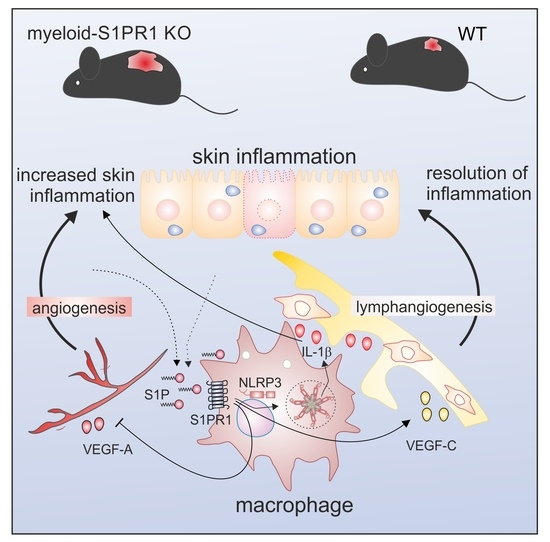
Macrophage S1PR1 Signaling Alters Angiogenesis and Lymphangiogenesis During Skin Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Reagents
2.2. Analysis of Publicly Available Datasets of Gene Expression in Psoriasis Patients
2.3. Psoriasiform Dermatitis Model
2.4. RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
2.5. Flow Cytometry
2.6. Multiplex Immunohistochemistry
2.7. Cytometric Bead Array
2.8. Macrophage Culturing and Stimulation
2.9. Genotyping PCR
2.10. Statistical Analysis
3. Results
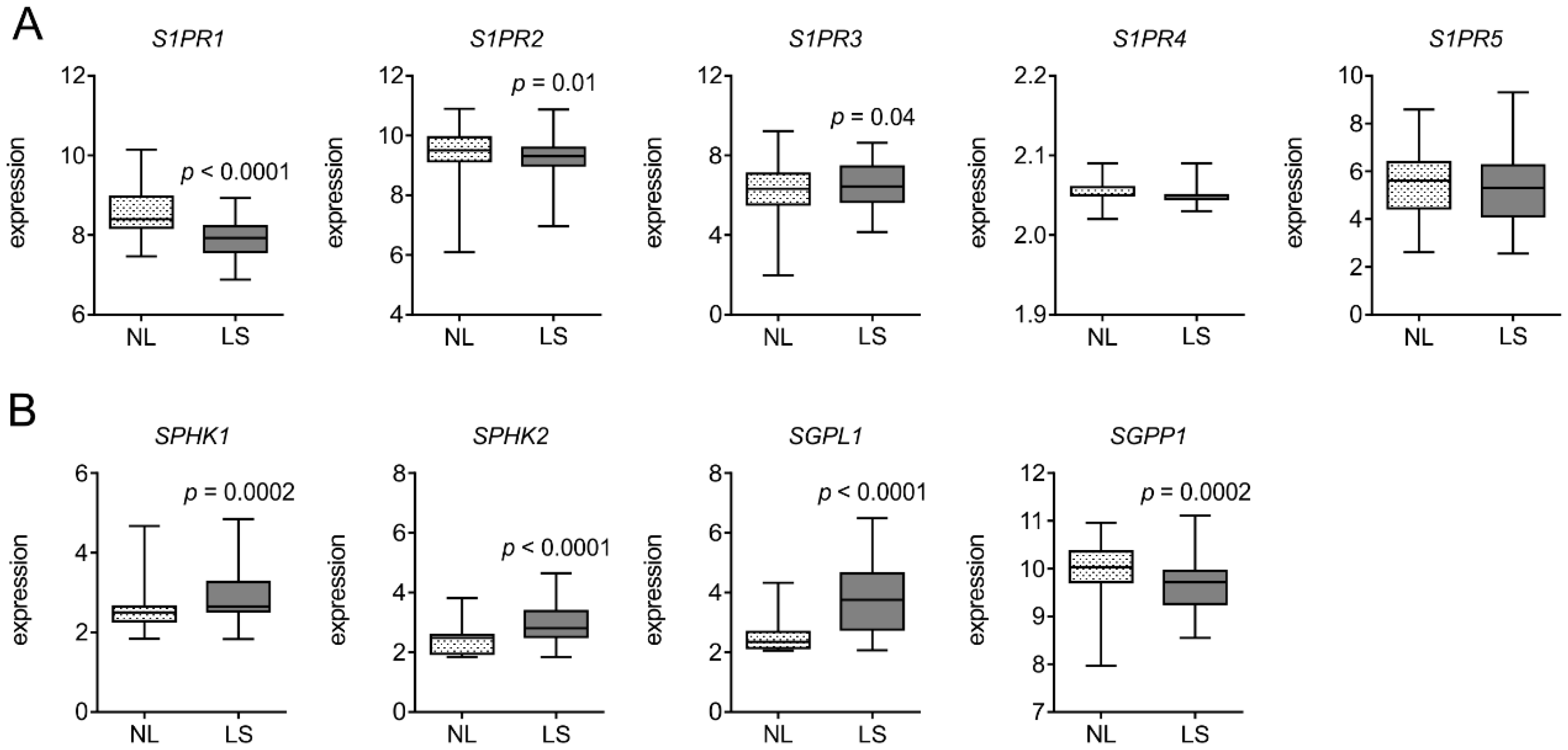
3.1. S1PR1 is Downregulated in Human Psoriatic Patients
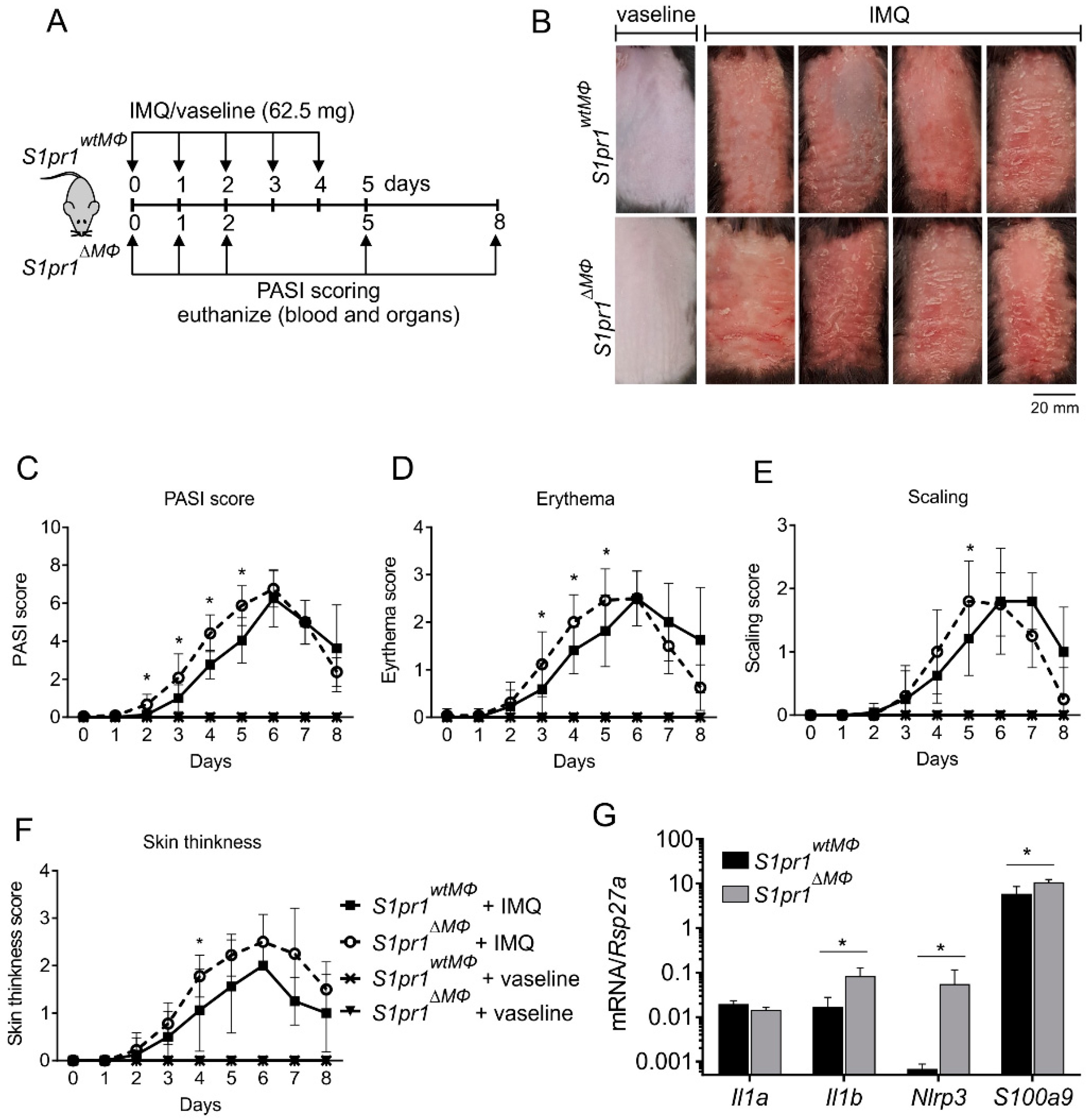
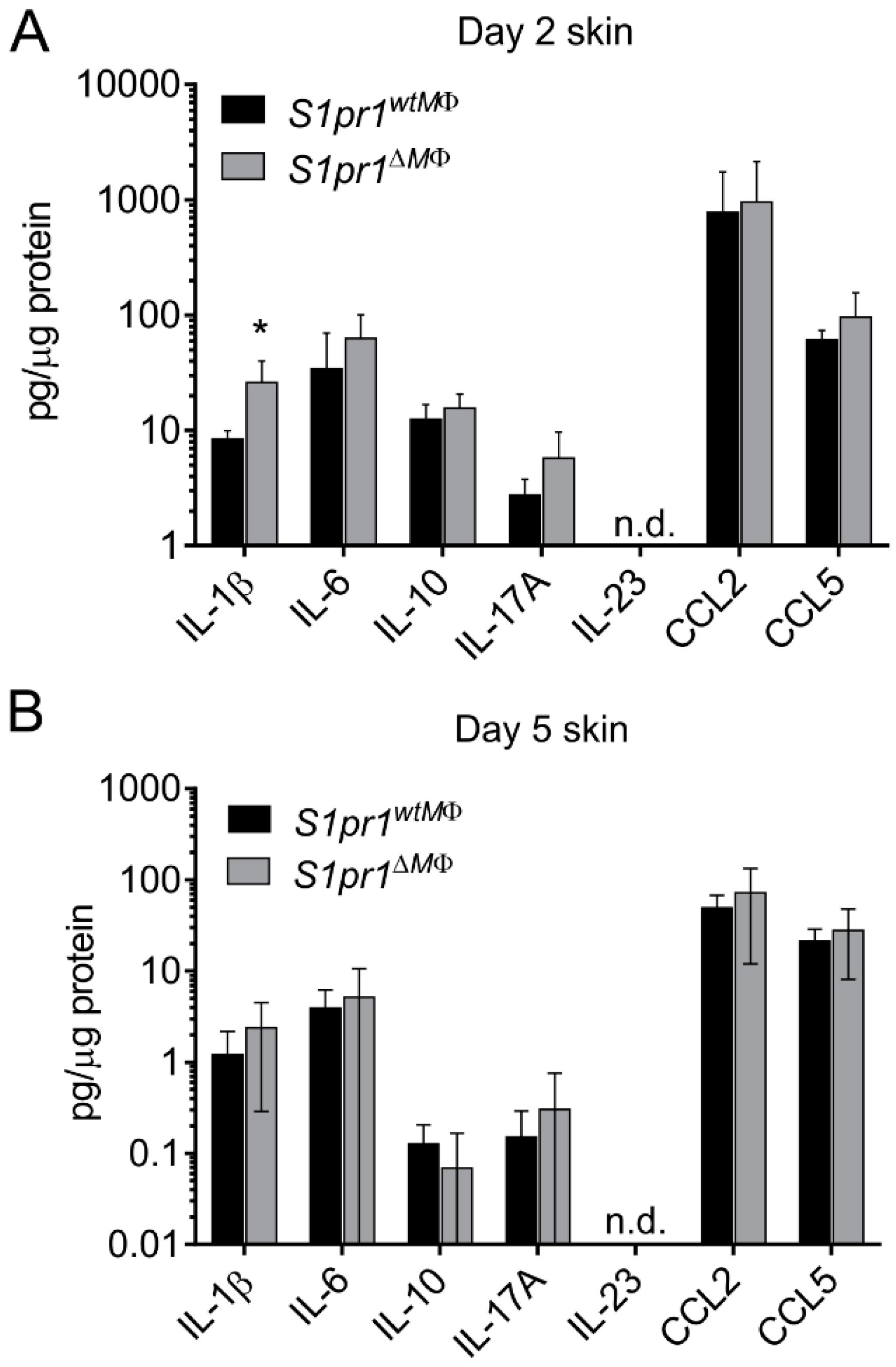
3.2. Myeloid-Specific S1PR1 Deletion Enhanced Early Inflammation in IMQ-Induced Psoriasis
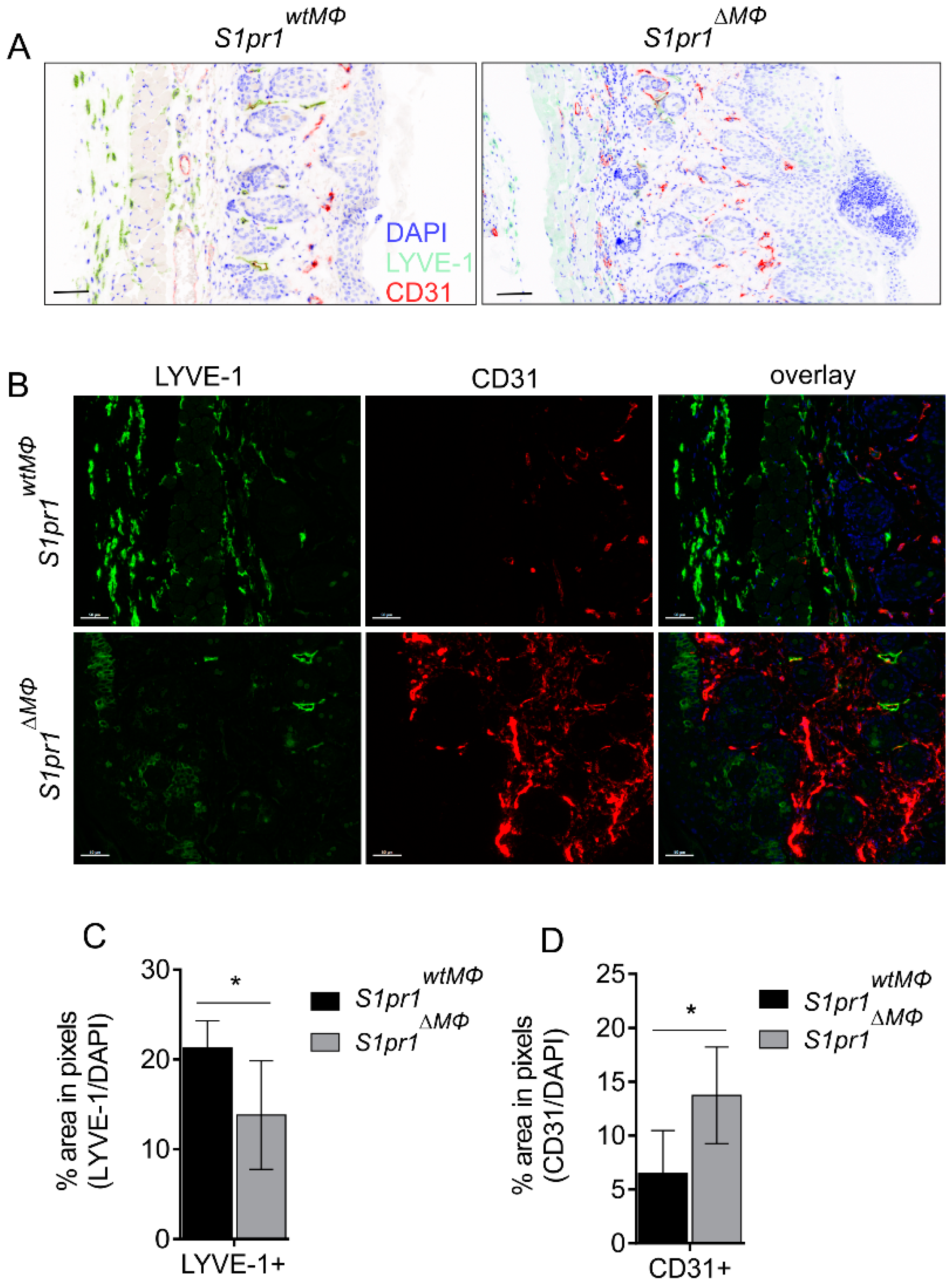
3.3. S1pr1∆MΦ Mice Show Reduced Lymph and Increased Blood Vessels
3.4. Human Psoriatic Clinical Data Corroborated a Reciprocal Regulation of Lymph vs. Blood Vessel Markers
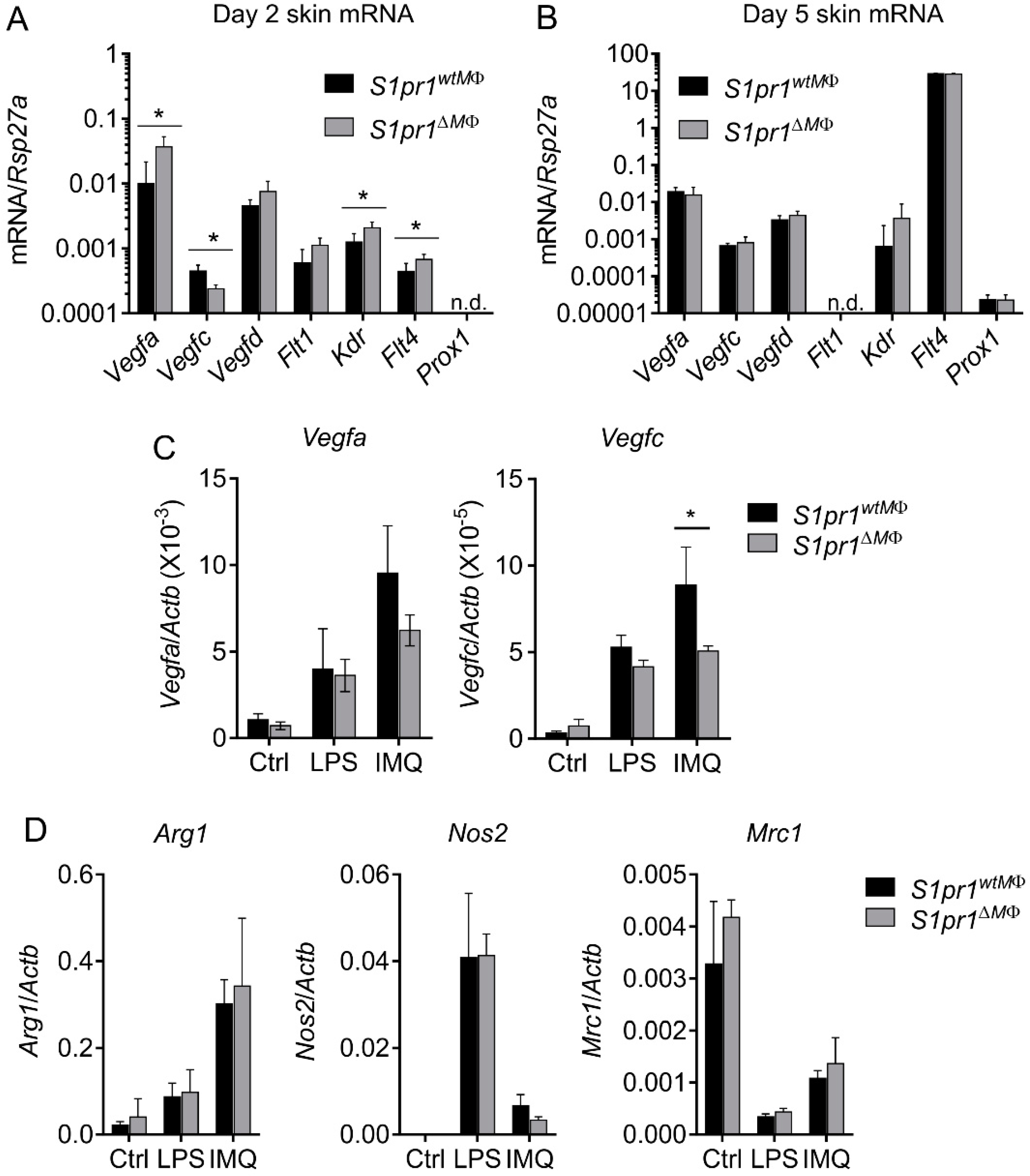
3.5. Reciprocal Angiogenesis and Lymphangiogenesis in S1pr1∆MΦ Is Reflected by Altered VEGFs and Their Receptors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griffiths, C.E.M.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Boyman, O.; Hefti, H.P.; Conrad, C.; Nickoloff, B.J.; Suter, M.; Nestle, F.O. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med. 2004, 199, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Stratis, A.; Pasparakis, M.; Rupec, R.A.; Markur, D.; Hartmann, K.; Scharffetter-Kochanek, K.; Peters, T.; van Rooijen, N.; Krieg, T.; Haase, I. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. J. Clin. Investig. 2006, 116, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peters, T.; Kess, D.; Sindrilaru, A.; Oreshkova, T.; van Rooijen, N.; Stratis, A.; Renkl, A.C.; Sunderkötter, C.; Wlaschek, M.; et al. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J. Clin. Investig. 2006, 116, 2105–2114. [Google Scholar] [CrossRef]
- Leite Dantas, R.; Masemann, D.; Schied, T.; Bergmeier, V.; Vogl, T.; Loser, K.; Brachvogel, B.; Varga, G.; Ludwig, S.; Wixler, V. Macrophage-mediated psoriasis can be suppressed by regulatory T lymphocytes. J. Pathol. 2016, 240, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Brüne, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; von Knethen, A.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef] [PubMed]
- Weigert, A.; Johann, A.M.; von Knethen, A.; Schmidt, H.; Geisslinger, G.; Brüne, B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood 2006, 108, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.N.; Jung, M.; Weigert, A.; Brüne, B. S1P Provokes Tumor Lymphangiogenesis via Macrophage-Derived Mediators Such as IL-1β or Lipocalin-2. Mediators Inflamm. 2017, 2017, 7510496. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Stevens, R.C.; Hanson, M.; Roberts, E.; Oldstone, M.B.A. Sphingosine-1-phosphate and its receptors: Structure, signaling, and influence. Annu. Rev. Biochem. 2013, 82, 637–662. [Google Scholar] [CrossRef]
- Weigert, A.; Weis, N.; Brüne, B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology 2009, 214, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Weichand, B.; Popp, R.; Dziumbla, S.; Mora, J.; Strack, E.; Elwakeel, E.; Frank, A.-C.; Scholich, K.; Pierre, S.; Syed, S.N.; et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J. Exp. Med. 2017, 214, 2695–2713. [Google Scholar] [CrossRef] [PubMed]
- Vaclavkova, A.; Chimenti, S.; Arenberger, P.; Holló, P.; Sator, P.-G.; Burcklen, M.; Stefani, M.; D’Ambrosio, D. Oral ponesimod in patients with chronic plaque psoriasis: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2014, 384, 2036–2045. [Google Scholar] [CrossRef]
- Schaper, K.; Dickhaut, J.; Japtok, L.; Kietzmann, M.; Mischke, R.; Kleuser, B.; Bäumer, W. Sphingosine-1-phosphate exhibits anti-proliferative and anti-inflammatory effects in mouse models of psoriasis. J. Dermatol. Sci. 2013, 71, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Hogmalm, A.; Bry, M.; Alitalo, K.; Bry, K.; McDonald, D.M. Transgenic Overexpression of Interleukin-1β Induces Persistent Lymphangiogenesis But Not Angiogenesis in Mouse Airways. Am. J. Pathol. 2013, 182, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.O.; Kovacicova, L.; Huggenberger, R.; Navarini, A.A.; Gitzelmann, G.; Amann-Vesti, B.R. Increased permeability of cutaneous lymphatic capillaries and enhanced blood flow in psoriatic plaques. Dermatology (Basel) 2013, 227, 118–125. [Google Scholar] [CrossRef]
- Allende, M.L.; Yamashita, T.; Proia, R.L. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 2003, 102, 3665–3667. [Google Scholar] [CrossRef]
- Weigert, A.; Weichand, B.; Sekar, D.; Sha, W.; Hahn, C.; Mora, J.; Ley, S.; Essler, S.; Dehne, N.; Brüne, B. HIF-1α is a negative regulator of plasmacytoid DC development in vitro and in vivo. Blood 2012, 120, 3001–3006. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Han, Y.; Mora, J.; Huard, A.; da Silva, P.; Wiechmann, S.; Putyrski, M.; Schuster, C.; Elwakeel, E.; Lang, G.; Scholz, A.; et al. IL-38 Ameliorates Skin Inflammation and Limits IL-17 Production from γδ T Cells. Cell Rep. 2019, 27, 835–846.e5. [Google Scholar] [CrossRef]
- Clausen, B.E.; Burkhardt, C.; Reith, W.; Renkawitz, R.; Förster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999, 8, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Li, K.; Fuentes-Duculan, J.; Hayden, K.; Brodmerkel, C.; Krueger, J.G. Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J. Investig. Dermatol. 2012, 132, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.P.; Duffin, K.C.; Helms, C.; Ding, J.; Stuart, P.E.; Goldgar, D.; Gudjonsson, J.E.; Li, Y.; Tejasvi, T.; Feng, B.-J.; et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet. 2009, 41, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Qian, A.S.; Tahir, U.; Yu, P.; Trigatti, B.L. Sphingosine-1-Phosphate Receptor 1, Expressed in Myeloid Cells, Slows Diet-Induced Atherosclerosis and Protects against Macrophage Apoptosis in Ldlr KO Mice. Int. J. Mol. Sci. 2017, 18, 2721. [Google Scholar] [CrossRef] [PubMed]
- Olesch, C.; Ringel, C.; Brüne, B.; Weigert, A. Beyond Immune Cell Migration: The Emerging Role of the Sphingosine-1-phosphate Receptor S1PR4 as a Modulator of Innate Immune Cell Activation. Mediators Inflamm. 2017, 2017, 6059203. [Google Scholar] [CrossRef] [PubMed]
- Rabeony, H.; Pohin, M.; Vasseur, P.; Petit-Paris, I.; Jégou, J.-F.; Favot, L.; Frouin, E.; Boutet, M.-A.; Blanchard, F.; Togbe, D.; et al. IMQ-induced skin inflammation in mice is dependent on IL-1R1 and MyD88 signaling but independent of the NLRP3 inflammasome. Eur. J. Immunol. 2015, 45, 2847–2857. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Johnston, A.; Carbajal, S.; Han, G.; Wohn, C.; Lu, J.; Xing, X.; Nair, R.P.; Voorhees, J.J.; Elder, J.T.; et al. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS ONE 2011, 6, e18266. [Google Scholar] [CrossRef]
- Frank, A.-C.; Ebersberger, S.; Fink, A.F.; Lampe, S.; Weigert, A.; Schmid, T.; Ebersberger, I.; Syed, S.N.; Brüne, B. Apoptotic tumor cell-derived microRNA-375 uses CD36 to alter the tumor-associated macrophage phenotype. Nat. Commun. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Moussai, D.; Mitsui, H.; Pettersen, J.S.; Pierson, K.C.; Shah, K.R.; Suárez-Fariñas, M.; Cardinale, I.R.; Bluth, M.J.; Krueger, J.G.; Carucci, J.A. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J. Investig. Dermatol. 2011, 131, 229–236. [Google Scholar] [CrossRef]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D.; Basham, B.; Smith, K.; Chen, T.; Morel, F.; et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007, 8, 950–957. [Google Scholar] [CrossRef]
- Venneri, M.A.; De Palma, M.; Ponzoni, M.; Pucci, F.; Scielzo, C.; Zonari, E.; Mazzieri, R.; Doglioni, C.; Naldini, L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 2007, 109, 5276–5285. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA. 2003, 100, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Lai, C.-Y.; Yeh, D.-W.; Liu, Y.-L.; Su, Y.-W.; Hsu, L.-C.; Chang, C.-H.; Catherine Jin, S.-L.; Chuang, T.-H. Involvement of M1 Macrophage Polarization in Endosomal Toll-Like Receptors Activated Psoriatic Inflammation. Mediators Inflamm. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Xue, N.; Lai, F.; Zhang, X.; Zhang, S.; Wang, Y.; Jin, J.; Chen, X. Validating a Selective S1P1 Receptor Modulator Syl930 for Psoriasis Treatment. Biol. Pharm. Bull. 2018, 41, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Cho, K.-A.; Hahn, S.; Lee, Y.; Kim, Y.-H.; Woo, S.-Y.; Ryu, K.-H.; Park, W.-J.; Park, J.-W. Inhibiting Sphingosine Kinase 2 Derived-sphingosine-1-phosphate Ameliorates Psoriasis-like Skin Disease via Blocking Th17 Differentiation of Naïve CD4 T Lymphocytes in Mice. Acta Derm. Venereol. 2019, 99, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, R.; Ullmann, S.; Proulx, S.T.; Pytowski, B.; Alitalo, K.; Detmar, M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J. Exp. Med. 2010, 207, 2255–2269. [Google Scholar] [CrossRef] [PubMed]
- Young, H.S.; Summers, A.M.; Bhushan, M.; Brenchley, P.E.C.; Griffiths, C.E.M. Single-nucleotide polymorphisms of vascular endothelial growth factor in psoriasis of early onset. J. Investig. Dermatol. 2004, 122, 209–215. [Google Scholar] [CrossRef]
- Creamer, D.; Allen, M.H.; Groves, R.W.; Barker, J.N. Circulating vascular permeability factor/vascular endothelial growth factor in erythroderma. Lancet 1996, 348, 1101. [Google Scholar] [CrossRef]
- Detmar, M.; Brown, L.F.; Claffey, K.P.; Yeo, K.T.; Kocher, O.; Jackman, R.W.; Berse, B.; Dvorak, H.F. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J. Exp. Med. 1994, 180, 1141–1146. [Google Scholar] [CrossRef]
- Trembath, R.C.; Clough, R.L.; Rosbotham, J.L.; Jones, A.B.; Camp, R.D.; Frodsham, A.; Browne, J.; Barber, R.; Terwilliger, J.; Lathrop, G.M.; et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum. Mol. Genet. 1997, 6, 813–820. [Google Scholar] [CrossRef]
- Kim, H.; Kataru, R.P.; Koh, G.Y. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol. 2012, 33, 350–356. [Google Scholar] [CrossRef]
- Yoon, C.M.; Hong, B.S.; Moon, H.G.; Lim, S.; Suh, P.-G.; Kim, Y.-K.; Chae, C.-B.; Gho, Y.S. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood 2008, 112, 1129–1138. [Google Scholar] [CrossRef]
- Nagahashi, M.; Ramachandran, S.; Kim, E.Y.; Allegood, J.C.; Rashid, O.M.; Yamada, A.; Zhao, R.; Milstien, S.; Zhou, H.; Spiegel, S.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012, 72, 726–735. [Google Scholar] [CrossRef]
- Kataru, R.P.; Kim, H.; Jang, C.; Choi, D.K.; Koh, B.I.; Kim, M.; Gollamudi, S.; Kim, Y.-K.; Lee, S.-H.; Koh, G.Y. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 2011, 34, 96–107. [Google Scholar] [CrossRef]
- Jang, C.; Koh, Y.J.; Lim, N.K.; Kang, H.J.; Kim, D.H.; Park, S.K.; Lee, G.M.; Jeon, C.J.; Koh, G.Y. Angiopoietin-2 exocytosis is stimulated by sphingosine-1-phosphate in human blood and lymphatic endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 401–407. [Google Scholar] [CrossRef]
- Gale, N.W.; Thurston, G.; Hackett, S.F.; Renard, R.; Wang, Q.; McClain, J.; Martin, C.; Witte, C.; Witte, M.H.; Jackson, D.; et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 2002, 3, 411–423. [Google Scholar] [CrossRef]
- Dellinger, M.; Hunter, R.; Bernas, M.; Gale, N.; Yancopoulos, G.; Erickson, R.; Witte, M. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev. Biol. 2008, 319, 309–320. [Google Scholar] [CrossRef]
- Pham, T.H.M.; Baluk, P.; Xu, Y.; Grigorova, I.; Bankovich, A.J.; Pappu, R.; Coughlin, S.R.; McDonald, D.M.; Schwab, S.R.; Cyster, J.G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010, 207, 17–27. [Google Scholar] [CrossRef]
- Kerjaschki, D. The crucial role of macrophages in lymphangiogenesis. J. Clin. Investig. 2005, 115, 2316–2319. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Birner, P.; Stöckl, J.; Kalt, R.; Ullrich, R.; Caucig, C.; Kriehuber, E.; Nagy, K.; Alitalo, K.; Kerjaschki, D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002, 161, 947–956. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Huttary, N.; Raab, I.; Regele, H.; Bojarski-Nagy, K.; Bartel, G.; Kröber, S.M.; Greinix, H.; Rosenmaier, A.; Karlhofer, F.; et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 2006, 12, 230–234. [Google Scholar] [CrossRef]
- Angeli, V.; Ginhoux, F.; Llodrà, J.; Quemeneur, L.; Frenette, P.S.; Skobe, M.; Jessberger, R.; Merad, M.; Randolph, G.J. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 2006, 24, 203–215. [Google Scholar] [CrossRef]
- Nestle, F.O.; Turka, L.A.; Nickoloff, B.J. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J. Clin. Investig. 1994, 94, 202–209. [Google Scholar] [CrossRef]
- Abram, C.L.; Roberge, G.L.; Hu, Y.; Lowell, C.A. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 2014, 408, 89–100. [Google Scholar] [CrossRef]
- Sumida, H.; Yanagida, K.; Kita, Y.; Abe, J.; Matsushima, K.; Nakamura, M.; Ishii, S.; Sato, S.; Shimizu, T. Interplay between CXCR2 and BLT1 facilitates neutrophil infiltration and resultant keratinocyte activation in a murine model of imiquimod-induced psoriasis. J. Immunol. 2014, 192, 4361–4369. [Google Scholar] [CrossRef]
- Schön, M.; Denzer, D.; Kubitza, R.C.; Ruzicka, T.; Schön, M.P. Critical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin mice. J. Investig. Dermatol. 2000, 114, 976–983. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Liu, Y.; Wada, R.; Yamashita, T.; Mi, Y.; Deng, C.X.; Hobson, J.P.; Rosenfeldt, H.M.; Nava, V.E.; Chae, S.S.; Lee, M.J.; et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 2000, 106, 951–961. [Google Scholar] [CrossRef]
- LaMontagne, K.; Littlewood-Evans, A.; Schnell, C.; O’Reilly, T.; Wyder, L.; Sanchez, T.; Probst, B.; Butler, J.; Wood, A.; Liau, G.; et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006, 66, 221–231. [Google Scholar] [CrossRef]
- Visentin, B.; Vekich, J.A.; Sibbald, B.J.; Cavalli, A.L.; Moreno, K.M.; Matteo, R.G.; Garland, W.A.; Lu, Y.; Yu, S.; Hall, H.S.; et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 2006, 9, 225–238. [Google Scholar] [CrossRef]
- Sweat, R.S.; Stapor, P.C.; Murfee, W.L. Relationships between lymphangiogenesis and angiogenesis during inflammation in rat mesentery microvascular networks. Lymphat. Res. Biol. 2012, 10, 198–207. [Google Scholar] [CrossRef]
- Fuentes-Duculan, J.; Suárez-Fariñas, M.; Zaba, L.C.; Nograles, K.E.; Pierson, K.C.; Mitsui, H.; Pensabene, C.A.; Kzhyshkowska, J.; Krueger, J.G.; Lowes, M.A. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J. Investig. Dermatol. 2010, 130, 2412–2422. [Google Scholar] [CrossRef]
- Alvarez, S.E.; Harikumar, K.B.; Hait, N.C.; Allegood, J.; Strub, G.M.; Kim, E.Y.; Maceyka, M.; Jiang, H.; Luo, C.; Kordula, T.; et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 2010, 465, 1084–1088. [Google Scholar] [CrossRef]
- Lichte, K.; Rossi, R.; Danneberg, K.; ter Braak, M.; Kürschner, U.; Jakobs, K.H.; Kleuser, B.; Meyer zu Heringdorf, D. Lysophospholipid receptor-mediated calcium signaling in human keratinocytes. J. Investig. Dermatol. 2008, 128, 1487–1498. [Google Scholar] [CrossRef]
- Manggau, M.; Kim, D.S.; Ruwisch, L.; Vogler, R.; Korting, H.C.; Schäfer-Korting, M.; Kleuser, B. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J. Investig. Dermatol. 2001, 117, 1241–1249. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, S.N.; Raue, R.; Weigert, A.; von Knethen, A.; Brüne, B. Macrophage S1PR1 Signaling Alters Angiogenesis and Lymphangiogenesis During Skin Inflammation. Cells 2019, 8, 785. https://doi.org/10.3390/cells8080785
Syed SN, Raue R, Weigert A, von Knethen A, Brüne B. Macrophage S1PR1 Signaling Alters Angiogenesis and Lymphangiogenesis During Skin Inflammation. Cells. 2019; 8(8):785. https://doi.org/10.3390/cells8080785
Chicago/Turabian StyleSyed, Shahzad Nawaz, Rebecca Raue, Andreas Weigert, Andreas von Knethen, and Bernhard Brüne. 2019. "Macrophage S1PR1 Signaling Alters Angiogenesis and Lymphangiogenesis During Skin Inflammation" Cells 8, no. 8: 785. https://doi.org/10.3390/cells8080785
APA StyleSyed, S. N., Raue, R., Weigert, A., von Knethen, A., & Brüne, B. (2019). Macrophage S1PR1 Signaling Alters Angiogenesis and Lymphangiogenesis During Skin Inflammation. Cells, 8(8), 785. https://doi.org/10.3390/cells8080785