Membrane-Bound Meet Membraneless in Health and Disease
Abstract
:1. Introduction
2. Stress Assemblies and the Inhibition of Trafficking in the Early Secretory Pathway
2.1. P-Bodies Form in Yeast Mutant for Secretory Pathway Components
2.2. Sec Body Formation and the ER Exit Sites (ERES)
3. The Complex Relationship between Stress Granules, Cyto-Nuclear Transport, and Amyotrophic Lateral Sclerosis (ALS)
3.1. Stress Granules
3.2. Stress Granules and ALS: A Role for FUS, TDP43, and C9orf72
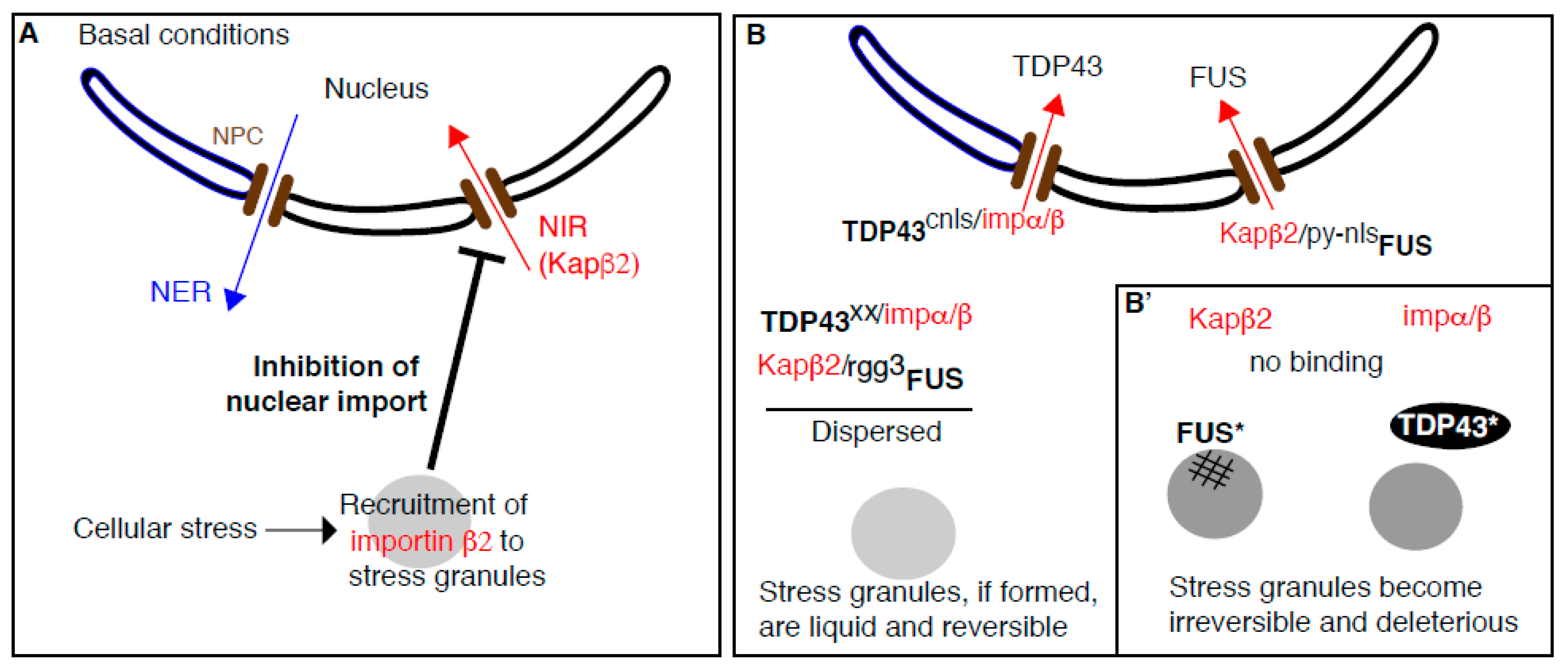
3.3. Stress Granules Assembly Negatively Regulates Cytoplasm to Nucleus Import
3.4. Beyond Nuclear Transport: NIRs Act as Chaperones to Prevent Stress-Granule-Related Pathological Aggregation in ALS (Figure 2B)
3.4.1. Purified FUS Phase Separation into Liquid Droplets is Specifically Inhibited by Karyopherin β2
3.4.2. Does Karyopherin β2 Antagonize the Coalescence/Aggregation of FUS in Cellulo?
3.4.3. Where Does Karyopherin β2 Bind FUS to Prevent Their Condensation/Fibrilization?
3.4.4. How Does This Relate to ALS and FTD? Could NIR be Used as A Treatment of ALS?
4. Membrane Enhances the Formation of Phase-Separated Condensates
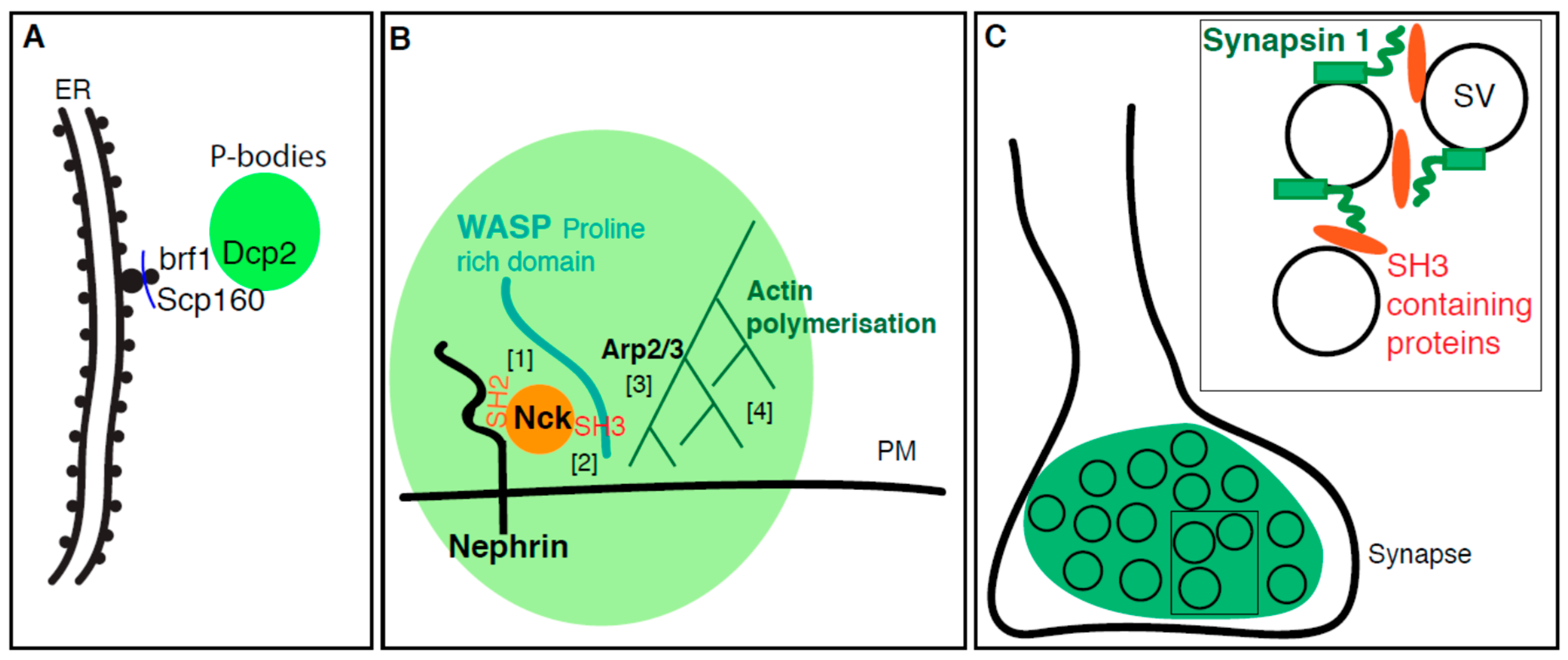
4.1. P-Body Formation is Regulated by ER Proteins
4.2. Plasma Membrane Receptors Promotes Phase Separation
4.3. The Synapse, A Phase Separation Mediated by Small Lipid Carriers?
5. The Cell Cycle Appears to be A Global Transition from An Interphasic Phase-Separated State to A Mitotic Dispersed State
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. The Early Secretory Pathway
Appendix B. The Cyto-Nuclear Trafficking
References
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming together to define membrane contact sites. Nat. Commun. 2019, 10, 1287. [Google Scholar] [CrossRef]
- Forman-Kay, J.D.; Kriwacki, R.W.; Seydoux, G. Phase separation in biology and disease. J. Mol. Biol. 2018, 430, 4603–4606. [Google Scholar] [CrossRef]
- Gomes, E.; Shorter, J. The molecular language of membraneless organelles. J. Biol. Chem. 2018, 294, 7115–7127. [Google Scholar] [CrossRef]
- Marnik, E.A.; Updike, D.L. Membraneless organelles: P granules in Caenorhabditis elegans. Traffic 2019. [Google Scholar] [CrossRef]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef] [Green Version]
- Zacharogianni, M.; Aguilera-Gomez, A.; Veenendaal, T.; Smout, J.; Rabouille, C. A stress assembly that confers cell viability by preserving ERES components during amino-acid starvation. eLife 2014, 3, e04132. [Google Scholar] [CrossRef]
- Petrovska, I.; Nuske, E.; Munder, M.C.; Kulasegaran, G.; Malinovska, L.; Kroschwald, S.; Richter, D.; Fahmy, K.; Gibson, K.; Verbavatz, J.M.; et al. Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. eLife 2014, 3, e02409. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P.P. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Banani, S.F.; Rice, A.M.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K.K. Compositional control of phase-separated cellular bodies. Cell 2016, 166, 651–663. [Google Scholar] [CrossRef]
- Ruff, K.M.; Pappu, R.V.; Holehouse, A.S.S. Conformational preferences and phase behavior of intrinsically disordered low complexity sequences: Insights from multiscale simulations. Curr. Med. Res. Opin. 2019, 56, 1–10. [Google Scholar] [CrossRef]
- Vernon, R.M.; Chong, P.A.; Tsang, B.; Kim, T.H.; Bah, A.; Farber, P.; Lin, H.; Forman-Kay, J.D.D. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. eLife 2018, 7, e31486. [Google Scholar] [CrossRef]
- Kroschwald, S.; Alberti, S.S. Gel or die: Phase separation as a survival strategy. Cell 2017, 168, 947–948. [Google Scholar] [CrossRef]
- Alberti, S.S. The wisdom of crowds: Regulating cell function through condensed states of living matter. J. Cell Sci. 2017, 130, 2789–2796. [Google Scholar] [CrossRef]
- Ji, S.; Luo, Y.; Cai, Q.; Cao, Z.; Zhao, Y.; Mei, J.; Li, C.; Xia, P.; Xie, Z.; Xia, Z.Z.; et al. LC Domain-mediated coalescence is essential for otu enzymatic activity to extend drosophila lifespan. Mol. Cell 2019, 74, 363–377. [Google Scholar] [CrossRef]
- Chong, P.A.; Forman-Kay, J.D. Liquid–liquid phase separation in cellular signaling systems. Curr. Med. Res. Opin. 2016, 41, 180–186. [Google Scholar] [CrossRef]
- Trcek, T.; Lehmann, R. Germ granules in Drosophila. Traffic 2019, 20, 650–660. [Google Scholar] [CrossRef]
- Wunder, T.; Oh, Z.G.; Mueller-Cajar, O. CO2-fixing liquid droplets: Towards a dissection of the microalgal pyrenoid. Traffic 2019, 20, 380–389. [Google Scholar] [CrossRef]
- Formicola, N.; Vijayakumar, J.; Besse, F. Neuronal ribonucleoprotein granules: Dynamic sensors of localized signals. Traffic 2019, 20, 639–649. [Google Scholar] [CrossRef]
- van Leeuwen, W.; Rabouille, C. Cellular stress leads to the formation of membraneless stress assemblies in eukaryotic animal cells. Traffic 2019, 20, 623–638. [Google Scholar]
- Parker, R.; Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 2007, 25, 635–646. [Google Scholar] [CrossRef]
- Hubstenberger, A.; Courel, M.; Bénard, M.; Souquere, S.; Ernoult-Lange, M.; Chouaib, R.; Yi, Z.; Morlot, J.-B.; Munier, A.; Fradet, M.; et al. P-body purification reveals the condensation of repressed mRNA regulons. Mol. Cell 2017, 68, 144–157. [Google Scholar] [CrossRef]
- Guzikowski, A.R.; Chen, Y.S.; Zid, B.M. Stress-induced mRNP granules: Form and function of processing bodies and stress granules. Wiley Interdiscip. Rev. RNA 2019, 10, e1524. [Google Scholar] [CrossRef]
- Teixeira, D.; Parker, R. Analysis of P-body assembly in saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 2274–2287. [Google Scholar] [CrossRef]
- Buchan, J.R.; Muhlrad, D.; Parker, R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J. Cell Biol. 2008, 183, 441–455. [Google Scholar] [CrossRef]
- Wang, C.; Schmich, F.; Srivatsa, S.; Weidner, J.; Beerenwinkel, N.; Spang, A. Context-dependent deposition and regulation of mRNAs in P-bodies. eLife 2018, 7, e29815. [Google Scholar] [CrossRef]
- Souquere, S.; Mollet, S.; Kress, M.; Dautry, F.; Pierron, G.; Weil, D. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells. J. Cell Sci. 2009, 122, 3619–3626. [Google Scholar] [CrossRef] [Green Version]
- Horvathova, I.; Voigt, F.; Kotrys, A.V.; Zhan, Y.; Artus-Revel, C.G.; Eglinger, J.; Stadler, M.B.; Giorgetti, L.; Chao, J.A. The dynamics of mRNA turnover revealed by single-molecule imaging in single cells. Mol. Cell 2017, 68, 615–625. [Google Scholar] [CrossRef]
- Yahara, N.; Ueda, T.; Sato, K.; Nakano, A. Multiple roles of Arf1 GTPase in the yeast exocytic and endocytic pathways. Mol. Biol. Cell 2001, 12, 221–238. [Google Scholar] [CrossRef]
- Kilchert, C.; Weidner, J.; Prescianotto-Baschong, C.; Spang, A. Defects in the secretory pathway and high Ca2+ induce multiple P-bodies. Mol. Biol. Cell 2010, 21, 2624–2638. [Google Scholar] [CrossRef]
- Aguilera-Gomez, A.; Rabouille, C. Intra-Golgi Transport. In Encyclopedia of Cell Biology; Bradshaw, R.A., Stahl, P.D., Eds.; Academic Press: Waltham, MA, USA, 2016; Volume 2, pp. 354–362. [Google Scholar]
- Jackson, C.L.; Bouvet, S. Arfs at a Glance. J. Cell Sci. 2014, 127, 4103. [Google Scholar] [CrossRef]
- Ackema, K.B.; Hench, J.; Bockler, S.; Wang, S.C.; Sauder, U.; Mergentaler, H.; Westermann, B.; Bard, F.; Frank, S.; Spang, A. The small GTPase Arf1 modulates mitochondrial morphology and function. EMBO J. 2014, 33, 2659–2675. [Google Scholar] [CrossRef]
- Walch, L.; Pellier, E.; Leng, W.; Lakisic, G.; Gautreau, A.; Contremoulins, V.; Verbavatz, J.-M.; Jackson, C.L. GBF1 and Arf1 interact with Miro and regulate mitochondrial positioning within cells. Sci. Rep. 2018, 8, 17121. [Google Scholar] [CrossRef]
- Batiza, A.F.; Schulz, T.; Masson, P.H. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 1996, 271, 23357–23362. [Google Scholar] [CrossRef]
- Matsumoto, T.K.; Ellsmore, A.J.; Cessna, S.G.; Low, P.S.; Pardo, J.M.; Bressan, R.A.; Hasegawa, P.M. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 33075–33080. [Google Scholar] [CrossRef]
- Decker, C.J.; Parker, R. P-bodies and stress granules: Possible roles in the control of translation and mRNA degradation. Cold Spring Harb. Perspect. Biol. 2012, 4, a012286. [Google Scholar] [CrossRef]
- Jeong, Y.T.; Simoneschi, D.; Keegan, S.; Melville, D.; Adler, N.S.; Saraf, A.; Florens, L.; Washburn, M.P.; Cavasotto, C.N.; Fenyö, D.; et al. The ULK1-FBXW5-SEC23B nexus controls autophagy. Elife 2018, 7, e42253. [Google Scholar] [CrossRef]
- Aguilera-Gomez, A.; Zacharogianni, M.; van Oorschot, M.M.; Genau, H.; Grond, R.; Veenendaal, T.; Sinsimer, K.S.; Gavis, E.R.; Behrends, C.; Rabouille, C. Phospho-rasputin stabilization by Sec16 is required for stress granule formation upon amino acid starvation. Cell Rep. 2017, 20, 935–948. [Google Scholar] [CrossRef]
- Ivan, V.; de Voer, G.; Xanthakis, D.; Spoorendonk, K.M.; Kondylis, V.; Rabouille, C. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol. Biol. Cell 2008, 19, 4352–4365. [Google Scholar] [CrossRef]
- Aguilera-Gomez, A.; van Oorschot, M.M.; Veenendaal, T.; Rabouille, C. In vivo vizualisation of mono-ADP-ribosylation by dPARP16 upon amino-acid starvation. eLife 2016, 5, e21475. [Google Scholar] [CrossRef]
- Di Paola, S.; Micaroni, M.; Di Tullio, G.; Buccione, R.; Di Girolamo, M. PARP16/ARTD15 is a novel endoplasmic-reticulum-associated mono-ADP-ribosyltransferase that interacts with, and modifies karyopherin-ss1. PLoS ONE 2012, 7, e37352. [Google Scholar] [CrossRef]
- Jwa, M.; Chang, P. PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK- and IRE1alpha-mediated unfolded protein response. Nat. Cell Biol. 2012, 14, 1223–1230. [Google Scholar] [CrossRef]
- Sprangers, J.; Rabouille, C. SEC16 in COPII coat dynamics at ER exit sites. Biochem. Soc. Trans. 2015, 43, 97–103. [Google Scholar] [CrossRef]
- Anderson, P.; Kedersha, N. Stressful initiations. J. Cell Sci. 2002, 115, 3227–3234. [Google Scholar]
- Anderson, P.; Kedersha, N. Stress granules: The Tao of RNA triage. Trends Biochem. Sci. 2008, 33, 141–150. [Google Scholar] [CrossRef]
- Protter, D.S.; Parker, R. Principles and properties of stress granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef]
- Anderson, P.; Kedersha, N. Stress granules. Curr. Biol. 2009, 19, R397–R398. [Google Scholar] [CrossRef] [Green Version]
- Buchan, J.R.; Parker, R. Eukaryotic stress granules: The ins and outs of translation. Mol. Cell 2009, 36, 932–941. [Google Scholar] [CrossRef]
- Scotter, E.L.; Chen, H.-J.; Shaw, C.E. TDP-43 proteinopathy and ALS: Insights into disease mechanisms and therapeutic targets. Neurotherapeutics 2015, 12, 352–363. [Google Scholar] [CrossRef]
- Birsa, N.; Bentham, M.P.; Fratta, P. Cytoplasmic functions of TDP-43 and FUS and their role in ALS. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Murray, D.T.; Kato, M.; Lin, Y.; Thurber, K.R.; Hung, I.; McKnight, S.L.; Tycko, R. Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell 2017, 171, 615–627. [Google Scholar] [CrossRef]
- Monahan, Z.; Ryan, V.H.; Janke, A.M.; Burke, K.A.; Rhoads, S.N.; Zerze, G.H.; Meally, R.; Dignon, G.L.; Conicella, A.E.; Zheng, W.; et al. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 2017, 36, 2951. [Google Scholar] [CrossRef]
- Guo, L.; Kim, H.J.; Wang, H.; Monaghan, J.; Freyermuth, F.; Sung, J.C.; O’Donovan, K.; Fare, C.M.; Diaz, Z.; Singh, N.; et al. Nuclear-import receptors reverse aberrant phase transitions of RNA-binding proteins with prion-like domains. Cell 2018, 173, 677–692. [Google Scholar] [CrossRef]
- Maharana, S.; Wang, J.; Papadopoulos, D.K.; Richter, D.; Pozniakovsky, A.; Poser, I.; Bickle, M.; Rizk, S.; Guillén-Boixet, J.; Franzmann, T.M.; et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 2018, 360, 918. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.-M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 2018, 174, 688–699. [Google Scholar] [CrossRef]
- Hughes, M.P.; Sawaya, M.R.; Boyer, D.R.; Goldschmidt, L.; Rodriguez, J.A.; Cascio, D.; Chong, L.; Gonen, T.; Eisenberg, D.S. Atomic structures of low-complexity protein segments reveal kinked β sheets that assemble networks. Science 2018, 359, 698. [Google Scholar] [CrossRef]
- Bowden, H.A.; Dormann, D. Altered mRNP granule dynamics in FTLD pathogenesis. J. Neurochem. 2016, 138, 112–133. [Google Scholar] [CrossRef]
- McGurk, L.; Gomes, E.; Guo, L.; Mojsilovic-Petrovic, J.; Tran, V.; Kalb, R.G.; Shorter, J.; Bonini, N.M. Poly (ADP-ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization. Mol. Cell 2018, 71, 703–717. [Google Scholar] [CrossRef]
- Li, Y.R.; King, O.D.; Shorter, J.; Gitler, A.D. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013, 201, 361–372. [Google Scholar] [CrossRef]
- Bigio, E.H. C9ORF72, the new gene on the block, causes C9FTD/ALS: New insights provided by neuropathology. Acta Neuropathol. 2011, 122, 653–655. [Google Scholar] [CrossRef]
- Fong, J.C.; Karydas, A.M.; Goldman, J.S. Genetic counseling for FTD/ALS caused by the C9ORF72 hexanucleotide expansion. Alzheimers Res. Ther. 2012, 4, 27. [Google Scholar] [CrossRef]
- Khan, B.K.; Yokoyama, J.S.; Takada, L.T.; Sha, S.J.; Rutherford, N.J.; Fong, J.C.; Karydas, A.M.; Wu, T.; Ketelle, R.S.; Baker, M.C.; et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. J. Neurol. Neurosurg. Psychiatry 2012, 83, 358–364. [Google Scholar] [CrossRef]
- Freibaum, B.D.; Lu, Y.; Lopez-Gonzalez, R.; Kim, N.C.; Almeida, S.; Lee, K.-H.; Badders, N.; Valentine, M.; Miller, B.L.; Wong, P.C.; et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 2015, 525, 129. [Google Scholar] [CrossRef]
- Zhang, K.; Donnelly, C.J.; Haeusler, A.R.; Grima, J.C.; Machamer, J.B.; Steinwald, P.; Daley, E.L.; Miller, S.J.; Cunningham, K.M.; Vidensky, S.; et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 2015, 525, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Jovičić, A.; Mertens, J.; Boeynaems, S.; Bogaert, E.; Chai, N.; Yamada, S.B.; Paul Iii, J.W.; Sun, S.; Herdy, J.R.; Bieri, G.; et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat. Neurosci. 2015, 18, 1226. [Google Scholar] [CrossRef]
- Boeynaems, S.; Bogaert, E.; Kovacs, D.; Konijnenberg, A.; Timmerman, E.; Volkov, A.; Guharoy, M.; De Decker, M.; Jaspers, T.; Ryan, V.H.; et al. Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol. Cell 2017, 65, 1044–1055. [Google Scholar] [CrossRef]
- Patterson, J.R.; Wood, M.P.; Schisa, J.A. Assembly of RNP granules in stressed and aging oocytes requires nucleoporins and is coordinated with nuclear membrane blebbing. Dev. Biol. 2011, 353, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Mahboubi, H.; Seganathy, E.; Kong, D.; Stochaj, U. Identification of novel stress granule components that are involved in nuclear transport. PLoS ONE 2013, 8, e68356. [Google Scholar] [CrossRef]
- Zhang, K.; Daigle, J.G.; Cunningham, K.M.; Coyne, A.N.; Ruan, K.; Grima, J.C.; Bowen, K.E.; Wadhwa, H.; Yang, P.; Rigo, F.; et al. Stress granule assembly disrupts nucleocytoplasmic transport. Cell 2018, 173, 958–971. [Google Scholar] [CrossRef]
- Gasset-Rosa, F.; Lu, S.; Yu, H.; Chen, C.C.; Melamed, Z.; Guo, L.; Shorter, J.; Da Cruz, S.; Cleveland, D.W. Cytoplasmic TDP-43 de-mixing independent of stress granules drives inhibition of nuclear import, loss of nuclear TDP-43, and cell death. Neuron 2019, 102, 339–357. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Ali, R.; Jiou, J.; Fung, H.Y.J.; Burke, K.A.; Kim, S.J.; Lin, Y.; Peeples, W.B.; Saltzberg, D.; Soniat, M.; et al. Nuclear import receptor inhibits phase separation of FUS through binding to multiple sites. Cell 2018, 173, 693–705. [Google Scholar] [CrossRef]
- Hofweber, M.; Hutten, S.; Bourgeois, B.; Spreitzer, E.; Niedner-Boblenz, A.; Schifferer, M.; Ruepp, M.-D.; Simons, M.; Niessing, D.; Madl, T.; et al. Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell 2018, 173, 706–719. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Chook, Y.M. Structural and energetic basis of ALS-causing mutations in the atypical proline–tyrosine nuclear localization signal of the Fused in Sarcoma protein (FUS). Proc. Natl. Acad. Sci. USA 2012, 109, 12017. [Google Scholar] [CrossRef]
- Couthouis, J.; Hart, M.P.; Shorter, J.; DeJesus-Hernandez, M.; Erion, R.; Oristano, R.; Liu, A.X.; Ramos, D.; Jethava, N.; Hosangadi, D.; et al. A yeast functional screen predicts new candidate ALS disease genes. Proc. Natl. Acad. Sci. USA 2011, 108, 20881. [Google Scholar] [CrossRef]
- Chong, P.A.; Vernon, R.M.; Forman-Kay, J.D. RGG/RG motif regions in RNA binding and phase separation. J. Mol. Biol. 2018, 430, 4650–4665. [Google Scholar] [CrossRef]
- Daigle, J.G.; Lanson, N.A., Jr.; Smith, R.B.; Casci, I.; Maltare, A.; Monaghan, J.; Nichols, C.D.; Kryndushkin, D.; Shewmaker, F.; Pandey, U.B. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum. Mol. Genet. 2012, 22, 1193–1205. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Fare, C.M.; Shorter, J. Therapeutic dissolution of aberrant phases by nuclear-import receptors. Trends Cell Biol. 2019, 29, 308–322. [Google Scholar] [CrossRef]
- Weil, T.T.; Parton, R.M.; Herpers, B.; Soetaert, J.; Veenendaal, T.; Xanthakis, D.; Dobbie, I.M.; Halstead, J.M.; Hayashi, R.; Rabouille, C.; et al. Drosophila patterning is established by differential association of mRNAs with P bodies. Nat. Cell Biol. 2012, 14, 1305–1313. [Google Scholar] [CrossRef]
- Weidner, J.; Wang, C.; Prescianotto-Baschong, C.; Estrada, A.F.; Spang, A. The polysome-associated proteins Scp160 and Bfr1 prevent P body formation under normal growth conditions. J. Cell Sci. 2014, 127, 1992–2004. [Google Scholar] [CrossRef] [Green Version]
- Lang, B.D.; Li, A.-M.; Black-Brewster, H.D.; Fridovich-Keil, J.L. The brefeldin A resistance protein Bfr1p is a component of polyribosome-associated mRNP complexes in yeast. Nucleic Acids Res. 2001, 29, 2567–2574. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Banjade, S.; Cheng, H.C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483, 336–340. [Google Scholar] [CrossRef]
- Jones, N.L.; Blasutig, I.M.; Eremina, V.; Ruston, J.M.; Bladt, F.; Li, H.; Huang, H.L.; Larose, L.; Li, S.S.C.; Takano, T.; et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 2006, 440, 818–823. [Google Scholar] [CrossRef]
- Douglass, A.D.; Vale, R.D. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 2005, 121, 937–950. [Google Scholar] [CrossRef]
- Su, X.; Ditlev, J.A.; Hui, E.; Xing, W.; Banjade, S.; Okrut, J.; King, D.S.; Taunton, J.; Rosen, M.K.; Vale, R.D. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 2016, 352, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Milovanovic, D.; Wu, Y.; Bian, X.; De Camilli, P. A liquid phase of synapsin and lipid vesicles. Science 2018, 361, 604. [Google Scholar] [CrossRef]
- Gerth, F.; Jäpel, M.; Pechstein, A.; Kochlamazashvili, G.; Lehmann, M.; Puchkov, D.; Onofri, F.; Benfenati, F.; Nikonenko, A.G.; Fredrich, K.; et al. Intersectin associates with synapsin and regulates its nanoscale localization and function. Proc. Natl. Acad. Sci. USA 2017, 114, 12057. [Google Scholar] [CrossRef]
- McPherson, P.S.; Czernik, A.J.; Chilcote, T.J.; Onofri, F.; Benfenati, F.; Greengard, P.; Schlessinger, J.; De Camilli, P. Interaction of Grb2 via its Src homology 3 domains with synaptic proteins including synapsin I. Proc. Natl. Acad. Sci. USA 1994, 91, 6486. [Google Scholar] [CrossRef]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The synapsins: Key actors of synapse function and plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef]
- Milovanovic, D.; De Camilli, P. Synaptic vesicle clusters at synapses: A distinct liquid phase? Neuron 2017, 93, 995–1002. [Google Scholar] [CrossRef]
- Rai, A.K.; Chen, J.-X.; Selbach, M.; Pelkmans, L. Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 2018, 559, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Wippich, F.; Bodenmiller, B.; Trajkovska, M.G.; Wanka, S.; Aebersold, R.; Pelkmans, L. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 2013, 152, 791–805. [Google Scholar] [CrossRef]
- Gomez-Navarro, N.; Miller, E. Protein sorting at the ER-Golgi interface. J. Cell Biol. 2016, 215, 769–778. [Google Scholar] [CrossRef]
- Peotter, J.; Kasberg, W.; Pustova, I.; Audhya, A. COPII-mediated trafficking at the ER/ERGIC interface. Traffic 2019, 20, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Barlowe, C. Membrane trafficking: ER export encounters dualism. Curr. Biol. 2015, 25, R151–R153. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.A.; Schekman, R. COPII - a flexible vesicle formation system. Curr. Opin. Cell Biol. 2013, 25, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Arakel, E.C.; Schwappach, B. Formation of COPI-coated vesicles at a glance. J. Cell Sci. 2018, 131, jcs209890. [Google Scholar] [CrossRef] [Green Version]
- Béthune, J.; Wieland, F.T. Assembly of COPI and COPII vesicular coat proteins on membranes. Annu. Rev. Biophys. 2018, 47, 63–83. [Google Scholar] [CrossRef]
- Kimura, M.; Imamoto, N. Biological significance of the importin-β family-dependent nucleocytoplasmic transport pathways. Traffic 2014, 15, 727–748. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Rabouille, C. Membrane-Bound Meet Membraneless in Health and Disease. Cells 2019, 8, 1000. https://doi.org/10.3390/cells8091000
Zhang C, Rabouille C. Membrane-Bound Meet Membraneless in Health and Disease. Cells. 2019; 8(9):1000. https://doi.org/10.3390/cells8091000
Chicago/Turabian StyleZhang, Chujun, and Catherine Rabouille. 2019. "Membrane-Bound Meet Membraneless in Health and Disease" Cells 8, no. 9: 1000. https://doi.org/10.3390/cells8091000
APA StyleZhang, C., & Rabouille, C. (2019). Membrane-Bound Meet Membraneless in Health and Disease. Cells, 8(9), 1000. https://doi.org/10.3390/cells8091000




