pleiotropic molecule regulating inflammation. Biochem. Pharmacol. 2010, 80, 1844–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandi-Perumal, S.R.; Zisapel, N.; Srinivasan, V.; Cardinali, D.P. Melatonin and sleep in aging population. Exp. Gerontol. 2005, 40, 911–925. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Pagano, E.S.; Bernasconi, P.S.; Reynoso, R.; Scacchi, P. Melatonin and mitochondrial dysfunction in the central nervous system. Horm. Behav. 2013, 63, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.H.C.; Leal, C.L.V.; Cruz, J.F.; Tan, D.-X.; Reiter, R.J. Essential actions of melatonin in protecting the ovary from oxidative damage. Theriogenology 2014, 82, 925–932. [Google Scholar] [CrossRef]
- Shen, Y.X.; Xu, S.Y.; Wei, W.; Sun, X.X.; Liu, L.H.; Yang, J.; Dong, C. The protective effects of melatonin from oxidative damage induced by amyloid beta-peptide 25–35 in middle-aged rats. J. Pineal Res. 2002, 32, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Dies, H.; Cheung, B.; Tang, J.; Rheinstädter, M.C. The organization of melatonin in lipid membranes. Biochim. Biophys. Acta 2015, 1848, 1032–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iriti, M.; Varoni, E.M. The good health of Bacchus: Melatonin in grapes, the unveiled myth. LWT-Food Sci. Technol. 2016, 65, 758–761. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef] [PubMed]
- Vielma, J.R.; Bonilla, E.; Bonilla, L.C.; Mora, M.; Leendertz, S.M.; Bravoa, Y. Effects of melatonin on oxidative stress, and resistance to bacterial, parasitic, and viral infections: A review. Acta Trop. 2014, 137, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Gogenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.; Maestroni, G.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Borjigin, J.; Zhang, L.S.; Calinescu, A.A. Circadian regulation of pineal gland rhythmicity. Mol. Cell. Endocrinol. 2012, 349, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Arendt, J.; Broadway, J. Light and melatonin as zeitgebers in man. Chronobiol. Int. 1987, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, P.; Mänttäri, S.; Leskinen, H.; Vakkuri, O.; Ruskoaho, H.; Saarela, S. Long-term postinfarction melatonin administration alters the expression of DHPR, RyR2, SERCA2, and MT2 and elevates the ANP level in the rat left ventricle. J. Pineal Res. 2008, 45, 61–69. [Google Scholar] [CrossRef]
- Singh, M.; Jadhav, H.R. Melatonin: Functions and ligands. Drug Discov. Today 2014, 19, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Gillette, M.U. Melatonin, sleep, and circadian rhythms: Rationale for development of specific melatonin agonists. Sleep Med. 2004, 5, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Lamont, K.; Nduhirabandi, F.; Adam, T.; Thomas, D.P.; Opie, L.H.; Lecour, S. Role of melatonin, melatonin receptors and STAT3 in the cardioprotective effect of chronic and moderate consumption of red wine. Biochem. Biophys. Res. Commun. 2015, 465, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Melatonin and retinoid orphan receptors: Demand for new interpretations after their exclusion as nuclear melatonin receptors. Melatonin Res. 2018, 1, 78–93. [Google Scholar] [CrossRef]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Drew, J.E.; Barrett, P.; Mercer, J.G.; Moar, K.M.; Canet, E.; Delagrange, P. Localization of the melatonin-related receptor in the rodent brain and peripheral tissues. J. Neuroendocr. 2001, 13, 453–458. [Google Scholar] [CrossRef]
- Shu, T.; Wu, T.; Pang, M.; Liu, C.; Wang, X.; Wang, J.; Liu, B.; Rong, L. Effects and mechanisms of melatonin on neural differentiation of induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 2016, 474, 566–571. [Google Scholar] [CrossRef]
- Favero, G.; Moretti, E.; Bonomini, F.; Reiter, R.J.; Rodella, L.F.; Rezzani, R. Promising Antineoplastic Actions of Melatonin. Front. Pharmacol. 2018, 9, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vriend, J.; Reiter, R.J. Melatonin, bone regulation and the ubiquitin-proteasome connection: A review. Life Sci. 2016, 145, 152–160. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X. Melatonin and male reproduction. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 446, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, W.; Ma, Y.; Wang, D.; Zhao, X.; Zeng, C.; Zhang, M.; Zeng, X.; Meng, Q.; Zhou, G. Improved development by melatonin treatment after vitrification of mouse metaphase II oocytes. Cryobiology 2016, 73, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Sahni, D.; Gupta, R.; Gupta, S.K. Expanding the horizons of melatonin use: An immunohistochemical neuroanatomic distribution of MT1 and MT2 receptors in human brain and retina. J. Anat. Soc. India 2017, 192, 9–18. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Jose, M.; Consuegra-Sanchez, L.; Piccolo, R.; Gonzalez-Gonzalez, J.; Garcia-Camarero, T.; del Mar Garcia-Saiz, M.; Aldea-Perona, A.; Reiter, R.J. Usefulness of early treatment with melatonin to reduce infarct Size in patients with ST-segment elevation myocardial infarction receiving percutaneous coronary intervention (from the Melatonin Adjunct in the Acute Myocardial Infarction Treated With Angioplasty Trial). Am. J. Cardiol. 2017, 120, 522–526. [Google Scholar] [PubMed]
- Zisapel, N. Melatonin-dopamine interactions: From basic neurochemistry to a clinical setting. Cell. Mol. Neurobiol. 2001, 21, 605–616. [Google Scholar] [CrossRef]
- Peschke, E.; Mühlbauer, E. New evidence for a role of melatonin in glucose regulation. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 829–841. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Salehi, B.; Iriti, M.; Roointan, A.; Mnayer, D.; Soltani-Nejad, A.; Afshari, A. In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica Poir. Cell. Mol. Biol. 2018, 64, 57–64. [Google Scholar] [CrossRef]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or Negative Actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Shaker, M.E.; Houssen, M.E.; Abo-Hashem, E.M. Comparison of vitamin E, L-carnitine and melatonin in ameliorating carbon tetrachloride and diabetes induced hepatic oxidative stress. J. Physiol. Biochem. 2009, 65, 225–233. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and its Metabolites versus Oxidative Stress: From Individual Actions to Collective Protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Ma, S.; Topal, T.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Glucose: A vital toxin and potential utility of melatonin in protecting against the diabetic state. Mol. Cell. Endocrinol. 2012, 349, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Coto-Montes, A.; Antonio Boga, J.; Rosales-Corral, S.; Fuentes-Broto, L.; Tan, D.-X.; Reiter, R.J. Role of melatonin in the regulation of autophagy and mitophagy: A review. Mol. Cell. Endocrinol. 2012, 361, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.M.; Schamne, M.G.; Sampaio, T.B.; Pertile, R.A.; Fernandes, P.A.; Markus, R.P.; Prediger, R.D. Melatoninergic System in Parkinson’s Disease: From Neuroprotection to the Management of Motor and Nonmotor Symptoms. Oxidative Med. Cell. Longev. 2016, 2016, 3472032. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Rueda, N.; Mediavilla, M.D.; Martinez-Cue, C.; Reiter, R.J. Clinical Uses of Melatonin in Neurological Diseases and Mental and Behavioural Disorders. Curr. Med. Chem. 2017, 24, 3851–3878. [Google Scholar] [CrossRef]
- Mahmood, D.; Muhammad, B.Y.; Alghani, M.; Anwar, J.; el-Lebban, N.; Haider, M. Advancing role of melatonin in the treatment of neuropsychiatric disorders. Egypt. J. Basic Appl. Sci. 2016, 3, 203–218. [Google Scholar] [Green Version]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.-X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Fan, C.; Pan, Y.; Yang, Y.; Di, S.; Jiang, S.; Ma, Z.; Li, T.; Zhang, Z.; Li, W.; Li, X.; et al. HDAC1 inhibition by melatonin leads to suppression of lung adenocarcinoma cells via induction of oxidative stress and activation of apoptotic pathways. J. Pineal Res. 2015, 59, 321–333. [Google Scholar] [CrossRef]
- Pita, R.; Marco-Contelles, J.; Ramos, E.; Pino, J.; Romero, A. Toxicity induced by chemical warfare agents: Insights on the protective role of melatonin. Chem.-Biol. Interact. 2013, 206, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.D.; Katkar, G.D.; Sundaram, M.S.; Swethakumar, B.; Girish, K.S.; Kemparaju, K. Melatonin inhibits snake venom and antivenom induced oxidative stress and augments treatment efficacy. Acta Trop. 2017, 169, 14–25. [Google Scholar] [CrossRef]
- Mehta, A.; Kaur, G. Potential role of melatonin in prevention and treatment of oral carcinoma. Indian J. Dent. 2014, 5, 56–61. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin and the von Hippel–Lindau/HIF-1 oxygen sensing mechanism: A review. Biochim. Biophys. Acta 2016, 1865, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [PubMed]
- Tosini, G.; Baba, K.; Hwang, C.K.; Iuvone, P.M. Melatonin: An underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 2012, 103, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, G.; Kröger, M.; Meier-Ewert, K. Effects of vitamin B12 on performance and circadian rhythm in normal subjects. Neuropsychopharmacology 1996, 15, 456. [Google Scholar] [CrossRef]
- Koziróg, M.; Poliwczak, A.R.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Broncel, M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 2010, 50, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.L.; Hermsdorff, H.H.M.; Bressan, J. Melatonin intake and potential chronobiological effects on human health. Crit. Rev. Food Sci. Nutr. 2017, 133–140. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Antiinflammatory Activity of Melatonin in Central Nervous System. Curr. Neuropharmacol. 2010, 8, 228–242. [Google Scholar] [CrossRef] [Green Version]
- El-Shenawy, S.M.; Abdel-Salam, O.M.; Baiuomy, A.R.; El-Batran, S.; Arbid, M.S. Studies on the anti-inflammatory and anti-nociceptive effects of melatonin in the rat. Pharmacol. Res. 2002, 46, 235–243. [Google Scholar] [CrossRef]
- Pugazhenthi, K.; Kapoor, M.; Clarkson, A.N.; Hall, I.; Appleton, I. Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J. Pineal Res. 2008, 44, 387–396. [Google Scholar] [CrossRef]
- Weishaupt, J.H.; Bartels, C.; Pölking, E.; Dietrich, J.; Rohde, G.; Poeggeler, B.; Mertens, N.; Sperling, S.; Bohn, M.; Hüther, G. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 2006, 41, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zaslavskaia, R.; Shcherbakov, E.; Logvinenko, S. Evaluation of different methods of treatment of patients with stable stenocardia combined with arterial hypertension according to echocardiographic data. Klin. Meditsina 2007, 85, 40–43. [Google Scholar]
- Thomale, U.-W.; Griebenow, M.; Kroppenstedt, S.-N.; Unterberg, A.W.; Stover, J.F. Small volume resuscitation with HyperHaes™ improves pericontusional perfusion and reduces lesion volume following controlled cortical impact injury in rats. J. Neurotrauma 2004, 21, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Traystman, R.J.; Kirsch, J.R.; Koehler, R.C. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J. Appl. Physiol. 1991, 71, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, A.; Okatani, Y.; Shinohara, K.; Ikenoue, N.; Kaneda, C.; Fukaya, T. Melatonin protects fetal rat brain against oxidative mitochondrial damage. J. Pineal Res. 2001, 30, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Tengattini, S.; Reiter, R.J.; Tan, D.X.; Terron, M.P.; Rodella, L.F.; Rezzani, R. Cardiovascular diseases: Protective effects of melatonin. J. Pineal Res. 2008, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J.; Qi, W.-B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Neurosignals 2000, 9, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.-K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, G.; Liu, L.; Xu, S. Suppressive effects of melatonin on amyloid-β-induced glial activation in rat hippocampus. Arch. Med. Res. 2007, 38, 284–290. [Google Scholar] [CrossRef]
- Ren, W.; Wang, P.; Yan, J.; Liu, G.; Zeng, B.; Hussain, T.; Peng, C.; Yin, J.; Li, T.; Wei, H. Melatonin alleviates weanling stress in mice: Involvement of intestinal microbiota. J. Pineal Res. 2018, 64, e12448. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X. Melatonin: A novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc. Res. 2003, 58, 10–19. [Google Scholar] [CrossRef]
- Grossman, E.; Laudon, M.; Yalcin, R.; Zengil, H.; Peleg, E.; Sharabi, Y.; Kamari, Y.; Shen-Orr, Z.; Zisapel, N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am. J. Med. 2006, 119, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Dwaich, K.H.; Al-Amran, F.G.; Al-Sheibani, B.I.; Al-Aubaidy, H.A. Melatonin effects on myocardial ischemia-reperfusion injury: Impact on the outcome in patients undergoing coronary artery bypass grafting surgery. Int. J. Cardiol. 2016, 221, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Gubin, G.D.; Gapon, L.I.; Weinert, D. Daily Melatonin Administration Attenuates Age-Dependent Disturbances of Cardiovascular Rhythms. Curr. Aging Sci. 2016, 9, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Parvez, S. Role of melatonin in traumatic brain injury and spinal cord injury. Sci. World J. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kilic, E.; Kilic, Ü.; Bacigaluppi, M.; Guo, Z.; Abdallah, N.B.; Wolfer, D.P.; Reiter, R.J.; Hermann, D.M.; Bassetti, C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res. 2008, 45, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration. Australian Public Assessment Report for Melatonin; RAD Data Australia Pty Ltd.: Melbourne, VIC, Australia, 2009. [Google Scholar]
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin receptors: Distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 2017, 222, 2921–2939. [Google Scholar] [CrossRef]
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: Melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 2004, 25, 951–960. [Google Scholar]
- Lee, B.-J.; Parrott, K.A.; Ayres, J.W. Development and characterization of an oral controlled-release delivery system for melatonin. Drug Dev. Ind. Pharm. 1996, 22, 269–274. [Google Scholar] [CrossRef]
- Yeleswaram, K.; McLaughlin, L.G.; Knipe, J.O.; Schabdach, D. Pharmacokinetics and oral bioavailability of exogenous melatonin in preclinical animal models and clinical implications. J. Pineal Res. 1997, 22, 45–51. [Google Scholar] [CrossRef]
- Neddegaard, F.; Kennaway, D.J. A method of achieving physiological plasma levels of melatonin in the chicken by oral administration. J. Pineal Res. 1999, 27, 129–138. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tana, D.-X.; Manchester, L.C.; Simopoulos, A.P.; Maldonado, M.D.; Floresa, L.J.; Terron, M.P. Melatonin in Edible Plants (Phytomelatonin): Identification, Concentrations, Bioavailability and Proposed Functions. World Rev. Nutr. Diet. 2007, 97, 211–230. [Google Scholar] [PubMed]
- Aguilera, Y.; Rebollo-Hernanz, M.; Herrera, T.; Cayuelas, L.T.; Rodriguez-Rodriguez, P.; de Pablo, A.L.L.; Arribas, S.M.; Martin-Cabrejas, M.A. Intake of bean sprouts influences melatonin and antioxidant capacity biomarker levels in rats. Food Funct. 2016, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.P.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Iriti, M.; Varoni, E.M. Commentary: Are the proposed benefits of melatonin-rich foods too hard to swallow? Front. Nutr. 2016, 3, 2. [Google Scholar] [CrossRef]
- Kennaway, D.J. Are the proposed benefits of melatonin-rich foods too hard to swallow? Crit. Rev. Food Sci. Nutr. 2017, 57, 958–962. [Google Scholar] [CrossRef]





| Common Name | Latin Name | Organ | Melatonin [ng g-1 DW](or FW*) | Reference |
|---|---|---|---|---|
| Coffee robusta | Coffea canephora Pierr. | Bean | 5800 | [41] |
| Coffee arabica | Coffea arabica (L.) | Bean | 6800 | [41] |
| Black pepper | Piper nigrum (L.) | Leaf | 1093 | [42] |
| Wolf berry (goji) | Lycium barbarum (L.) | Fruit | 530 | [43] |
| White radish | Raphanus sativus (L.) | Bulb | 485 | [43] |
| White mustard | Sinapis alba (L.) | Seed | 189 | [1] |
| Black mustard | Brassica nigra (L.) | Seed | 129 | [1] |
| Curcuma | Curcuma aeruginosa Roxb. | Root | 120 | [43] |
| Wolf berry | Lycium barbarum | Seed | 103 | [1] |
| Burmese grape | Baccaurea ramiflora Lour. | Leaf | 43.2 | [42] |
| Fenugreek | Trigonella foenum-graecum (L.) | Seed | 43 | [1] |
| Almond | Prunus amygdalus (Batsch) | Seed | 39 | [1] |
| Sunflower | Helianthus annuus (L.) | Seed | 29 | [1] |
| Fennel | Foeniculum vulgare (Gilib.) | Seed | 28 | [1] |
| Agati | Sesbania glandiflora (L.) Desv. | Leaf | 26.3 | [42] |
| Bitter melon | Momordica charantia (L.) | Leaf | 21.4 | [42] |
| Alfalfa | Medicago sativum (L.) | Seed | 16 | [1] |
| Green cardamom | Elettaria cardamomum (White et Maton) | Seed | 15 | [1] |
| Flax | Linum usitatissimum (L.) | Seed | 12 | [1] |
| Linseed (flax) | Linum usitatissimum (L.) | Seed | 12 | [1] |
| Java bean | Senna tora (L.) Roxb. | Leaf | 10.5 | [42] |
| Sesban | Sesbania sesban (L.) Merr. | Leaf | 8.7 | [42] |
| Anise | Pimpinela anisum (L.) | Seed | 7 | [1] |
| Celery | Apium graveolens (L.) | Seed | 7 | [1] |
| Coriander | Coriandrum sativum (L.) | Seed | 7 | [1] |
| Poppy | Papaver somniferum (L.) | Seed | 6 | [1] |
| Walnut | Juglans regia (L.) | Seed | 3.5 | [44] |
| Milk thistle | Silybum marianum (L.) | Seed | 2 | [1] |
| Sweet cherries | Prunus avium (L.) | Fruit | 120* | [45] |
| Tart cherries | Prunus cerasus (L.) | Fruit | 19.5* | [46] |
| Grapevine | Vitis vinifera (L.) | Fruit | 18* | [47] |
| Cherry | Prunus cerasus (L.) | Fruit | 18* | [46] |
| Corn | Zea mays (L) | Seed | 14-53* | [24] |
| Cucumber | Cucumis sativus (L) | Seed | 11-80* | [24] |
| Strawberry | Fragaria x ananassa (Duch.) | Fruit | 11.3* | [48] |
| Pomegranate | Punica granatum (L.) | Fruit | 5.5* | [49] |
| Tall fescue | Festuca arundinacea | Seed | 5.3* | [10] |
| St. John’s wort | Hypericum perforatum (L.) | Flower | 4* | [50] |
| Lupine | Lupinus albus (L.) | Seed | 3.8* | [36] |
| Tomato | Solanum lycopersicum (L.) | Fruit | 2.5* | [51] |
| Fever few | Tanacetum parthenium (L.) | Leaf | 2* | [50] |
| St. John’s wort | Hypericum perforatum (L.) | Leaf | 2* | [50] |
| Oat | Avena sativa (L.) | Seed | 1.8* | [10] |
| Corn | Zea mays (L.) | Seed | 1.4* | [10] |
| Grapevine | Vitis vinifera (L.) | Fruit | 1.2* | [52] |
| Rice | Oryza sativa japonica (L.) | Seed | 1* | [10] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.d.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. https://doi.org/10.3390/cells8070681
Salehi B, Sharopov F, Fokou PVT, Kobylinska A, Jonge Ld, Tadio K, Sharifi-Rad J, Posmyk MM, Martorell M, Martins N, et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells. 2019; 8(7):681. https://doi.org/10.3390/cells8070681
Chicago/Turabian StyleSalehi, Bahare, Farukh Sharopov, Patrick Valere Tsouh Fokou, Agnieszka Kobylinska, Lilian de Jonge, Kathryn Tadio, Javad Sharifi-Rad, Malgorzata M. Posmyk, Miquel Martorell, Natália Martins, and et al. 2019. "Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans" Cells 8, no. 7: 681. https://doi.org/10.3390/cells8070681
APA StyleSalehi, B., Sharopov, F., Fokou, P. V. T., Kobylinska, A., Jonge, L. d., Tadio, K., Sharifi-Rad, J., Posmyk, M. M., Martorell, M., Martins, N., & Iriti, M. (2019). Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells, 8(7), 681. https://doi.org/10.3390/cells8070681