The Abnormal CD4+T Lymphocyte Subset Distribution and Vbeta Repertoire in New-onset Rheumatoid Arthritis Can Be Modulated by Methotrexate Treament
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Protocol
2.5. Isolation and Analysis of Peripheral Blood Mononuclear Cells
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics at Baseline
3.2. Recently Diagnosed, Dmard-Naïve Ar Patients Showed Increased Numbers of Tn, Tem and Cd4+ Te and Cd4+Cd28− T Lymphocytes,
3.3. MTX Responder and Non-Responder Recently Diagnosed Ra Patients Showed Different CD4+ T Lymphocyte Distributions at Basal Conditions and after 3 and 6 Months of Treatment
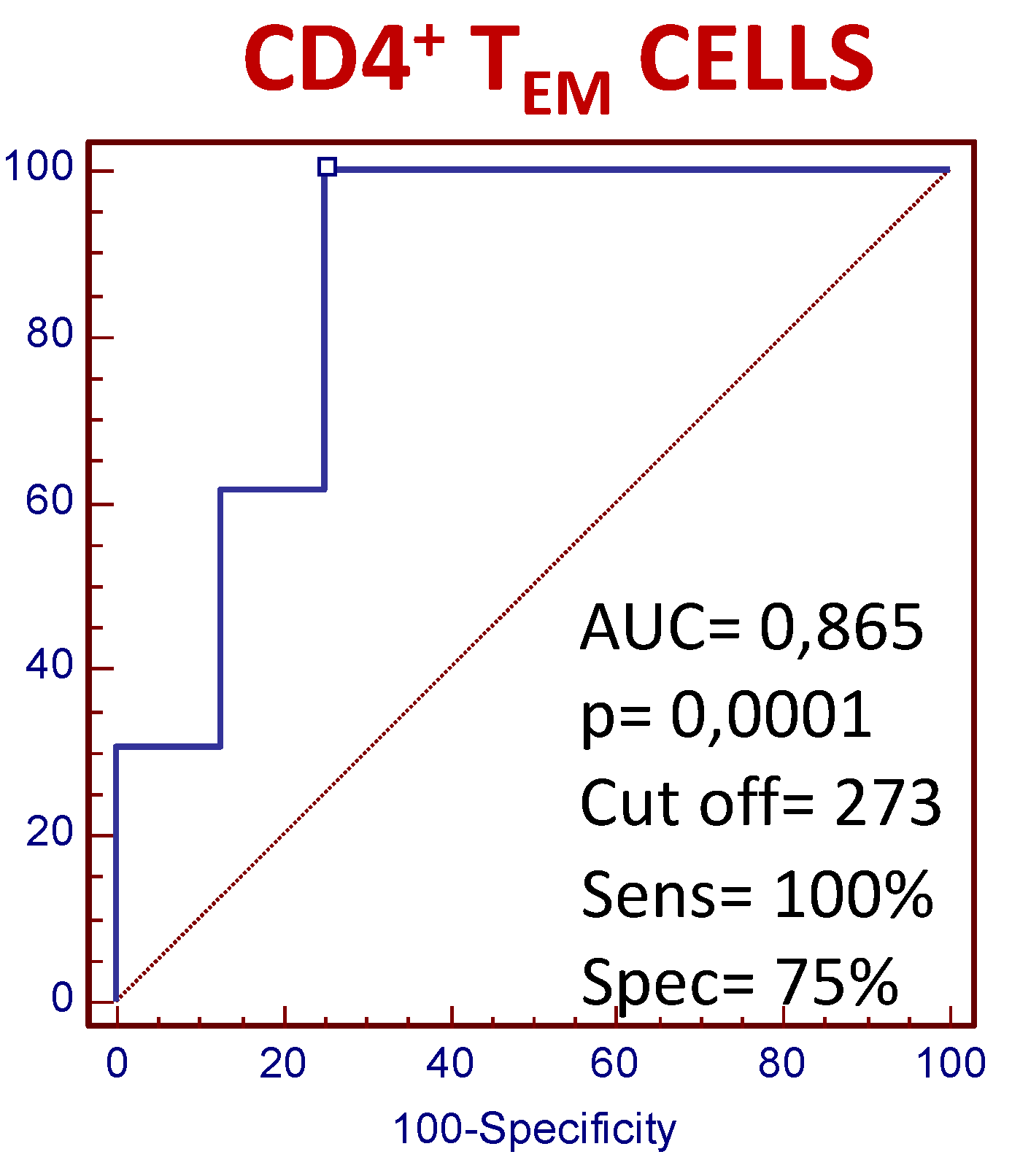
3.4. The Number of Circulating CD4+ TEM Lymphocytes Predict the Clinical Response to MTX Treatment in Recently Diagnosed Dmard-Naive Patients
3.5. MTX Responder and Non-Responder Recently Diagnosed Ar Patients Show Different Pattern of Distribution of the Vβ T-Cell Repertoire in Circulating CD4+ T Lymphocyte
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DAS28 | Disease Activity Score 28 |
| DMARDS | disease-modifying antirheumatic drugs |
| EULAR | European League Against Rheumatism |
| HAQ | Health Assessment Questionnaire |
| mAb | monoclonal antibodies |
| PBMC | peripheral blood mononuclear cells |
| FITC | fluorescein-isothiocyanate |
| PE | phycoerythrin |
| PerCP | peridinin chlorophyll protein conjugate |
| APC | allophycocyanin |
| APC-Alexa780 | allophycocyanin-alexa-780 |
| PE-CY7 | phycoerythrin-cyanine seven |
| RA | rheumatoid arthritis |
| RPMI | Roswell Park Memorial Institute |
| SD | standard desviation |
| S.E.M. | standard error of the mean |
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1098. [Google Scholar] [CrossRef]
- Gibbons, L.J.; Hyrich, K.L. Biologic Therapy for Rheumatoid Arthritis: Clinical Efficacy and Predictors of Response; Springer: Berlin/Heidelberg, Germany, 2009; Volume 23, pp. 111–124. [Google Scholar]
- Smolen, J.S.; Landewe, R.; Breedveld, F.C. C. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 Update. Ann. Rheum. Dis. 2014, 73, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Rau, R. Efficacy of methotrexate in comparison to biologics in rheumatoid arthritis. Clin. Exp. Rheumatol. 2010, 28, S58–S64. [Google Scholar] [PubMed]
- Bansard, C.; Lequerre, T.; Daveau, M. Can rheumatoid arthritis responsiveness to methotrexate and biologics be predicted? Rheumatology (Oxford) 2009, 48, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzabin, S. Williams RO Effector T cells in rheumatoid arthritis: Lessons from animal models. Febs Lett. 2011, 585, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.P. T cells in rheumatoid arthritis. Arthritis Res. 2008, 10, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Bryl, E.; Goronzy, J.J. The role of T cells in rheumatoid arthritis. Arch. Immunol. Exp. (Warsz) 2000, 48, 429–435. [Google Scholar]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur. J. Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef]
- Moro-Garcia, M.A.; Alonso-Arias, R.; Lopez-Larrea, C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front. Immunol. 2013, 4, 107. [Google Scholar] [CrossRef]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Kaech, S.M.; Wherry, E.J. Ahmed R Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Taylor, J.J.; Jenkins, M.K. CD4+ memory T cell survival. Curr Opin Immunol 2011, 23, 319–323. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur. J. Immunol. 2009, 39, 2076–2082. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012, 51, 3–11. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Lee, W.W. Weyand CM Aging and T-cell diversity. Exp. Gerontol. 2007, 42, 400–406. [Google Scholar] [CrossRef]
- Koetz, K.; Bryl, E.; Spickschen, K.; O’Fallon, W.M.; Goronzy, J.J. Weyand CM T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9203–9208. [Google Scholar] [CrossRef]
- Pawlowska, J.; Smolenska, Z.; Daca, A.; Witkowski, J.M.; Bryl, E. Older age of rheumatoid arthritis onset is associated with higher activation status of peripheral blood CD4(+) T cells and disease activity. Clin. Exp. Immunol. 2011, 163, 157–164. [Google Scholar] [CrossRef]
- Salazar-Fontana, L.I.; Sanz, E.; Merida, I. Cell surface CD28 levels define four CD4+ T cell subsets: Abnormal expression in rheumatoid arthritis. Clin. Immunol. 2001, 99, 253–265. [Google Scholar] [CrossRef]
- Fekete, A.; Soos, L.; Szekanecz, Z. Disturbances in B- and T-cell homeostasis in rheumatoid arthritis: Suggested relationships with antigen-driven immune responses. J. Autoimmun. 2007, 29, 154–163. [Google Scholar] [CrossRef]
- Kohem, C.L.; Brezinschek, R.I.; Wisbey, H.; Tortorella, C.; Lipsky, P.E. Oppenheimer-Marks N Enrichment of differentiated CD45RBdim. Arthritis Rheum. 1996, 39, 844–854. [Google Scholar] [CrossRef]
- Neidhart, M.; Pataki, F.; Schonbachler, J. Bruhlmann P Flow cytometric characterisation of the "false naive" (CD45RA+, CD45RO-, CD29 bright+) peripheral blood T-lymphocytes in health and in rheumatoid arthritis. Rheumatol. Int. 1996, 16, 77–87. [Google Scholar] [CrossRef]
- Neidhart, M.; Fehr, K.; Pataki, F. Michel BA The levels of memory (CD45RA-, RO+) CD4+ and CD8+ peripheral blood T-lymphocytes correlate with IgM rheumatoid factors in rheumatoid arthritis. Rheumatol. Int. 1996, 15, 201–209. [Google Scholar] [CrossRef]
- Ponchel, F.; Morgan, A.W.; Bingham, S.J. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood 2002, 100, 4550–4556. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, D.; Goronzy, J.J. Weyand CM CD4+ CD7. J. Clin. Invest. 1996, 97, 2027–2037. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Shao, L. Weyand CM Immune aging and rheumatoid arthritis. Rheum. Dis. Clin. North Am. 2010, 36, 297–310. [Google Scholar] [CrossRef]
- Prescott, J.; Karlson, E.W.; Orr, E.H.; Zee, R.Y.; De V, I. Costenbader KH A Prospective Study Investigating Prediagnostic Leukocyte Telomere Length and Risk of Developing Rheumatoid Arthritis in Women. J. Rheumatol. 2016, 43, 282–288. [Google Scholar] [CrossRef]
- Davis, M.M.; Bjorkman, P.J. T-cell antigen receptor genes and T-cell recognition. Nature 1988, 334, 395–402. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Zhao, M. T cell receptor beta repertoires as novel diagnostic markers for systemic lupus erythematosus and rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 1070–1078. [Google Scholar] [CrossRef]
- Sun, W.; Nie, H.; Li, N. Skewed T-cell receptor BV14 and BV16 expression and shared CDR3 sequence and common sequence motifs in synovial T cells of rheumatoid arthritis. Genes Immun. 2005, 6, 248–261. [Google Scholar] [CrossRef]
- Jia, X.; Wang, B.; Zhai, T.; Yao, Q.; Li, Q.; Zhang, J.A. T cell receptor revision and immune repertoire changes in autoimmune diseases. Clin. Immunol. 2018. [Google Scholar] [CrossRef]
- Davey, M.P.; Munkirs, D.D. Patterns of T-cell receptor variable beta gene expression by synovial fluid and peripheral blood T-cells in rheumatoid arthritis. Clin. Immunol. Immunopathol. 1993, 68, 79–87. [Google Scholar] [CrossRef]
- Genevee, C.; Diu, A.; Nierat, J. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur. J. Immunol. 1992, 22, 1261–1269. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Zettl, A. Weyand CM T cell receptor repertoire in rheumatoid arthritis. Int. Rev. Immunol. 1998, 17, 339–363. [Google Scholar] [CrossRef]
- Hall, F.C.; Thomson, K.; Procter, J.; McMichael, A.J. Wordsworth BP TCR beta spectratyping in RA: Evidence of clonal expansions in peripheral blood lymphocytes. Ann. Rheum. Dis. 1998, 57, 319–322. [Google Scholar] [CrossRef]
- Klarenbeek, P.L.; de Hair, M.J.; Doorenspleet, M.E. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann. Rheum. Dis. 2012, 71, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Pluschke, G.; Ginter, A.; Taube, H.; Melchers, I.; Peter, H.H. Krawinkel U Analysis of T cell receptor V beta regions expressed by rheumatoid synovial T lymphocytes. Immunobiology 1993, 188, 330–339. [Google Scholar] [CrossRef]
- Sottini, A.; Imberti, L.; Bettinardi, A.; Mazza, C.; Gorla, R.; Primi, D. Selection of T lymphocytes in two rheumatoid arthritis patients defines different T-cell receptor V beta repertoires in CD4+ and CD8+ T-cell subsets. J. Autoimmun. 1993, 6, 621–637. [Google Scholar] [CrossRef]
- Spreafico, R.; Rossetti, M.; van, L.J. A circulating reservoir of pathogenic-like CD4+ T cells shares a genetic and phenotypic signature with the inflamed synovial micro-environment. Ann. Rheum. Dis. 2016, 75, 459–465. [Google Scholar] [CrossRef]
- Striebich, C.C.; Falta, M.T.; Wang, Y.; Bill, J.; Kotzin, B.L. Selective accumulation of related CD4+ T cell clones in the synovial fluid of patients with rheumatoid arthritis. J. Immunol. 1998, 161, 4428–4436. [Google Scholar]
- Zagon, G.; Tumang, J.R.; Li, Y.; Friedman, S.M. Crow MK Increased frequency of V beta 17-positive T cells in patients with rheumatoid arthritis. Arthritis Rheum. 1994, 37, 1431–1440. [Google Scholar] [CrossRef]
- Zhou, J.; Kong, C.; Yu, J.; Dong, H.; Jin, C. Song Q Skewness of TCR Vbeta of peripheral blood and synovial fluid of patients with rheumatoid arthritis. J. Immunoass. Immunochem. 2014, 35, 207–219. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Gonzalez, V.M.; Stewart, A.; Ritter, P.L.; Lorig, K. Translation and validation of arthritis outcome measures into Spanish. Arthritis Rheum. 1995, 38, 1429–1446. [Google Scholar] [CrossRef]
- van Gestel, A.M.; Prevoo, M.L.; van Hof, M.A.; van Rijswijk, M.H.; van de Putte, L.B.; van Riel, P.L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996, 39, 34–40. [Google Scholar]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J. Clin. Lab. Invest. 1968, 97, 77–89. [Google Scholar]
- Roederer, M. Compensation in flow cytometry. Curr. Protoc. Cytom. 2002. [Google Scholar] [CrossRef]
- Roederer, M.; Darzynkiewicz, Z. Parks DR Guidelines for the presentation of flow cytometric data. Methods Cell. Biol. 2004, 75, 241–256. [Google Scholar]
- Firestein GS Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J. Clin. Rheumatol. 2005, 11, S39–S44. [CrossRef]
- Skapenko, A.; Lipsky, P.E.; Schulze-Koops, H. T cell activation as starter and motor of rheumatic inflammation. Curr. Top. Microbiol. Immunol. 2006, 305, 195–211. [Google Scholar]
- Appay, V.; van Lier, R.A.; Sallusto, F.; Roederer, M. Phenotype and function of human T lymphocyte subsets: Consensus and issues. Cytom. A 2008, 73, 975–983. [Google Scholar] [CrossRef]
- Okada, R.; Kondo, T.; Matsuki, F.; Takata, H. Takiguchi M Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int. Immunol. 2008, 20, 1189–1199. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Baeten, D.; Houbiers, J.; Kruithof, E. Synovial inflammation does not change in the absence of effective treatment: Implications for the use of synovial histopathology as biomarker in early phase clinical trials in rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 990–997. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, B.; Zhaopeng, Z. Elevated circulating T cell subsets and cytokines expression in patients with rheumatoid arthritis. Clin. Rheumatol. 2019, 38, 1831–1839. [Google Scholar] [CrossRef]
- Weng, N.P.; Akbar, A.N.; Goronzy, J. CD28(-) T cells: Their role in the age-associated decline of immune function. Trends Immunol. 2009, 30, 306–312. [Google Scholar] [CrossRef]
- Effros, R.B.; Dagarag, M. Valenzuela HF In vitro senescence of immune cells. Exp. Gerontol. 2003, 38, 1243–1249. [Google Scholar] [CrossRef]
- Goronzy, J.J. Weyand CM Rheumatoid arthritis. Immunol. Rev. 2005, 204, 55–73. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Henel, G.; Sawai, H. Costimulatory pathways in rheumatoid synovitis and T-cell senescence. Ann. N. Y. Acad. Sci. 2005, 1062, 182–194. [Google Scholar] [CrossRef]
- Bomberger, C.; Singh-Jairam, M.; Rodey, G. Lymphoid reconstitution after autologous PBSC transplantation with FACS-sorted CD34+ hematopoietic progenitors. Blood 1998, 91, 2588–2600. [Google Scholar]
- Gorski, J.; Yassai, M.; Zhu, X. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J. Immunol. 1994, 152, 5109–5119. [Google Scholar]
- Kluin-Nelemans, H.C.; Kester, M.G. Correction of abnormal T-cell receptor repertoire during interferon-alpha therapy in patients with hairy cell leukemia. Blood 1998, 91, 4224–4231. [Google Scholar]
- Liu, X.; Chesnokova, V.; Forman, S.J. Diamond DJ Molecular analysis of T-cell receptor repertoire in bone marrow transplant recipients: Evidence for oligoclonal T-cell expansion in graft-versus-host disease lesions. Blood 1996, 87, 3032–3044. [Google Scholar]
- de, V.N.; Prinsen, C.F.; Mensink, E.B.; van Riel, P.L.; van Hof, M.A.; van de Putte, L.B.A. T cell receptor beta chain variable region polymorphism associated with radiographic progression in rheumatoid arthritis. Ann. Rheum. Dis. 1993, 52, 327–331. [Google Scholar]
- Wilson, K.B.; Quayle, A.J.; Suleyman, S. Heterogeneity of the TCR repertoire in synovial fluid T lymphocytes responding to BCG in a patient with early rheumatoid arthritis. Scand. J. Immunol. 1993, 38, 102–112. [Google Scholar] [CrossRef]
- Vandevyver, C.; Gu, X.X.; Geusens, P. HLA class II and T-cell receptor beta chain polymorphisms in Belgian patients with rheumatoid arthritis: No evidence for disease association with the TCRBC2, TCRBV8 and TCRBV11 polymorphisms. Ann. Rheum. Dis. 1994, 53, 580–586. [Google Scholar] [CrossRef]
- Chara, L.; Sanchez-Atrio, A.; Perez, A. The number of circulating monocytes as biomarkers of the clinical response to methotrexate in untreated patients with rheumatoid arthritis. J. Transl. Med. 2015, 13, 2. [Google Scholar] [CrossRef]
- Chara, L.; Sanchez-Atrio, A.; Perez, A. Monocyte populations as markers of response to adalimumab plus MTX in rheumatoid arthritis. Arthritis Res. 2012, 14, R175. [Google Scholar] [CrossRef]

 ) responders (including eventual at baseline) and (
) responders (including eventual at baseline) and (  ) non-responders (including eventual at baseline) at up to 6 months of MTX treatment. The dotted line represents the mean of the results obtained for each variable in this group of subjects in the three different studies performed (
) non-responders (including eventual at baseline) at up to 6 months of MTX treatment. The dotted line represents the mean of the results obtained for each variable in this group of subjects in the three different studies performed (  ). All values are expressed as mean cell counts ± S.E.M. *, p < 0.05 for responders or non-responders (including eventual) vs. healthy controls; †, p < 0.05 for responders vs. non-responders (including eventual), σ, p < 0.05 for values at 3 months of treatment compared to baseline, ‡, p < 0.05 at 6 months of treatment time compared to 3 months, and δ, p < 0.05 at 6 months of treatment time compared to baseline.
). All values are expressed as mean cell counts ± S.E.M. *, p < 0.05 for responders or non-responders (including eventual) vs. healthy controls; †, p < 0.05 for responders vs. non-responders (including eventual), σ, p < 0.05 for values at 3 months of treatment compared to baseline, ‡, p < 0.05 at 6 months of treatment time compared to 3 months, and δ, p < 0.05 at 6 months of treatment time compared to baseline.
 ) responders (including eventual at baseline) and (
) responders (including eventual at baseline) and (  ) non-responders (including eventual at baseline) at up to 6 months of MTX treatment. The dotted line represents the mean of the results obtained for each variable in this group of subjects in the three different studies performed (
) non-responders (including eventual at baseline) at up to 6 months of MTX treatment. The dotted line represents the mean of the results obtained for each variable in this group of subjects in the three different studies performed (  ). All values are expressed as mean cell counts ± S.E.M. *, p < 0.05 for responders or non-responders (including eventual) vs. healthy controls; †, p < 0.05 for responders vs. non-responders (including eventual), σ, p < 0.05 for values at 3 months of treatment compared to baseline, ‡, p < 0.05 at 6 months of treatment time compared to 3 months, and δ, p < 0.05 at 6 months of treatment time compared to baseline.
). All values are expressed as mean cell counts ± S.E.M. *, p < 0.05 for responders or non-responders (including eventual) vs. healthy controls; †, p < 0.05 for responders vs. non-responders (including eventual), σ, p < 0.05 for values at 3 months of treatment compared to baseline, ‡, p < 0.05 at 6 months of treatment time compared to 3 months, and δ, p < 0.05 at 6 months of treatment time compared to baseline.

 ) responders (including eventual at baseline) and (
) responders (including eventual at baseline) and (  ) non-responders (including eventual at baseline) at up to 6 months of MTX treatment. The dotted line represents the mean of the results obtained for each variable in this group of subjects in the three different studies performed (
) non-responders (including eventual at baseline) at up to 6 months of MTX treatment. The dotted line represents the mean of the results obtained for each variable in this group of subjects in the three different studies performed (  ). All values are expressed as mean cell counts ± S.E.M. *, p < 0.05 for responders or non-responders (including eventual) vs. healthy controls; †, p < 0.05 for responders vs. non-responders (including eventual), σ p < 0.05 for values at 3 months of treatment compared to baseline, ‡, p < 0.05 at 6 months of treatment time compared to 3 months, and δ, p < 0.05 at 6 months of treatment time compared to baseline.
). All values are expressed as mean cell counts ± S.E.M. *, p < 0.05 for responders or non-responders (including eventual) vs. healthy controls; †, p < 0.05 for responders vs. non-responders (including eventual), σ p < 0.05 for values at 3 months of treatment compared to baseline, ‡, p < 0.05 at 6 months of treatment time compared to 3 months, and δ, p < 0.05 at 6 months of treatment time compared to baseline.
 ) responders (including eventual at baseline) and (
) responders (including eventual at baseline) and (  ) non-responders (including eventual at baseline) at up to 6 months of MTX treatment. The dotted line represents the mean of the results obtained for each variable in this group of subjects in the three different studies performed (
) non-responders (including eventual at baseline) at up to 6 months of MTX treatment. The dotted line represents the mean of the results obtained for each variable in this group of subjects in the three different studies performed (  ). All values are expressed as mean cell counts ± S.E.M. *, p < 0.05 for responders or non-responders (including eventual) vs. healthy controls; †, p < 0.05 for responders vs. non-responders (including eventual), σ p < 0.05 for values at 3 months of treatment compared to baseline, ‡, p < 0.05 at 6 months of treatment time compared to 3 months, and δ, p < 0.05 at 6 months of treatment time compared to baseline.
). All values are expressed as mean cell counts ± S.E.M. *, p < 0.05 for responders or non-responders (including eventual) vs. healthy controls; †, p < 0.05 for responders vs. non-responders (including eventual), σ p < 0.05 for values at 3 months of treatment compared to baseline, ‡, p < 0.05 at 6 months of treatment time compared to 3 months, and δ, p < 0.05 at 6 months of treatment time compared to baseline.
 )responders and (
)responders and (  ) non-responders at up to 6 months of MTX treatment. All values are expressed as percentages ± S.E.M. †, p<0.05 for responders vs. non-responders.
) non-responders at up to 6 months of MTX treatment. All values are expressed as percentages ± S.E.M. †, p<0.05 for responders vs. non-responders.
 )responders and (
)responders and (  ) non-responders at up to 6 months of MTX treatment. All values are expressed as percentages ± S.E.M. †, p<0.05 for responders vs. non-responders.
) non-responders at up to 6 months of MTX treatment. All values are expressed as percentages ± S.E.M. †, p<0.05 for responders vs. non-responders.

| Variables | Healthy Controls (n = 24) (mean ± SD) | Eventual Responders (n = 48) (mean ± SD) | Eventual Non-Responders (n = 20) (mean ± SD) |
|---|---|---|---|
| Age (years) | 49.30 ± 8.40 | 51.05 ± 9.72 | 52.80 ± 9.75 |
| Sex (women) | 70.83% | 72.91% | 75.00% |
| CRP (mg/dL) | 15.40 ± 6.51 | 16.51 ± 6.11 | |
| Rheumatoid factor (+) | 90.76% | 92185% | |
| Anti-CCP (UI/mL) | 425.61 ± 389.25 | 433.99 ± 295.60 | |
| DAS28 | 3.61 ± 0.62 | 3.75 ± 0.65 | |
| Erosions (+) | 26.17% | 27.07% | |
| HAQ | 0.82 ± 0.51 | 0.81 ± 0.55 |
| n°/μL (%) | Controls | Patients at Baseline | Controls | Patients at Baseline | |
|---|---|---|---|---|---|
| Subsets | CD4+ | CD4+CD28− | |||
| CD4+ Total (% with respect to total lymphocytes) | 770.29 ± 61.59 (39.62 ± 3.07) | 1265.96 ± 95.10 * (50.99 ± 2.24) * | (% with respect to total CD4+ lymphocytes) | 12.16 ± 3.76 (1.72 ± 0.66) | 101.77 ± 36.88 * (10.10 ± 4.14) * |
| TN(% with respect to total CD4+ lymphocytes) | 460.55 ± 34.99 (60.61 ± 2.8) | 737.13 ± 80.82 * (58.58 ± 4.00) | TN(% with respect to total CD4+CD28- lymphocytes) | 1.8 ± 0.45 (0.40 ± 0.12) | 2.87 ± 0.57 (0.7 ± 0.2) |
| TEM | 37.44 ± 6.6 (4.63 ± 0.66) | 164.43 ± 37.00 * (12.58 ± 3.07) | TEM | 5.25 ± 2.31 (14.00 ± 4.5) | 35.04 ± 19.03 (26.8 ± 5.6) * |
| TE | 11.79 ± 5.85 (1.62 ± 0.71) | 60.54 ± 17.46 * (5.40 ± 1.31) * | TE | 4.24 ± 2.09 (46.89 ± 8.81) | 62.54 ± 28.98 * (81.2 ± 4.50) * |
| TCM | 260.5 ± 31.23 (33.14 ± 2.07) | 303.84 ± 37.62 (23.42 ± 1.38) * | TCM | 0.85 ± 0.41 (0.50 ± 0.30) | 1.31 ± 0.52 (0.6 ± 0.30) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat, J.; Bohórquez, C.; Gómez Lahoz, A.M.; Movasat, A.; Pérez, A.; Ruíz, L.; Díaz, D.; Chara, L.; Sánchez, A.I.; Albarrán, F.; et al. The Abnormal CD4+T Lymphocyte Subset Distribution and Vbeta Repertoire in New-onset Rheumatoid Arthritis Can Be Modulated by Methotrexate Treament. Cells 2019, 8, 871. https://doi.org/10.3390/cells8080871
Monserrat J, Bohórquez C, Gómez Lahoz AM, Movasat A, Pérez A, Ruíz L, Díaz D, Chara L, Sánchez AI, Albarrán F, et al. The Abnormal CD4+T Lymphocyte Subset Distribution and Vbeta Repertoire in New-onset Rheumatoid Arthritis Can Be Modulated by Methotrexate Treament. Cells. 2019; 8(8):871. https://doi.org/10.3390/cells8080871
Chicago/Turabian StyleMonserrat, Jorge, Cristina Bohórquez, Ana María Gómez Lahoz, Atusa Movasat, Ana Pérez, Lucía Ruíz, David Díaz, Luis Chara, Ana Isabel Sánchez, Fernando Albarrán, and et al. 2019. "The Abnormal CD4+T Lymphocyte Subset Distribution and Vbeta Repertoire in New-onset Rheumatoid Arthritis Can Be Modulated by Methotrexate Treament" Cells 8, no. 8: 871. https://doi.org/10.3390/cells8080871
APA StyleMonserrat, J., Bohórquez, C., Gómez Lahoz, A. M., Movasat, A., Pérez, A., Ruíz, L., Díaz, D., Chara, L., Sánchez, A. I., Albarrán, F., Sanz, I., & Álvarez-Mon, M. (2019). The Abnormal CD4+T Lymphocyte Subset Distribution and Vbeta Repertoire in New-onset Rheumatoid Arthritis Can Be Modulated by Methotrexate Treament. Cells, 8(8), 871. https://doi.org/10.3390/cells8080871







