Single Cell Gene Co-Expression Network Reveals FECH/CROT Signature as a Prognostic Marker
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Preprocessing
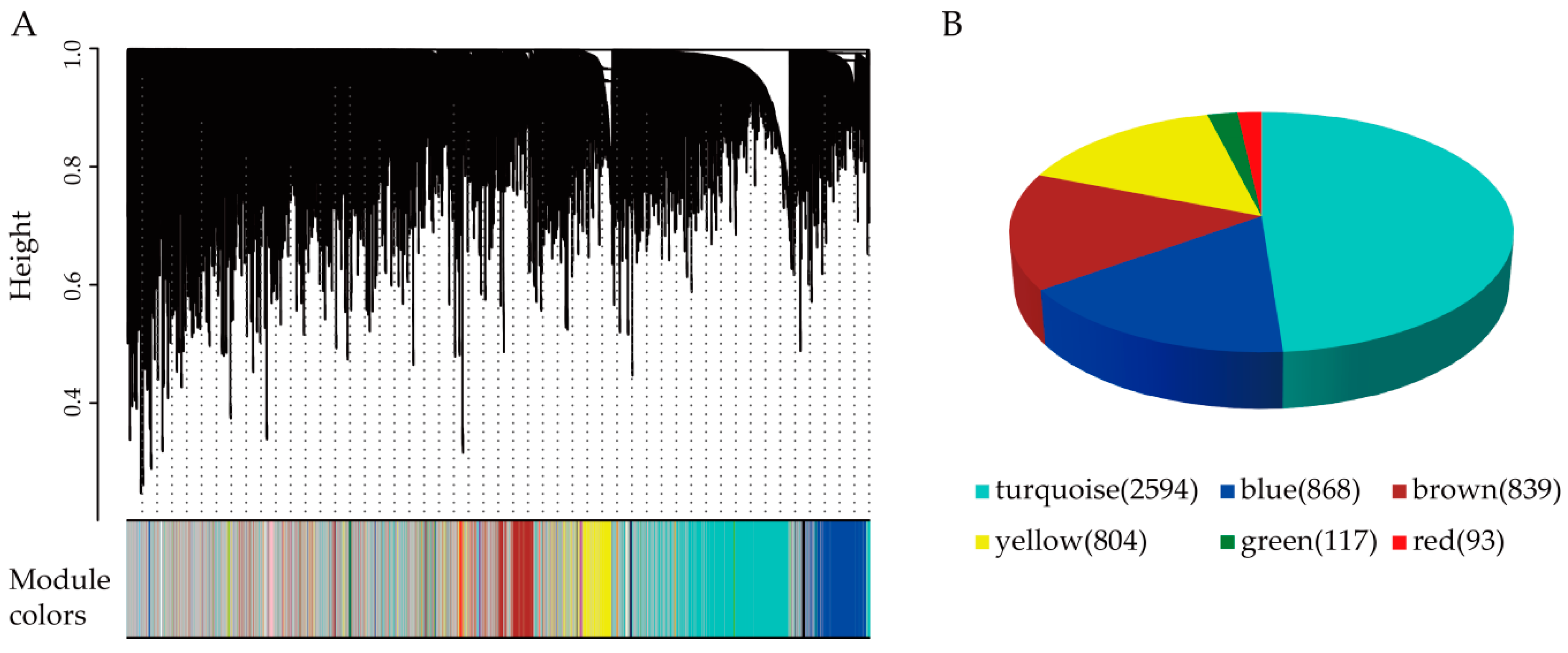
2.2. Weighted Correlation Network Analysis (WGCNA)
2.3. Identifying Genes with AR Binding Sites Based on ChIP-seq Dataset
2.4. Androgen Regulated Genes
2.5. Gene Ontology Annotation and Enrichment Analysis
2.6. Statistic Method for Differential Expression and Survival Analysis
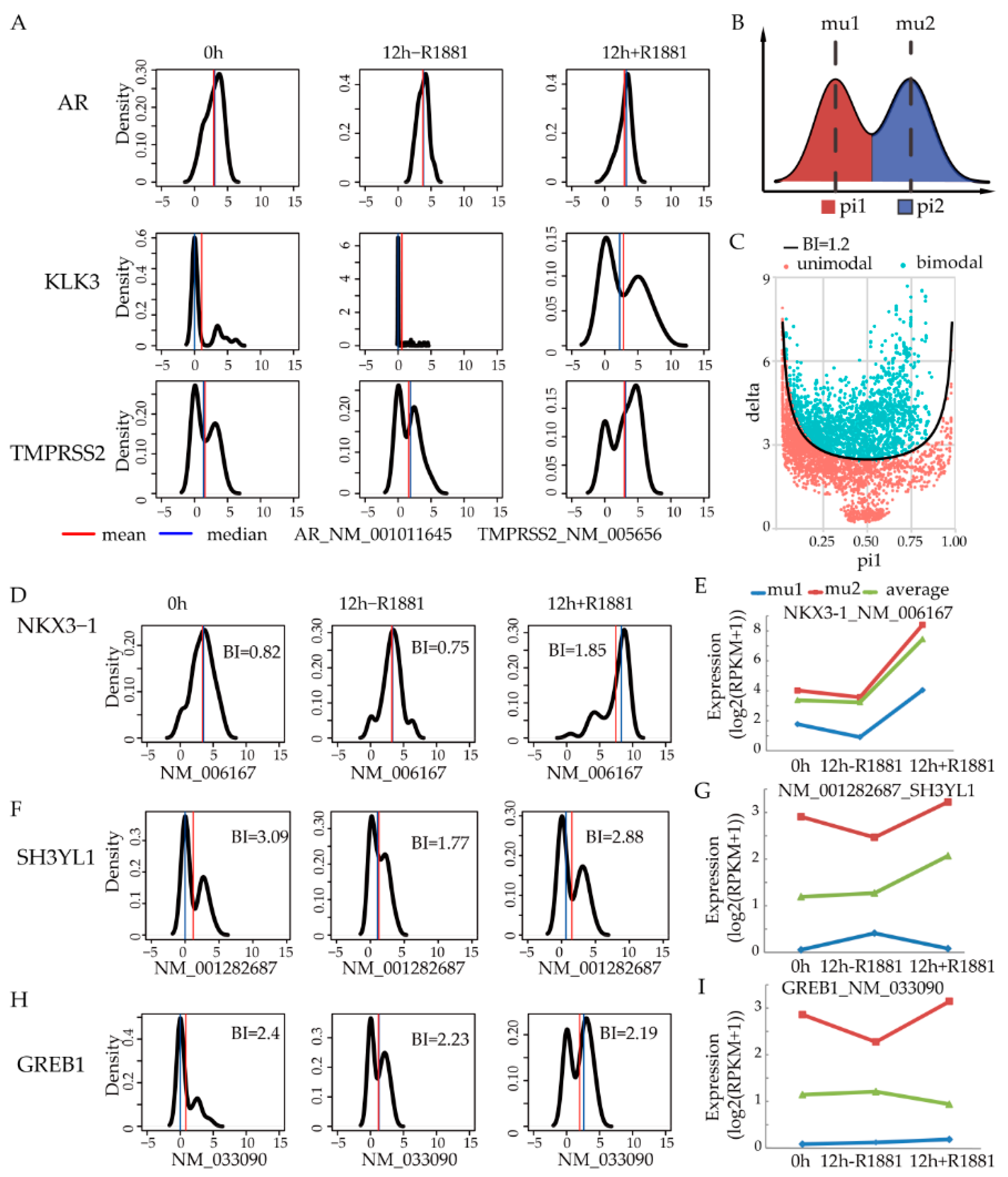
2.7. Gene Expression Bimodality Analysis
3. Results
3.1. Bimodal Gene Expression Among Single Cells Reveals Averaging Pitfalls
3.2. Twenty-Nine Gene Modules Are Identified Using WGCNA
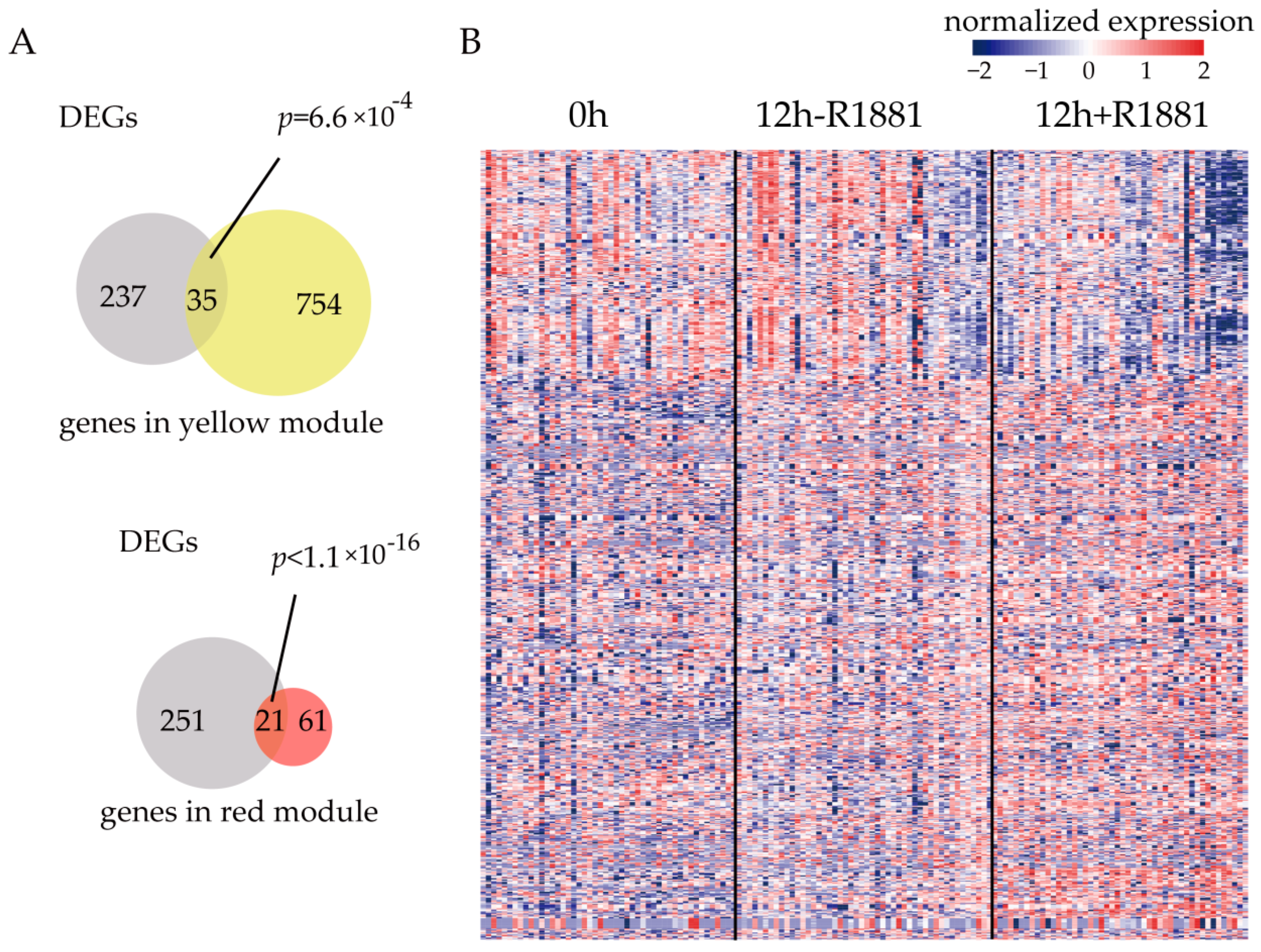
3.3. Modules Significantly Regulated by Androgen Receptor
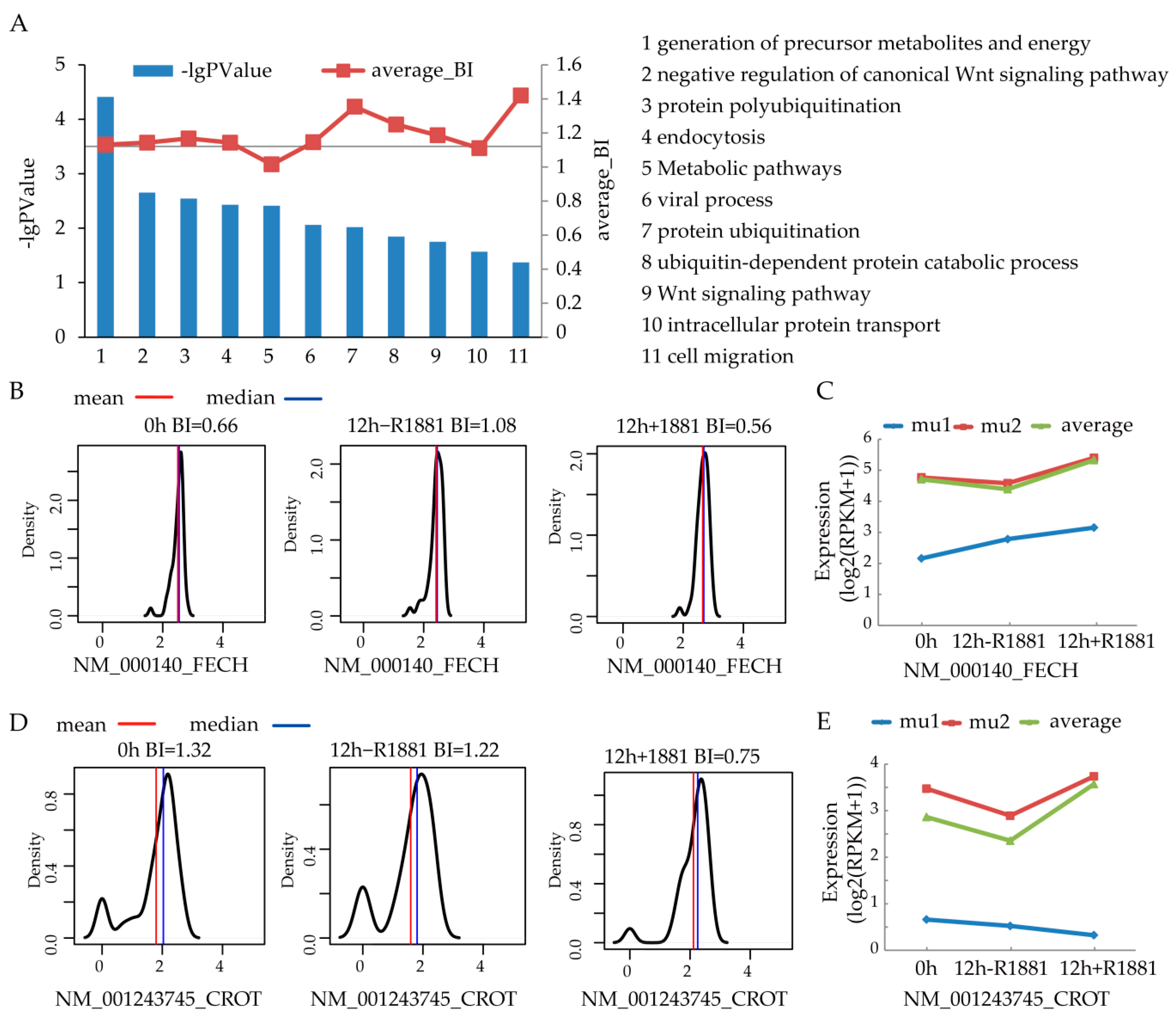
3.4. Biological Functions of Modules Significantly Regulated by Androgen Receptor
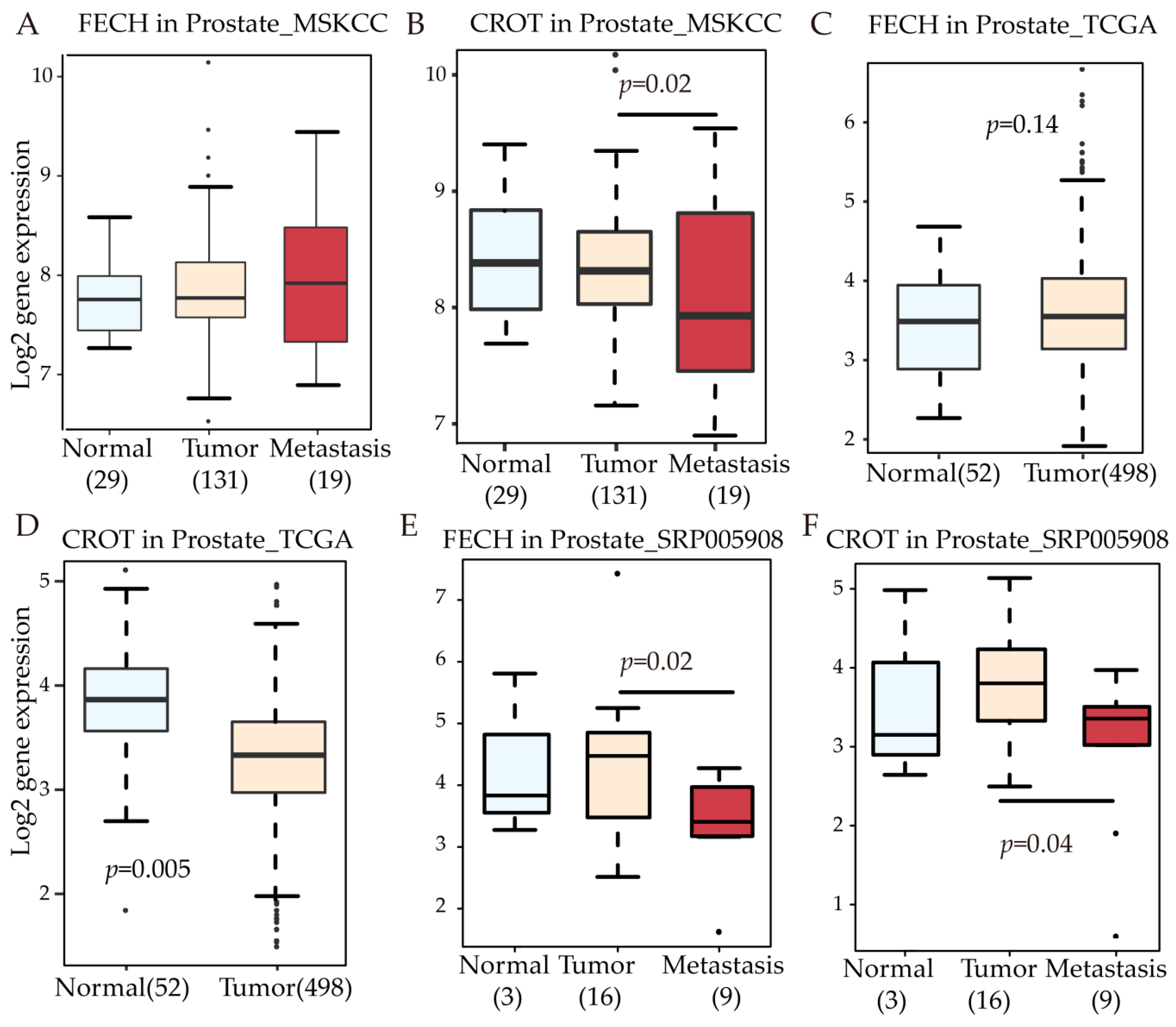
3.5. FECH and CROT Expression in Patients
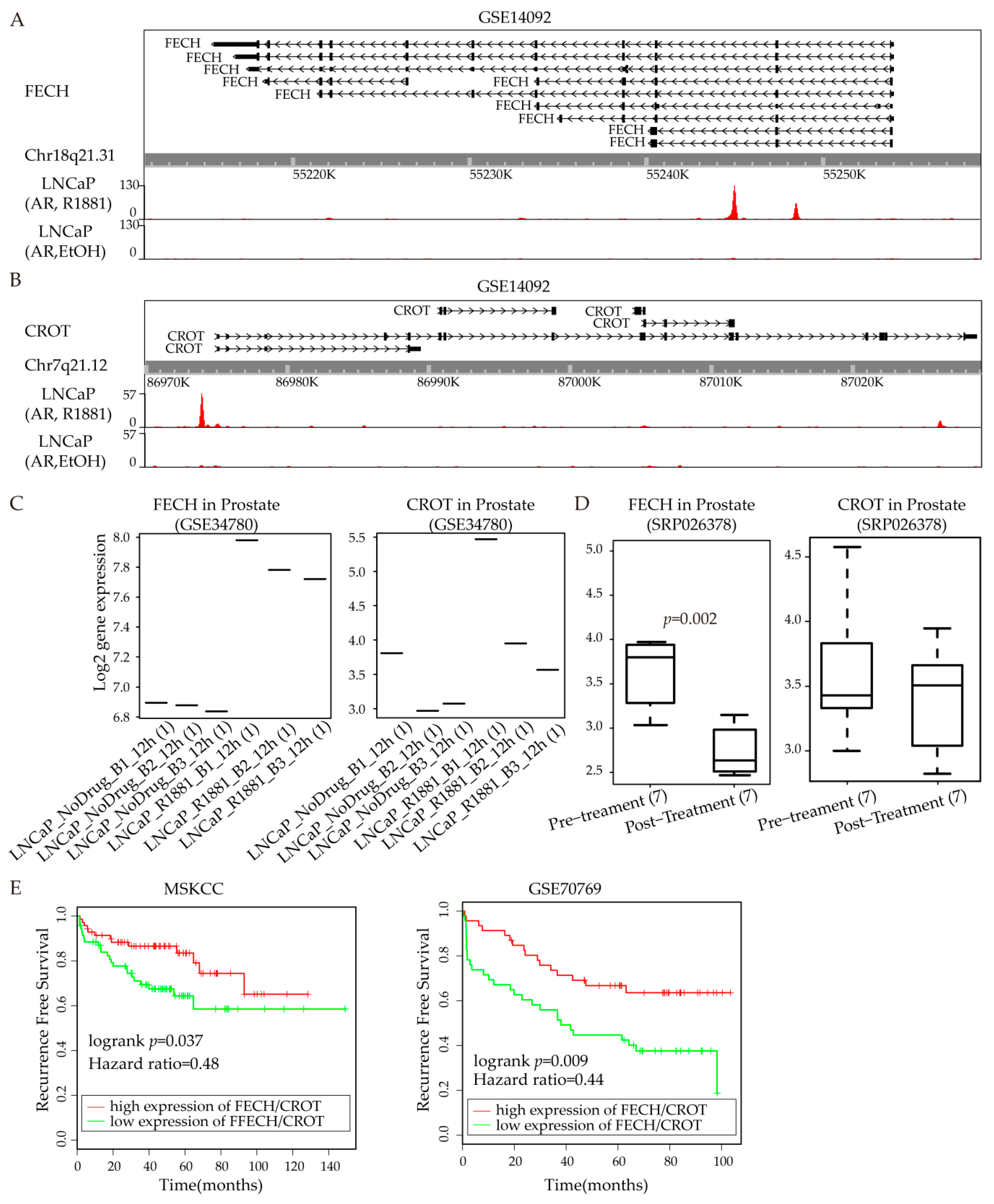
3.6. FECH/CROT Signature Is Potent Prognostic Marker Regulated by AR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Garzon, V.; Kypta, R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017, 14, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Takeda, D.Y.; Spisak, S.; Seo, J.H.; Bell, C.; O’Connor, E.; Korthauer, K.; Ribli, D.; Csabai, I.; Solymosi, N.; Szallasi, Z.; et al. A Somatically Acquired Enhancer of the Androgen Receptor Is a Noncoding Driver in Advanced Prostate Cancer. Cell 2018, 174, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Poirion, O.B.; Zhu, X.; Ching, T.; Garmire, L. Single-Cell Transcriptomics Bioinformatics and Computational Challenges. Front. Genet. 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Karaayvaz, M.; Cristea, S.; Gillespie, S.M.; Patel, A.P.; Mylvaganam, R.; Luo, C.C.; Specht, M.C.; Bernstein, B.E.; Michor, F.; Ellisen, L.W. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun. 2018, 9, 3588. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.N.; Phipson, B.; Lawlor, K.T.; Dorison, A.; Patrick, R.; Zappia, L.; Harvey, R.P.; Oshlack, A.; Little, M.H. Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk. Development 2019, 146, dev178673. [Google Scholar] [CrossRef]
- Qin, Y.; Sukumaran, S.K.; Jyotaki, M.; Redding, K.; Jiang, P.; Margolskee, R.F. Gli3 is a negative regulator of Tas1r3-expressing taste cells. PLoS Genet. 2018, 14, e1007058. [Google Scholar] [CrossRef]
- Tanaka, N.; Katayama, S.; Reddy, A.; Nishimura, K.; Niwa, N.; Hongo, H.; Ogihara, K.; Kosaka, T.; Mizuno, R.; Kikuchi, E.; et al. Single-cell RNA-seq analysis reveals the platinum resistance gene COX7B and the surrogate marker CD63. Cancer Med. 2018, 7, 6193–6204. [Google Scholar] [CrossRef]
- Sonawane, A.R.; Weiss, S.T.; Glass, K.; Sharma, A. Network Medicine in the Age of Biomedical Big Data. Front. Genet. 2019, 10, 294. [Google Scholar] [CrossRef]
- Magani, F.; Bray, E.R.; Martinez, M.J.; Zhao, N.; Copello, V.A.; Heidman, L.; Peacock, S.O.; Wiley, D.J.; D’Urso, G.; Burnstein, K.L. Identification of an oncogenic network with prognostic and therapeutic value in prostate cancer. Mol. Syst. Biol. 2018, 14, e8202. [Google Scholar] [CrossRef]
- Sun, X.; Clermont, P.L.; Jiao, W.; Helgason, C.D.; Gout, P.W.; Wang, Y.; Qu, S. Elevated expression of the centromere protein-A(CENP-A)-encoding gene as a prognostic and predictive biomarker in human cancers. Int. J. Cancer 2016, 139, 899–907. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Coskun, V.; Liang, A.; Yu, J.; Cheng, L.; Ge, W.; Shi, Z.; Zhang, K.; Li, C.; Cui, Y.; et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell 2015, 161, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, S.; Yu, J.; Li, Y.; Zhang, X.Y.; Yang, L.; Zhang, H.; Hou, Q.; Jiang, M.; Brunicardi, F.C.; et al. Single-cell Transcriptome Analyses Reveal Molecular Signals to Intrinsic and Acquired Paclitaxel Resistance in Esophageal Squamous Cancer Cells. Cancer Lett. 2018, 420, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, K.; Zhou, L.; Gao, X.; Wang, J.; Yao, Y.; He, F.; Luo, Y.; Yu, Y.; Li, S.; et al. Coupled electrophysiological recording and single cell transcriptome analyses revealed molecular mechanisms underlying neuronal maturation. Protein Cell 2016, 7, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yandim, C.; Karakulah, G. Expression dynamics of repetitive DNA in early human embryonic development. BMC Genom. 2019, 20, 439. [Google Scholar] [CrossRef] [PubMed]
- Horning, A.M.; Wang, Y.; Lin, C.K.; Louie, A.D.; Jadhav, R.R.; Hung, C.N.; Wang, C.M.; Lin, C.L.; Kirma, N.B.; Liss, M.A.; et al. Single-Cell RNA-seq Reveals a Subpopulation of Prostate Cancer Cells with Enhanced Cell-Cycle-Related Transcription and Attenuated Androgen Response. Cancer Res. 2018, 78, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Miao, Z.; Divate, M.; Zhao, Z.; Cheung, E. KM-express: An integrated online patient survival and gene expression analysis tool for the identification and functional characterization of prognostic markers in breast and prostate cancers. Database 2018, 2018. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Z.Y.; Yang, C.; Xie, K.; Sun, W.J.; Xie, S.L. Sparse Gene Coexpression Network Analysis Reveals EIF3J-AS1 as a Prognostic Marker for Breast Cancer. Complexity 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Kharchenko, P.V.; Silberstein, L.; Scadden, D.T. Bayesian approach to single-cell differential expression analysis. Nat. Methods 2014, 11, 740–742. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Chen, Y.; Su, X.; Coombes, K.R. SIBER: Systematic identification of bimodally expressed genes using RNAseq data. Bioinformatics 2013, 29, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Blessing, A.M.; Ganesan, S.; Rajapakshe, K.; Ying Sung, Y.; Reddy Bollu, L.; Shi, Y.; Cheung, E.; Coarfa, C.; Chang, J.T.; McDonnell, D.P.; et al. Identification of a Novel Coregulator, SH3YL1, That Interacts With the Androgen Receptor N-Terminus. Mol. Endocrinol. 2015, 29, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ma, Y.; Chen, C.; Fu, X.; Yang, S.; Li, X.; Yu, G.; Mao, Y.; Xie, Y.; Li, Y. Androgen-responsive gene database: Integrated knowledge on androgen-responsive genes. Mol. Endocrinol. 2009, 23, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Tyleckova, J.; Hrabakova, R.; Mairychova, K.; Halada, P.; Radova, L.; Dzubak, P.; Hajduch, M.; Gadher, S.J.; Kovarova, H. Cancer cell response to anthracyclines effects: Mysteries of the hidden proteins associated with these drugs. Int. J. Mol. Sci. 2012, 13, 15536–15564. [Google Scholar] [CrossRef] [PubMed]
- Manikova, D.; Vlasakova, D.; Letavayova, L.; Klobucnikova, V.; Griac, P.; Chovanec, M. Selenium toxicity toward yeast as assessed by microarray analysis and deletion mutant library screen: A role for DNA repair. Chem. Res. Toxicol. 2012, 25, 1598–1608. [Google Scholar] [CrossRef]
- Yoshioka, E.; Chelakkot, V.S.; Licursi, M.; Rutihinda, S.G.; Som, J.; Derwish, L.; King, J.J.; Pongnopparat, T.; Mearow, K.; Larijani, M.; et al. Enhancement of Cancer-Specific Protoporphyrin IX Fluorescence by Targeting Oncogenic Ras/MEK Pathway. Theranostics 2018, 8, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Itkonen, H.M.; Engedal, N.; Babaie, E.; Luhr, M.; Guldvik, I.J.; Minner, S.; Hohloch, J.; Tsourlakis, M.C.; Schlomm, T.; Mills, I.G. UAP1 is overexpressed in prostate cancer and is protective against inhibitors of N-linked glycosylation. Oncogene 2015, 34, 3744–3750. [Google Scholar] [CrossRef]
- Munkley, J.; Vodak, D.; Livermore, K.E.; James, K.; Wilson, B.T.; Knight, B.; McCullagh, P.; McGrath, J.; Crundwell, M.; Harries, L.W.; et al. Glycosylation is an Androgen-Regulated Process Essential for Prostate Cancer Cell Viability. EBioMedicine 2016, 8, 103–116. [Google Scholar] [CrossRef]
- Munkley, J. Glycosylation is a global target for androgen control in prostate cancer cells. Endocr. Relat. Cancer 2017, 24, R49–R64. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, H.; Gao, A.C.; Marshall, J.R.; Ip, C. Androgen receptor signaling intensity is a key factor in determining the sensitivity of prostate cancer cells to selenium inhibition of growth and cancer-specific biomarkers. Mol. Cancer Ther. 2005, 4, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Hernando, C.; Suarez, Y.; Rayner, K.J.; Moore, K.J. MicroRNAs in lipid metabolism. Curr. Opin. Lipidol. 2011, 22, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.N.; Ehlers, N.S.; Zhu, S.; Thomsen, M.B.; Nielsen, R.L.; Liu, D.; Wang, G.; Hou, Y.; Zhang, X.; Xu, X.; et al. The stepwise evolution of the exome during acquisition of docetaxel resistance in breast cancer cells. BMC Genom. 2016, 17, 442. [Google Scholar] [CrossRef] [PubMed]
- Hage-Sleiman, R.; Bahmad, H.; Kobeissy, H.; Dakdouk, Z.; Kobeissy, F.; Dbaibo, G. Genomic alterations during p53-dependent apoptosis induced by gamma-irradiation of Molt-4 leukemia cells. PLoS ONE 2017, 12, e0190221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Juric, D.; Francisco, B.; Yu, R.X.; Duran, G.E.; Chen, K.G.; Chen, X.; Sikic, B.I. Regional activation of chromosomal arm 7q with and without gene amplification in taxane-selected human ovarian cancer cell lines. Genes Chromosom. Cancer 2006, 45, 365–374. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hu, L.; Wang, Y.; Sun, W.; Yang, C. Single Cell Gene Co-Expression Network Reveals FECH/CROT Signature as a Prognostic Marker. Cells 2019, 8, 698. https://doi.org/10.3390/cells8070698
Chen X, Hu L, Wang Y, Sun W, Yang C. Single Cell Gene Co-Expression Network Reveals FECH/CROT Signature as a Prognostic Marker. Cells. 2019; 8(7):698. https://doi.org/10.3390/cells8070698
Chicago/Turabian StyleChen, Xin, Lingling Hu, Yuan Wang, Weijun Sun, and Chao Yang. 2019. "Single Cell Gene Co-Expression Network Reveals FECH/CROT Signature as a Prognostic Marker" Cells 8, no. 7: 698. https://doi.org/10.3390/cells8070698
APA StyleChen, X., Hu, L., Wang, Y., Sun, W., & Yang, C. (2019). Single Cell Gene Co-Expression Network Reveals FECH/CROT Signature as a Prognostic Marker. Cells, 8(7), 698. https://doi.org/10.3390/cells8070698




