Cellular and Synaptic Dysfunctions in Parkinson’s Disease: Stepping Out of the Striatum
Abstract
1. Introduction
2. Extra-Striatal Nuclei Neurons Share Specific Features
2.1. ESN Neurons are Fast-Spiking Autonomous Pacemakers
2.2. GABAergic Transmission Efficiently Sculpts the Activity of ESN Neurons
2.3. ESN Receive Functional Dopaminergic Innervation
3. The Globus Pallidus
3.1. Neuronal Diversity in the GP
3.2. Ionic Conductances Underlying Pacemaking in GP Neurons
3.3. Dopamine Modulation of Intrinsic Excitability of GP Neurons
3.4. Dopamine Modulation of Gabaergic Transmission in the GP
3.5. Astrocyte-Dependent Alteration of Gabaergic Inhibition in the GP
4. The Subthalamic Nucleus
4.1. Dopamine Modulation of STN Neuron Excitability
4.2. Alteration of STN Autonomous Pacemaking in DA Depleted Rodents
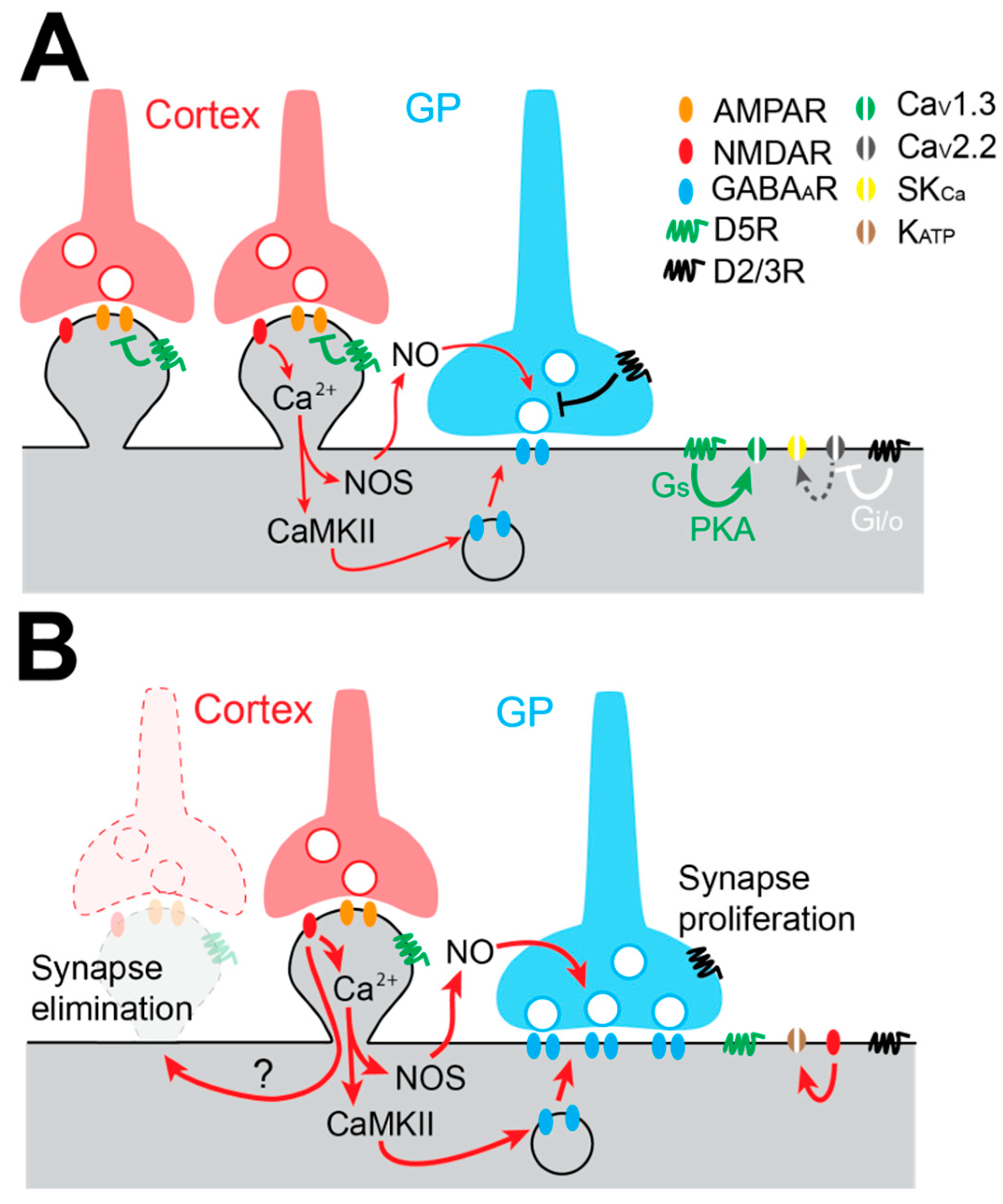
4.3. Augmentation of Pallido-Subthalamic Transmission in Experimental Parkinson’s Disease Models
4.4. Loss of the Cortico-Subthalamic Pathway in Experimental Parkinson’s Disease Models
5. The Substantia Nigra Pars Reticulata
5.1. Neuronal Diversity in the Substantia Nigra Pars Reticulata
5.2. Ionic Conductances Underlying Pacemaking in Snr Neurons
5.3. Dopamine Modulation of Intrinsic Excitability of Snr Neurons
5.4. Alteration of Gabaergic and Glutamatergic Transmission in Snr Neurons in Experimental Parkinson’s Disease Models
6. The Entopeduncular Nucleus
6.1. Anatomical Organization and Cellular Diversity in the EPN
6.2. Autonomous Pacemaking in EPN Neurons.
6.3. Dopamine Modulation of Gabaergic and Glutamatergic Transmission in the EPN
7. Consequences of Cellular and Synaptic Dysfunctions for Abnormal Neural Dynamics in the Basal Ganglia during Parkinson’s Disease
8. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Graybiel, A.M. The basal ganglia: Learning new tricks and loving it. Curr. Opin. Neurobiol. 2005, 15, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Packard, M.G.; Knowlton, B.J. Learning and Memory Functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Graves, S.M.; Shen, W. Dopaminergic modulation of striatal networks in health and Parkinson’s disease. Curr. Opin. Neurobiol. 2014, 29, 109–117. [Google Scholar] [PubMed]
- Zhai, S.; Shen, W.; Graves, S.M.; Surmeier, D.J. Dopaminergic modulation of striatal function and Parkinson’s disease. J. Neural Transm. 2019, 126, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.; Kieval, J.Z. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000, 23, S28–S33. [Google Scholar] [PubMed]
- Tepper, J.M.; Bolam, J.P. Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 2004, 14, 685–692. [Google Scholar] [PubMed]
- Tepper, J.M.; Koós, T.; Ibanez-Sandoval, O.; Tecuapetla, F.; Faust, T.W.; Assous, M. Heterogeneity and Diversity of Striatal GABAergic Interneurons: Update 2018. Front. Neuroanat. 2018, 12, 91. [Google Scholar] [CrossRef]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Freeze, B.S.; Parker, P.R.L.; Kay, K.; Thwin, M.T.; Deisseroth, K.; Kreitzer, A.C. Regulation of parkinsonian motor behaviors by optogenetic control of basal ganglia circuitry. Nature 2010, 466, 622–626. [Google Scholar] [CrossRef]
- Cui, G.; Jun, S.B.; Jin, X.; Pham, M.D.; Vogel, S.S.; Lovinger, D.M.; Costa, R.M. Concurrent Activation of Striatal Direct and Indirect Pathways During Action Initiation. Nature 2013, 494, 238–242. [Google Scholar]
- Mink, J.W. The Basal Ganglia and involuntary movements: Impaired inhibition of competing motor patterns. Arch. Neurol. 2003, 60, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A.; Tokuno, H.; Hamada, I.; Kita, H.; Imanishi, M.; Akazawa, T.; Ikeuchi, Y.; Hasegawa, N. Excitatory Cortical Inputs to Pallidal Neurons Via the Subthalamic Nucleus in the Monkey. J. Neurophysiol. 2000, 84, 289–300. [Google Scholar]
- Bevan, M. Move to the rhythm: Oscillations in the subthalamic nucleus–external globus pallidus network. Trends Neurosci. 2002, 25, 525–531. [Google Scholar] [CrossRef]
- Mallet, N.; Micklem, B.R.; Henny, P.; Brown, M.T.; Williams, C.; Bolam, J.P.; Nakamura, K.C.; Magill, P.J. Dichotomous Organization of the External Globus Pallidus. Neuron 2012, 74, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Mallet, N.; Schmidt, R.; Leventhal, D.; Chen, F.; Amer, N.; Boraud, T.; Berke, J.D. Arkypallidal cells send a Stop signal to Striatum. Neuron 2016, 89, 308–316. [Google Scholar] [PubMed]
- Cazorla, M.; De Carvalho, F.D.; Chohan, M.O.; Shegda, M.; Chuhma, N.; Rayport, S.; Ahmari, S.E.; Moore, H.; Kellendonk, C. Dopamine D2 Receptors Regulate the Anatomical and Functional Balance of Basal Ganglia Circuitry. Neuron 2014, 81, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Wilson, C.; Emson, P. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J. Neurosci. 1990, 10, 3421–3438. [Google Scholar] [CrossRef]
- Atherton, J.F.; Bevan, M.D. Ionic Mechanisms Underlying Autonomous Action Potential Generation in the Somata and Dendrites of GABAergic Substantia Nigra Pars Reticulata Neurons In Vitro. J. Neurosci. 2005, 25, 8272–8281. [Google Scholar] [CrossRef] [PubMed]
- Beurrier, C.; Bioulac, B.; Hammond, C. Slowly Inactivating Sodium Current (I NaP) Underlies Single-Spike Activity in Rat Subthalamic Neurons. J. Neurophysiol. 2000, 83, 1951–1957. [Google Scholar]
- Bevan, M.D.; Wilson, C.J. Mechanisms Underlying Spontaneous Oscillation and Rhythmic Firing in Rat Subthalamic Neurons. J. Neurosci. 1999, 19, 7617–7628. [Google Scholar]
- Chan, C.S.; Shigemoto, R.; Mercer, J.N.; Surmeier, D.J. HCN2 and HCN1 Channels Govern the Regularity of Autonomous Pacemaking and Synaptic Resetting in Globus Pallidus Neurons. J. Neurosci. 2004, 24, 9921–9932. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, G.; Tkatch, T.; Nagata, K.; Yeh, J.Z.; Surmeier, D.J. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat. Neurosci. 2003, 6, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, M.A.; Lacey, M.G. A Kv3-like persistent, outwardly rectifying, Cs+-permeable, K+ current in rat subthalamic nucleus neurones. J. Physiol. 2000, 527, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Nisenbaum, E.; Wilson, C. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 1995, 15, 4449–4463. [Google Scholar] [CrossRef] [PubMed]
- Nisenbaum, E.S.; Xu, Z.C.; Wilson, C.J. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J. Neurophysiol. 1994, 71, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Pidoux, M.; Mahon, S.; Deniau, J.M.; Charpier, S. Integration and propagation of somatosensory responses in the corticostriatal pathway: An intracellular study in vivo. J. Physiol. 2011, 589, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, J.L.; Day, M.; Surmeier, D.J. Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nat. Neurosci. 2011, 14, 881–888. [Google Scholar] [PubMed]
- Reig, R.; Silberberg, G. Multisensory Integration in the Mouse Striatum. Neuron 2014, 83, 1200–1212. [Google Scholar] [CrossRef]
- Sippy, T.; Lapray, D.; Crochet, S.; Petersen, C.C. Cell-Type-Specific Sensorimotor Processing in Striatal Projection Neurons during Goal-Directed Behavior. Neuron 2015, 88, 298–305. [Google Scholar]
- Surmeier, D.J.; Mercer, J.N.; Chan, C.S. Autonomous pacemakers in the basal ganglia: Who needs excitatory synapses anyway? Curr. Opin. Neurobiol. 2005, 15, 312–318. [Google Scholar] [CrossRef]
- Bevan, M.D.; Magill, P.J.; Hallworth, N.E.; Bolam, J.P.; Wilson, C.J. Regulation of the Timing and Pattern of Action Potential Generation in Rat Subthalamic Neurons In Vitro by GABA-A IPSPs. J. Neurophysiol. 2002, 87, 1348–1362. [Google Scholar] [CrossRef] [PubMed]
- Farries, M.A.; Wilson, C.J. Phase response curves of subthalamic neurons measured with synaptic input and current injection. J. Neurophysiol. 2012, 108, 1822–1837. [Google Scholar] [PubMed]
- Wilson, C.J. Active Decorrelation in the Basal Ganglia. Neuroscience 2013, 250, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.D.; Hallworth, N.E.; Baufreton, J. GABAergic control of the subthalamic nucleus. Prog. Brain Res. 2007, 160, 173–188. [Google Scholar] [PubMed]
- Chan, C.S.; Surmeier, D.J.; Yung, W.-H. Striatal Information Signaling and Integration in Globus Pallidus: Timing Matters. Neurosignals 2005, 14, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.D.; Wilson, C.J.; Bolam, J.P.; Magill, P.J. Equilibrium potential of GABA(A) current and implications for rebound burst firing in rat subthalamic neurons in vitro. J. Neurophysiol. 2000, 83, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Hallworth, N.E.; Bevan, M.D. Globus Pallidus Neurons Dynamically Regulate the Activity Pattern of Subthalamic Nucleus Neurons through the Frequency-Dependent Activation of Postsynaptic GABAA and GABAB Receptors. J. Neurosci. 2005, 25, 6304–6315. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kita, H. Short-term plasticity shapes activity pattern-dependent striato-pallidal synaptic transmission. J. Neurophysiol. 2013, 109, 932–939. [Google Scholar] [CrossRef]
- Matsuda, W.; Furuta, T.; Nakamura, K.C.; Hioki, H.; Fujiyama, F.; Arai, R.; Kaneko, T. Single Nigrostriatal Dopaminergic Neurons Form Widely Spread and Highly Dense Axonal Arborizations in the Neostriatum. J. Neurosci. 2009, 29, 444–453. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Carrillo-Reid, L.; Bargas, J. Dopaminergic modulation of striatal neurons, circuits and assemblies. Neuroscience 2011, 198, 3–18. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Plotkin, J.; Shen, W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr. Opin. Neurobiol. 2009, 19, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Cossette, M.; Levesque, M.; Parent, A. Extrastriatal dopaminergic innervation of human basal ganglia. Neurosci. Res. 1999, 34, 51–54. [Google Scholar] [CrossRef]
- Cragg, S.J.; Baufreton, J.; Xue, Y.; Bolam, J.P.; Bevan, M.D. Synaptic release of dopamine in the subthalamic nucleus. Eur. J. Neurosci. 2004, 20, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Debeir, T.; Ginestet, L.; François, C.; Laurens, S.; Martel, J.-C.; Chopin, P.; Marien, M.; Colpaert, F.; Raisman-Vozari, R. Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp. Neurol. 2005, 193, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Hassani, O.-K.; François, C.; Yelnik, J.; Féger, J. Evidence for a dopaminergic innervation of the subthalamic nucleus in the rat. Brain Res. 1997, 749, 88–94. [Google Scholar] [CrossRef]
- Rommelfanger, K.S.; Wichmann, T. Extrastriatal Dopaminergic Circuits of the Basal Ganglia. Front. Neuroanat. 2010, 4, 139. [Google Scholar] [CrossRef]
- Cáceres-Chávez, V.A.; Hernández-Martinez, R.; Pérez-Ortega, J.; Herrera-Valdez, M.A.; Aceves, J.J.; Galarraga, E.; Bargas, J. Acute dopamine receptor blockade in substantia nigra pars reticulata: A possible model for drug-induced Parkinsonism. J. Neurophysiol. 2018, 120, 2922–2938. [Google Scholar] [CrossRef]
- Hassani, O.-K.; Féger, J. Effects of intrasubthalamic injection of dopamine receptor agonists on subthalamic neurons in normal and 6-hydroxydopamine-lesioned rats: An electrophysiological and c-Fos study. Neuroscience 1999, 92, 533–543. [Google Scholar] [CrossRef]
- Mamad, O.; Delaville, C.; Benjelloun, W.; Benazzouz, A. Dopaminergic Control of the Globus Pallidus through Activation of D2 Receptors and Its Impact on the Electrical Activity of Subthalamic Nucleus and Substantia Nigra Reticulata Neurons. PLOS ONE 2015, 10, e0119152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-C.; Xue, Y.; Diao, H.-L.; Chen, H.; Liu, H.-Y.; Han, X.-H.; Chen, L. Direct modulation of firing activity by dopamine D2 like receptors in the globus pallidus of both normal and parkinsonian rats. Sheng li xue bao [Acta Physiol. Sin.] 2016, 68, 699–707. [Google Scholar]
- Cooper, A.; Stanford, I. Calbindin D-28k positive projection neurones and calretinin positive interneurones of the rat globus pallidus. Brain Res. 2002, 929, 243–251. [Google Scholar] [CrossRef]
- Cooper, A.J.; Stanford, I.M. Electrophysiological and morphological characteristics of three subtypes of rat globus pallidus neurone in vitro. J. Physiol. 2000, 527, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Günay, C.; Edgerton, J.R.; Jaeger, D. Channel Density Distributions Explain Spiking Variability in the Globus Pallidus: A Combined Physiology and Computer Simulation Database Approach. J. Neurosci. 2008, 28, 7476–7491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoover, B.; Marshall, J. Further characterization of preproenkephalin mRNA-containing cells in the rodent globus pallidus. Neuroscience 2002, 111, 111–125. [Google Scholar] [CrossRef]
- Hoover, B.R.; Marshall, J.F. Molecular, chemical, and anatomical characterization of globus pallidus dopamine D2 receptor mRNA-containing neurons. Synapse 2004, 52, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Kita, H.; Kitai, S. Intracellular study of rat globus pallidus neurons: Membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res. 1991, 564, 296–305. [Google Scholar] [CrossRef]
- Bugaysen, J.; Bronfeld, M.; Tischler, H.; Bar-Gad, I.; Korngreen, A. Electrophysiological Characteristics of Globus Pallidus Neurons. PLOS ONE 2010, 5, e12001. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega-Pereira, S.; Gelman, D.; Bartolini, G.; Pla, R.; Pierani, A.; Marín, O. Origin and Molecular Specification of Globus Pallidus Neurons. J. Neurosci. 2010, 30, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; De Rivera, H.; Bien, E.; Baum, M.; Bortolin, L.; Wang, S.; et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 2018, 174, 1015–1030. [Google Scholar] [CrossRef]
- Assaf, F.; Schiller, Y. A chemogenetic approach for treating experimental Parkinson’s disease. Mov. Disord. 2019, 34, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Mastro, K.J.; Zitelli, K.T.; Willard, A.M.; Leblanc, K.H.; Kravitz, A.V.; Gittis, A.H. Cell-Specific Pallidal Intervention Induces Long-Lasting Motor Recovery in Dopamine Depleted Mice. Nat. Neurosci. 2017, 20, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yan, Y.; Xi, W.; Zhou, R.; Lou, H.; Duan, S.; Chen, J.F.; Zhang, B. Optogenetic Stimulation of GABAergic Neurons in the Globus Pallidus Produces Hyperkinesia. Front. Behav. Neurosci. 2018, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Mallet, N.; Mohamed, F.Y.; Sharott, A.; Dodson, P.D.; Nakamura, K.C.; Suri, S.; Avery, S.V.; Larvin, J.T.; Garas, F.N.; et al. Prototypic and Arkypallidal Neurons in the Dopamine-Intact External Globus Pallidus. J. Neurosci. 2015, 35, 6667–6688. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, K.P.; Lovinger, D.M. Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice. J. Physiol. 2018, 596, 4219–4235. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, V.M.; Hegeman, D.J.; Cui, Q.; Kelver, D.A.; Fiske, M.P.; Glajch, K.E.; Pitt, J.E.; Huang, T.Y.; Justice, N.J.; Chan, C.S. Parvalbumin+ Neurons and Npas1+ Neurons Are Distinct Neuron Classes in the Mouse External Globus Pallidus. J. Neurosci. 2015, 35, 11830–11847. [Google Scholar] [CrossRef] [PubMed]
- Mastro, K.J.; Bouchard, R.S.; Holt, H.A.K.; Gittis, A.H. Transgenic Mouse Lines Subdivide External Segment of the Globus Pallidus (GPe) Neurons and Reveal Distinct GPe Output Pathways. J. Neurosci. 2014, 34, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Hegeman, D.J.; Hong, E.S.; Hernández, V.M.; Chan, C.S. The External Globus Pallidus: Progress and Perspectives. Eur. J. Neurosci. 2016, 43, 1239–1265. [Google Scholar] [PubMed]
- Fujiyama, F.; Nakano, T.; Matsuda, W.; Furuta, T.; Udagawa, J.; Kaneko, T. A single-neuron tracing study of arkypallidal and prototypic neurons in healthy rats. Brain Struct. Funct. 2016, 221, 4733–4740. [Google Scholar] [PubMed]
- Saunders, A.; Huang, K.W.; Sabatini, B.L. Globus Pallidus Externus Neurons Expressing parvalbumin Interconnect the Subthalamic Nucleus and Striatal Interneurons. PLOS ONE 2016, 11, e0149798. [Google Scholar] [CrossRef] [PubMed]
- Dodson, P.D.; Larvin, J.T.; Duffell, J.M.; Garas, F.N.; Doig, N.M.; Kessaris, N.; Duguid, I.C.; Bogacz, R.; Butt, S.J.; Magill, P.J. Distinct Developmental Origins Manifest in the Specialized Encoding of Movement by Adult Neurons of the External Globus Pallidus. Neuron 2015, 86, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Mallet, N.; Pogosyan, A.; Márton, L.F.; Bolam, J.P.; Brown, P.; Magill, P.J. Parkinsonian Beta Oscillations in the External Globus Pallidus and Their Relationship with Subthalamic Nucleus Activity. J. Neurosci. 2008, 28, 14245–14258. [Google Scholar] [CrossRef] [PubMed]
- Kita, H.; Kita, T. Role of Striatum in the Pause and Burst Generation in the Globus Pallidus of 6-OHDA-Treated Rats. Front. Syst. Neurosci. 2011, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Sharott, A.; Vinciati, F.; Nakamura, K.C.; Magill, P.J. A Population of Indirect Pathway Striatal Projection Neurons Is Selectively Entrained to Parkinsonian Beta Oscillations. J. Neurosci. 2017, 37, 9977–9998. [Google Scholar]
- Zold, C.L.; Ballion, B.; Riquelme, L.A.; Gonon, F.; Murer, M.G. Nigrostriatal lesion induces D2-modulated phase-locked activity in the basal ganglia of rats. Eur. J. Neurosci. 2007, 25, 2131–2144. [Google Scholar] [PubMed]
- Yuan, X.S.; Wang, L.; Dong, H.; Qu, W.M.; Yang, S.R.; Cherasse, Y.; Lazarus, M.; Schiffmann, S.N.; d’Exaerde, A.K.; Li, R.X.; et al. Striatal adenosine A2A receptor neurons control active-period sleep via parvalbumin neurons in external globus pallidus. ELife 2017, 6, e29055. [Google Scholar] [CrossRef] [PubMed]
- Filion, M.; Tremblay, L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991, 547, 140–144. [Google Scholar]
- Raz, A.; Frechter-Mazar, V.; Feingold, A.; Abeles, M.; Vaadia, E.; Bergman, H. Activity of Pallidal and Striatal Tonically Active Neurons Is Correlated in MPTP-Treated Monkeys But Not in Normal Monkeys. J. Neurosci. 2001, 21, RC128. [Google Scholar] [CrossRef]
- Raz, A.; Vaadia, E.; Bergman, H. Firing Patterns and Correlations of Spontaneous Discharge of Pallidal Neurons in the Normal and the Tremulous 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Vervet Model of Parkinsonism. J. Neurosci. 2000, 20, 8559–8571. [Google Scholar]
- Deister, C.A.; Dodla, R.; Barraza, D.; Kita, H.; Wilson, C.J. Firing rate and pattern heterogeneity in the globus pallidus arise from a single neuronal population. J. Neurophysiol. 2013, 109, 497–506. [Google Scholar] [CrossRef]
- Mercer, J.N.; Chan, C.S.; Tkatch, T.; Held, J.; Surmeier, D.J. Nav1.6 Sodium Channels Are Critical to Pacemaking and Fast Spiking in Globus Pallidus Neurons. J. Neurosci. 2007, 27, 13552–13566. [Google Scholar] [CrossRef]
- Deister, C.A.; Chan, C.S.; Surmeier, D.J.; Wilson, C.J. Calcium-activated SK channels influence voltage-gated ion channels to determine the precision of firing in globus pallidus neurons. J. Neurosci. 2009, 29, 8452–8461. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.Y.; Sims, J.R.; Bhide, P.G. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and post-natal development. Brain Res. 2007, 1156, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Hauber, W. Reverse microdialysis of ionotropic glutamate receptor agonists in the rat globus pallidus increased extracellular dopamine. Neurosci. Lett. 2003, 343, 37–40. [Google Scholar] [CrossRef]
- Hauber, W.; Fuchs, H. Dopamine release in the rat globus pallidus characterised by in vivo microdialysis. Behav. Brain Res. 2000, 111, 39–44. [Google Scholar] [CrossRef]
- Meszaros, J.; Cheung, T.; Erler, M.M.; Kang, U.J.; Sames, D.; Kellendonk, C.; Sulzer, D. Evoked transients of pH-sensitive fluorescent false neurotransmitter reveal dopamine hot spots in the globus pallidus. eLife 2018, 7, e42383. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Spadoni, F.; Martorana, A.; Lavaroni, F.; Martella, G.; Sancesario, G.; Bernardi, G. D2-mediated modulation of N-type calcium currents in rat globus pallidus neurons following dopamine denervation. Eur. J. Neurosci. 2002, 15, 815–825. [Google Scholar]
- Napier, T.C.; Simson, P.E.; Givens, B.S. Dopamine electrophysiology of ventral pallidal/substantia innominata neurons: Comparison with the dorsal globus pallidus. J. Pharmacol. Exp. Ther. 1991, 258, 249–262. [Google Scholar] [PubMed]
- Chan, C.S.; Glajch, K.E.; Gertler, T.S.; Guzman, J.N.; Mercer, J.N.; Lewis, A.S.; Goldberg, A.B.; Tkatch, T.; Shigemoto, R.; Fleming, S.M.; et al. HCN channelopathy in external globus pallidus neurons in models of Parkinson’s disease. Nat. Neurosci. 2011, 14, 85–92. [Google Scholar] [CrossRef]
- Glajch, K.E.; Kelver, D.A.; Hegeman, D.J.; Cui, Q.; Xenias, H.S.; Augustine, E.C.; Hernandez, V.M.; Verma, N.; Huang, T.Y.; Luo, M.; et al. Npas1+ Pallidal Neurons Target Striatal Projection Neurons. J. Neurosci. 2016, 36, 5472–5488. [Google Scholar] [CrossRef]
- Mizutani, K.; Takahashi, S.; Okamoto, S.; Karube, F.; Fujiyama, F. Substance P effects exclusively on prototypic neurons in mouse globus pallidus. Brain Struct. Funct. 2017, 222, 4089–4110. [Google Scholar] [CrossRef]
- Sadek, A.R.; Magill, P.J.; Bolam, J.P. A Single-Cell Analysis of Intrinsic Connectivity in the Rat Globus Pallidus. J. Neurosci. 2007, 27, 6352–6362. [Google Scholar] [CrossRef] [PubMed]
- Ingham, C.A.; Hood, S.H.; Mijnster, M.J.; Baldock, R.A.; Arbuthnott, G.W.; Baldock, R. Plasticity of striatopallidal terminals following unilateral lesion of the dopaminergic nigrostriatal pathway: A morphological study. Exp. Brain Res. 1997, 116, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Miguelez, C.; Morin, S.; Martinez, A.; Goillandeau, M.; Bezard, E.; Bioulac, B.; Baufreton, J. Altered pallido-pallidal synaptic transmission leads to aberrant firing of globus pallidus neurons in a rat model of Parkinson’s disease. J. Physiol. 2012, 590, 5861–5875. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.E.; Woodhall, G.L.; Wilson, C.L.; Stanford, I.M. Functional characterization of GABAergic pallidopallidal and striatopallidal synapses in the rat globus pallidus in vitro. Eur. J. Neurosci. 2008, 28, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Charara, A.; Smith, Y.; Parent, A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J. Comp. Neurol. 1996, 364, 254–266. [Google Scholar] [CrossRef]
- Chen, L.; Chan, S.C.Y.; Yung, W.H. Rotational behavior and electrophysiological effects induced by GABA(B) receptor activation in rat globus pallidus. Neuroscience 2002, 114, 417–425. [Google Scholar] [CrossRef]
- Engler, B.; Freiman, I.; Urbanski, M.; Szabo, B. Effects of exogenous and endogenous cannabinoids on GABAergic neurotransmission between the caudate-putamen and the globus pallidus in the mouse. J. Pharmacol. Exp. Ther. 2006, 316, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, K.; Kita, H. Synaptically Released GABA Activates Both Pre- and Postsynaptic GABABReceptors in the Rat Globus Pallidus. J. Neurophysiol. 2005, 94, 1104–1114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stanford, I.M.; Cooper, A.J. Presynaptic mu and delta opioid receptor modulation of GABAA IPSCs in the rat globus pallidus in vitro. J. Neurosci. 1999, 19, 4796–4803. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Kita, H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience 2003, 122, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Kita, H. Dynorphin Exerts Both Postsynaptic and Presynaptic Effects in the Globus Pallidus of the Rat. J. Neurophysiol. 2000, 83, 3366–3376. [Google Scholar] [CrossRef] [PubMed]
- Valenti, O.; Marino, M.J.; Wittmann, M.; Lis, E.; DiLella, A.G.; Kinney, G.G.; Conn, P.J. Group III Metabotropic Glutamate Receptor-Mediated Modulation of the Striatopallidal Synapse. J. Neurosci. 2003, 23, 7218–7226. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Stanford, I.M. Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacology 2001, 41, 62–71. [Google Scholar] [CrossRef]
- Bugaysen, J.; Bar-Gad, I.; Korngreen, A. Continuous Modulation of Action Potential Firing by a Unitary GABAergic Connection in the Globus Pallidus In Vitro. J. Neurosci. 2013, 33, 12805–12809. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.-M.; Masuda, M.; Miura, M.; Sano, H.; Shirasawa, T.; Song, W.-J.; Kobayashi, K.; Aosaki, T. Dopamine D4 receptor-induced postsynaptic inhibition of GABAergic currents in mouse globus pallidus neurons. J. Neurosci. 2003, 23, 11662–11672. [Google Scholar] [CrossRef] [PubMed]
- Charron, G.; Doudnikoff, E.; Canron, M.H.; Li, Q.; Vega, C.; Marais, S.; Baufreton, J.; Vital, A.; Oliet, S.H.; Bezard, E. Astrocytosis in parkinsonism: Considering tripartite striatal synapses in physiopathology? Front. Aging Neurosci. 2014, 6, 258. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Okuda, H.; Morita-Takemura, S.; Tanaka, T.; Isonishi, A.; Shinjo, T.; Terada, Y.; Wanaka, A. Voluntary Exercise Induces Astrocytic Structural Plasticity in the Globus Pallidus. Front. Cell. Neurosci. 2016, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Barres, B.A. Neuroscience: Glia—More than just brain glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Fellin, T.; Takano, H.; Dong, J.-H.; Haydon, P.G. Synaptic Islands Defined by the Territory of a Single Astrocyte. J. Neurosci. 2007, 27, 6473–6477. [Google Scholar] [CrossRef] [PubMed]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef]
- Galvan, A.; Hu, X.; Smith, Y.; Wichmann, T. Localization and function of GABA transporters in the globus pallidus of parkinsonian monkeys. Exp. Neurol. 2010, 223, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-T.; Pare, J.-F.; Smith, Y. Differential localization and function of GABA transporters, GAT-1 and GAT-3, in the rat globus pallidus. Eur. J. Neurosci. 2011, 33, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Pitt, J.E.; Pamukcu, A.; Poulin, J.-F.; Mabrouk, O.S.; Fiske, M.P.; Fan, I.B.; Augustine, E.C.; Young, K.A.; Kennedy, R.T.; et al. Blunted mGluR Activation Disinhibits Striatopallidal Transmission in Parkinsonian Mice. Cell Rep. 2016, 17, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Chazalon, M.; Paredes-Rodriguez, E.; Morin, S.; Martinez, A.; Cristóvão-Ferreira, S.; Vaz, S.; Sebastião, A.; Panatier, A.; Boué-Grabot, E.; Miguelez, C.; et al. GAT-3 Dysfunction Generates Tonic Inhibition in External Globus Pallidus Neurons in Parkinsonian Rodents. Cell Rep. 2018, 23, 1678–1690. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Koga, K.; Kurokawa, M.; Kase, H.; Nakamura, J.; Kuwana, Y. Systemic administration of adenosine A2A receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Neuroscience 2000, 100, 53–62. [Google Scholar] [CrossRef]
- Ramanathan, S.; Tkatch, T.; Atherton, J.F.; Wilson, C.J.; Bevan, M.D. D2-Like Dopamine Receptors Modulate SKCaChannel Function in Subthalamic Nucleus Neurons Through Inhibition of Cav2.2 Channels. J. Neurophysiol. 2008, 99, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Le Moine, C. Dopamine D1/5 receptor stimulation induces c- fos expression in the subthalamic nucleus: Possible involvement of local D5 receptors. Eur. J. Neurosci. 2002, 15, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Baufreton, J.; Zhu, Z.-T.; Garret, M.; Bioulac, B.; Johnson, S.W.; Taupignon, A.I. Dopamine receptors set the pattern of activity generated in subthalamic neurons. FASEB J. 2005, 19, 1771–1777. [Google Scholar] [CrossRef]
- Beurrier, C.; Congar, P.; Bioulac, B.; Hammond, C. Subthalamic Nucleus Neurons Switch from Single-Spike Activity to Burst-Firing Mode. J. Neurosci. 1999, 19, 599–609. [Google Scholar] [CrossRef]
- Baufreton, J.; Garret, M.; Rivera, A.; De La Calle, A.; Gonon, F.; Dufy, B.; Bioulac, B.; Taupignon, A. D5 (Not D1) Dopamine Receptors Potentiate Burst-Firing in Neurons of the Subthalamic Nucleus by Modulating an L-Type Calcium Conductance. J. Neurosci. 2003, 23, 816–825. [Google Scholar] [CrossRef]
- Loucif, K.C.; Wilson, C.L.; Baig, R.; Lacey, M.G.; Stanford, I.M. Functional interconnectivity between the globus pallidus and the subthalamic nucleus in the mouse brain slice. J. Physiol. 2005, 567, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Hutchison, W.D.; Lozano, A.M.; Dostrovsky, J.O. High-frequency Synchronization of Neuronal Activity in the Subthalamic Nucleus of Parkinsonian Patients with Limb Tremor. J. Neurosci. 2000, 20, 7766–7775. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Cash, D.; Galley, K.; Chapman, H.; Lacey, M.; Stanford, I.M. Subthalamic nucleus neurones in slices from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mice show irregular, dopamine-reversible firing pattern changes, but without synchronous activity. Neuroscience 2006, 143, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bartol, M.; Shen, K.; Johnson, S.W. Excitatory effects of dopamine on subthalamic nucleus neurons: In vitro study of rats pretreated with 6-hydroxydopamine and levodopa. Brain Res. 2002, 945, 31–40. [Google Scholar] [CrossRef]
- McIver, E.L.; Atherton, J.F.; Chu, H.-Y.; Cosgrove, K.E.; Kondapalli, J.; Wokosin, D.; Surmeier, D.J.; Bevan, M.D. Maladaptive Downregulation of Autonomous Subthalamic Nucleus Activity following the Loss of Midbrain Dopamine Neurons. Cell Rep. 2019, 28, 992–1002.e4. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-Z.; Johnson, S.W. Chronic dopamine depletion augments the functional expression of K-ATP channels in the rat subthalamic nucleus. Neurosci. Lett. 2012, 531, 104–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, C.; Zhang, J.-R.; Chen, L.; Ge, S.-N.; Wang, J.-L.; Yan, Z.-Q.; Jia, D.; Zhu, J.-L.; Gao, G.-D. Decreased HCN2 expression in STN contributes to abnormal high-voltage spindles in the cortex and globus pallidus of freely moving rats. Brain Res. 2015, 1618, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Baufreton, J.; Kirkham, E.; Atherton, J.F.; Menard, A.; Magill, P.J.; Bolam, J.P.; Bevan, M.D. Sparse but Selective and Potent Synaptic Transmission From the Globus Pallidus to the Subthalamic Nucleus. J. Neurophysiol. 2009, 102, 532–545. [Google Scholar] [CrossRef]
- Urbain, N.; Gervasoni, D.; Soulière, F.; Lobo, L.; Rentero, N.; Windels, F.; Astier, B.; Savasta, M.; Fort, P.; Renaud, B.; et al. Unrelated course of subthalamic nucleus and globus pallidus neuronal activities across vigilance states in the rat. Eur. J. Neurosci. 2000, 12, 3361–3374. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.F.; Menard, A.; Urbain, N.; Bevan, M.D. Short-term depression of external globus pallidus-subthalamic nucleus synaptic transmission and implications for patterning subthalamic activity. J. Neurosci. 2013, 33, 7130–7144. [Google Scholar] [CrossRef] [PubMed]
- Baufreton, J.; Bevan, M.D. D2-like dopamine receptor-mediated modulation of activity-dependent plasticity at GABAergic synapses in the subthalamic nucleus. J. Physiol. 2008, 586, 2121–2142. [Google Scholar] [PubMed]
- Abbott, L.F.; Regehr, W.G. Synaptic computation. Nature 2004, 431, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.Y.; Baufreton, J.; Surmeier, D.J.; Chan, C.S.; Bevan, M.D. Proliferation of external globus pallidus-subthalamic nucleus synapses following degeneration of midbrain dopamine neurons. J. Neurosci. 2012, 32, 13718–13728. [Google Scholar] [PubMed]
- Chu, H.-Y.; Atherton, J.F.; Wokosin, D.; Surmeier, D.J.; Bevan, M.D. Heterosynaptic Regulation of External Globus Pallidus Inputs to the Subthalamic Nucleus by the Motor Cortex. Neuron 2015, 85, 364–376. [Google Scholar] [CrossRef]
- Bevan, M.D.; Francis, C.M.; Bolam, J.P. The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat: Convergence with GABA-positive terminals. J. Comp. Neurol. 1995, 361, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Mathai, A.; Smith, Y. The Corticostriatal and Corticosubthalamic Pathways: Two Entries, One Target. So What? Front. Syst. Neurosci. 2011, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Froux, L.; Le Bon-Jego, M.; Miguelez, C.; Normand, E.; Morin, S.; Fioramonti, S.; Barresi, M.; Frick, A.; Baufreton, J.; Taupignon, A. D5 dopamine receptors control glutamatergic AMPA transmission between the motor cortex and subthalamic nucleus. Sci. Rep. 2018, 8, 8858. [Google Scholar] [CrossRef]
- Shen, K.-Z.; Johnson, S.W. Presynaptic dopamine D2 and muscarine M3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. J. Physiol. 2000, 525, 331–341. [Google Scholar]
- Chu, H.-Y.; McIver, E.L.; Kovaleski, R.F.; Atherton, J.F.; Bevan, M.D. Loss Of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration Of Midbrain Dopamine Neurons. Neuron 2017, 95, 1306–1318.e5. [Google Scholar] [CrossRef]
- Mathai, A.; Ma, Y.; Paré, J.-F.; Villalba, R.M.; Wichmann, T.; Smith, Y. Reduced cortical innervation of the subthalamic nucleus in MPTP-treated parkinsonian monkeys. Brain 2015, 138, 946–962. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, Y.; Jiang, H.-F.; Liu, J.-H.; Jia, J.; Wang, K.; Zhao, F.; Luo, M.-H.; Luo, M.-M.; Wang, X.-M. Impaired glutamatergic projection from the motor cortex to the subthalamic nucleus in 6-hydroxydopamine-lesioned hemi-parkinsonian rats. Exp. Neurol. 2018, 300, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, V.; Mogri, M.; Thompson, K.R.; Henderson, J.M.; Deisseroth, K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science 2009, 324, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.H.; Jaeger, D. Optogenetic Stimulation Of Cortico-Subthalamic Projections Is Sufficient To Ameliorate Bradykinesia In 6-Ohda Lesioned Mice. Neurobiol. Dis. 2016, 95, 225–237. [Google Scholar] [PubMed]
- Kha, H.T.; Finkelstein, D.I.; Tomas, D.; Drago, J.; Pow, D.V.; Horne, M.K. Projections from the substantia nigra pars reticulata to the motor thalamus of the rat: Single axon reconstructions and immunohistochemical study. J. Comp. Neurol. 2001, 440, 20–30. [Google Scholar] [PubMed]
- Cebrián, C.; Parent, A.; Prensa, L. Patterns of axonal branching of neurons of the substantia nigra pars reticulata and pars lateralis in the rat. J. Comp. Neurol. 2005, 492, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, O. GABAergic output of the basal ganglia. Prog. Brain Res. 2007, 160, 209–226. [Google Scholar] [PubMed]
- Deniau, J.; Mailly, P.; Maurice, N.; Charpier, S. The pars reticulata of the substantia nigra: A window to basal ganglia output. Prog. Brain Res. 2007, 160, 151–172. [Google Scholar]
- Brown, J.; Pan, W.-X.; Dudman, J.T. The inhibitory microcircuit of the substantia nigra provides feedback gain control of the basal ganglia output. eLife 2014, 3, e02397. [Google Scholar] [CrossRef]
- Higgs, M.H.; Wilson, C.J. Unitary synaptic connections among substantia nigra pars reticulata neurons. J. Neurophysiol. 2016, 115, 2814–2829. [Google Scholar]
- González-Hernández, T.; Rodríguez, M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J. Comp. Neurol. 2000, 421, 107–135. [Google Scholar] [CrossRef]
- Rajakumar, N.; Elisevich, K.; Flumerfelt, B.A. Parvalbumin-containing GABAergic neurons in the basal ganglia output system of the rat. J. Comp. Neurol. 1994, 350, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Anderson, K.D. Co-occurrence of gamma-aminobutyric acid, parvalbumin and the neurotensin-related neuropeptide LANT6 in pallidal, nigral and striatal neurons in pigeons and monkeys. Brain Res. 1993, 624, 317–325. [Google Scholar] [CrossRef]
- Liang, C.-L.; Sinton, C.; German, D. Midbrain dopaminergic neurons in the mouse: Co-localization with Calbindin-D28k and calretinin. Neuroscience 1996, 75, 523–533. [Google Scholar] [CrossRef]
- McRitchie, D.; Hardman, C.; Halliday, G.; Halliday, G. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. J. Comp. Neurol. 1996, 364, 121–150. [Google Scholar] [CrossRef]
- Martïanez-Murillo, R.; Villalba, R.; Montero-Caballero, M.I.; Rodrigo, J. Cholinergic somata and terminals in the rat substantia nigra: An immunocytochemical study with optical and electron microscopic techniques. J. Comp. Neurol. 1989, 281, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.; Yung, K.K. Distinct cellular distribution of GABA(B)R1 and GABA(A)alpha1 receptor immunoreactivity in the rat substantia nigra. Neuroscience 2000, 99, 65–76. [Google Scholar] [CrossRef]
- Ng, T.K.; Yung, K.K. Subpopulations of neurons in rat substantia nigra display GABA(B)R2 receptor immunoreactivity. Brain Res. 2001, 920, 210–216. [Google Scholar] [CrossRef]
- Amadio, S.; Montilli, C.; Picconi, B.; Calabresi, P.; Volonté, C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: An immunohistological study. Purinergic Signal. 2007, 3, 389–398. [Google Scholar] [CrossRef]
- Rodríguez, M.; González-Hernández, T. Electrophysiological and Morphological Evidence for a GABAergic Nigrostriatal Pathway. J. Neurosci. 1999, 19, 4682–4694. [Google Scholar]
- Lee, C.R.; Tepper, J.M. Morphological and physiological properties of parvalbumin- and calretinin-containing gamma-aminobutyric acidergic neurons in the substantia nigra. J. Comp. Neurol. 2007, 500, 958–972. [Google Scholar] [CrossRef]
- Barter, J.W.; Castro, S.; Sukharnikova, T.; Rossi, M.A.; Yin, H.H. The role of the substantia nigra in posture control. Eur. J. Neurosci. 2014, 39, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Barter, J.W.; Li, S.; Sukharnikova, T.; Rossi, M.A.; Bartholomew, R.A.; Yin, H.H. Basal Ganglia Outputs Map Instantaneous Position Coordinates during Behavior. J. Neurosci. 2015, 35, 2703–2716. [Google Scholar] [CrossRef] [PubMed]
- Bodor, Á.L.; Giber, K.; Rovó, Z.; Ulbert, I.; Acsády, L. Structural Correlates of Efficient GABAergic Transmission in the Basal Ganglia-Thalamus Pathway. J. Neurosci. 2008, 28, 3090–3102. [Google Scholar] [CrossRef] [PubMed]
- Kase, D.; Uta, D.; Ishihara, H.; Imoto, K. Inhibitory synaptic transmission from the substantia nigra pars reticulata to the ventral medial thalamus in mice. Neurosci. Res. 2015, 97, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, E.; Fujiyama, F.; Nakamura, K.C.; Tanaka, Y.; Hioki, H.; Kaneko, T. Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur. J. Neurosci. 2011, 33, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Lutas, A.; Birnbaumer, L.; Yellen, G. Metabolism Regulates the Spontaneous Firing of Substantia Nigra Pars Reticulata Neurons via KATP and Nonselective Cation Channels. J. Neurosci. 2014, 34, 16336–16347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-W.; Matta, S.G.; Zhou, F.-M. Constitutively Active TRPC3 Channels Regulate Basal Ganglia Output Neurons. J. Neurosci. 2008, 28, 473–482. [Google Scholar] [CrossRef]
- Lutas, A.; Lahmann, C.; Soumillon, M.; Yellen, G. The leak channel NALCN controls tonic firing and glycolytic sensitivity of substantia nigra pars reticulata neurons. eLife 2016, 5, e15271. [Google Scholar] [CrossRef]
- Ding, S.; Matta, S.G.; Zhou, F.M. Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. J. Neurophysiol. 2011, 105, 554–570. [Google Scholar] [CrossRef]
- Zhou, F.-M.; Lee, C.R. Intrinsic and integrative properties of substantia nigra pars reticulata neurons. Neuroscience 2011, 198, 69–94. [Google Scholar] [CrossRef]
- Rice, M.E.; Patel, J.C. Somatodendritic dopamine release: Recent mechanistic insights. Philos. Trans. R. Soc. B: Boil. Sci. 2015, 370, 20140185. [Google Scholar] [CrossRef] [PubMed]
- Ciliax, B.J.; Nash, N.; Heilman, C.; Sunahara, R.; Hartney, A.; Tiberi, M.; Rye, D.B.; Caron, M.G.; Niznik, H.B.; Levey, A.I. Dopamine D5 receptor immunolocalization in rat and monkey brain. Synapse 2000, 37, 125–145. [Google Scholar] [CrossRef]
- Khan, Z.; Gutierrez, A.; Martín, R.; Penafiel, A.; Rivera, A.; De La Calle, A.; Khan, Z. Dopamine D5 receptors of rat and human brain. Neuroscience 2000, 100, 689–699. [Google Scholar] [CrossRef]
- Kliem, M.A.; Pare, J.-F.; Khan, Z.U.; Wichmann, T.; Smith, Y. Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur. J. Neurosci. 2010, 31, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Mrzljak, L.; Bergson, C.; Pappy, M.; Huff, R.; Levenson, R.; Goldman-Rakic, P.S. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 1996, 381, 245–248. [Google Scholar] [CrossRef]
- Rivera, A.; Trías, S.; Peñafiel, A.; Narváez, J.A.; Díaz-Cabiale, Z.; Moratalla, R.; De La Calle, A. Expression of D4 dopamine receptors in striatonigral and striatopallidal neurons in the rat striatum. Brain Res. 2003, 989, 35–41. [Google Scholar] [CrossRef][Green Version]
- Nagatomo, K.; Suga, S.; Saitoh, M.; Kogawa, M.; Kobayashi, K.; Yamamoto, Y.; Yamada, K. Dopamine D1 Receptor Immunoreactivity on Fine Processes of GFAP-Positive Astrocytes in the Substantia Nigra Pars Reticulata of Adult Mouse. Front. Neuroanat. 2017, 11, 182. [Google Scholar] [CrossRef]
- Zhou, F.-W.; Jin, Y.; Matta, S.G.; Xu, M.; Zhou, F.-M. An ultra-short dopamine pathway regulates basal ganglia output. J. Neurosci. 2009, 29, 10424–10435. [Google Scholar] [CrossRef]
- Lobb, C.; Jaeger, D. Bursting activity of substantia nigra pars reticulata neurons in mouse parkinsonism in awake and anesthetized states. Neurobiol. Dis. 2015, 75, 177–185. [Google Scholar] [CrossRef][Green Version]
- Seeger-Armbruster, S.; von Ameln-Mayerhofer, A. Short- and long-term unilateral 6-hydroxydopamine lesions in rats show different changes in characteristics of spontaneous firing of substantia nigra pars reticulata neurons. Exp. Brain Res. 2013, 224, 15–24. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.J.; Liu, J.; Ali, U.; Gui, Z.H.; Hui, Y.P.; Chen, L.; Wang, T. Changes in firing rate and pattern of GABAergic neurons in subregions of the substantia nigra pars reticulata in rat models of Parkinson’s disease. Brain Res. 2010, 1324, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, T.; Soares, J. Neuronal Firing Before and After Burst Discharges in the Monkey Basal Ganglia Is Predictably Patterned in the Normal State and Altered in Parkinsonism. J. Neurophysiol. 2006, 95, 2120–2133. [Google Scholar] [CrossRef]
- Willard, A.M.; Isett, B.R.; Whalen, T.C.; Mastro, K.J.; Ki, C.S.; Mao, X.; Gittis, A.H. State transitions in the substantia nigra reticulata predict the onset of motor deficits in models of progressive dopamine depletion in mice. eLife 2019, 8, e42746. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.; Bevan, M.D.; Shink, E.; Bolam, J.P. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 1998, 86, 353–387. [Google Scholar] [PubMed]
- Bolam, J.P.; Smith, Y. The striatum and the globus pallidus send convergent synaptic inputs onto single cells in the entopeduncular nucleus of the rat: A double anterograde labelling study combined with postembedding immunocytochemistry for GABA. J. Comp. Neurol. 1992, 321, 456–476. [Google Scholar] [CrossRef] [PubMed]
- Von Krosigk, M.; Smith, Y.; Bolam, J.P.; Smith, A. Synaptic organization of gabaergic inputs from the striatum and the globus pallidus onto neurons in the substantia nigra and retrorubral field which project to the medullary reticular formation. Neuroscience 1992, 50, 531–549. [Google Scholar] [CrossRef]
- Bolam, J.P.; Smith, Y.; Ingham, C.A.; von Krosigk, M.; Smith, A.D. Convergence of synaptic terminals from the striatum and the globus pallidus onto single neurones in the substantia nigra and the entopeduncular nucleus. Prog. Brain. Res. 1993, 99, 73–88. [Google Scholar]
- Connelly, W.M.; Schulz, J.M.; Lees, G.; Reynolds, J.N.J. Differential Short-Term Plasticity at Convergent Inhibitory Synapses to the Substantia Nigra Pars Reticulata. J. Neurosci. 2010, 30, 14854–14861. [Google Scholar] [CrossRef]
- Galarraga, E.; Aceves, J.J.; Rueda-Orozco, P.E.; Hernandez-Martinez, R.; Bargas, J. Bidirectional plasticity in striatonigral synapses: A switch to balance direct and indirect basal ganglia pathways. Learn. Mem. 2011, 18, 764–773. [Google Scholar]
- Erlij, D.; Acosta-García, J.; Rojas-Márquez, M.; González-Hernández, B.; Escartín-Perez, E.; Aceves, J.; Florán, B. Dopamine D4 receptor stimulation in GABAergic projections of the globus pallidus to the reticular thalamic nucleus and the substantia nigra reticulata of the rat decreases locomotor activity. Neuropharmacology 2012, 62, 1111–1118. [Google Scholar] [CrossRef]
- Acosta-García, J.; Hernández-Chan, N.; Paz-Bermúdez, F.; Sierra, A.; Erlij, D.; Aceves, J.; Florán, B. D4 and D1 dopamine receptors modulate [3H]GABA release in the substantia nigra pars reticulata of the rat. Neuropharmacology 2009, 57, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Chuhma, N.; Tanaka, K.F.; Hen, R.; Rayport, S. Functional Connectome of the Striatal Medium-Spiny Neuron. J. Neurosci. 2011, 31, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Radnikow, G.; Misgeld, U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J. Neurosci. 1998, 18, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Borgkvist, A.; Avegno, E.M.; Wong, M.Y.; Kheirbek, M.A.; Sonders, M.S.; Hen, R.; Sulzer, D. Loss of Striatonigral GABAergic Presynaptic Inhibition Enables Motor Sensitization in Parkinsonian Mice. Neuron 2015, 87, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.P.; Feyder, M.; Miguelez, C.; Garcia, L.; Morin, S.; Choquet, D.; Hosy, E.; Bezard, E.; Fisone, G.; Bioulac, B.H.; et al. Dopamine-Dependent Long-Term Depression at Subthalamo-Nigral Synapses Is Lost in Experimental Parkinsonism. J. Neurosci. 2013, 33, 14331–14341. [Google Scholar] [CrossRef] [PubMed]
- Ibanez-Sandoval, O.; Hernández, A.; Florán, B.; Galarraga, E.; Tapia, D.; Valdiosera, R.; Erlij, D.; Aceves, J.; Bargas, J.; Hernandez-Cortes, A. Control of the Subthalamic Innervation of Substantia Nigra Pars Reticulata by D1and D2Dopamine Receptors. J. Neurophysiol. 2006, 95, 1800–1811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ammari, R.; Lopez, C.; Bioulac, B.; Garcia, L.; Hammond, C. Subthalamic nucleus evokes similar long lasting glutamatergic excitations in pallidal, entopeduncular and nigral neurons in the basal ganglia slice. Neuroscience 2010, 166, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-Z.; Johnson, S.W. Subthalamic stimulation evokes complex EPSCs in the rat substantia nigra pars reticulata in vitro. J. Physiol. 2006, 573, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Gouty-Colomer, L.A.; Michel, F.J.; Baude, A.; Lopez-Pauchet, C.; Dufour, A.; Cossart, R.; Hammond, C. Mouse subthalamic nucleus neurons with local axon collaterals. J. Comp. Neurol. 2018, 526, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-Z.; Johnson, S.W. Regulation of polysynaptic subthalamonigral transmission by D2, D3 and D4 dopamine receptors in rat brain slices. J. Physiol. 2012, 590, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.P.; Bioulac, B.H.; Baufreton, J. Long-term depression at distinct glutamatergic synapses in the basal ganglia. Rev. Neurosci. 2014, 25, 741–754. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Fornaro, M.; Valchera, A.; Cavuto, M.; Perna, G.; Di Nicola, M.; Serafini, G.; Carano, A.; Pompili, M.; Vellante, F.; et al. Eradicating Suicide at Its Roots: Preclinical Bases and Clinical Evidence of the Efficacy of Ketamine in the Treatment of Suicidal Behaviors. Int. J. Mol. Sci. 2018, 19, 2888. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Iasevoli, F.; Buonaguro, E.F.; De Berardis, D.; Fornaro, M.; Fiengo, A.L.C.; Martinotti, G.; Orsolini, L.; Valchera, A.; Di Giannantonio, M.; et al. Treating the Synapse in Major Psychiatric Disorders: The Role of Postsynaptic Density Network in Dopamine-Glutamate Interplay and Psychopharmacologic Drugs Molecular Actions. Int. J. Mol. Sci. 2017, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Hontanilla, B.; Parent, A.; Heras, S.D.L.; Giménez-Amaya, J.M. Distribution of calbindin D-28k and parvalbumin neurons and fibers in the rat basal ganglia. Brain Res. Bull. 1998, 47, 107–116. [Google Scholar] [CrossRef]
- Hontanilla, B.; Parent, A.; Giménez-Amaya, J.M. Parvalbumin and calbindin D-28k in the entopeduncular nucleus, subthalamic nucleus, and substantia nigra of the rat as revealed by double-immunohistochemical methods. Synapse 1997, 25, 359–367. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Fukuda, T. Immunohistochemical study on the neuronal diversity and three-dimensional organization of the mouse entopeduncular nucleus. Neurosci. Res. 2015, 94, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.L.; Saunders, A.; Huang, K.W.; Philson, A.C.; Goldman, M.; Macosko, E.Z.; McCarroll, S.A.; Sabatini, B.L. Genetically distinct parallel pathways in the entopeduncular nucleus for limbic and sensorimotor output of the basal ganglia. Neuron 2017, 94, 138–152.e5. [Google Scholar] [CrossRef]
- Stephenson-Jones, M.; Yu, K.; Ahrens, S.; Tucciarone, J.M.; Van Huijstee, A.N.; Mejia, L.A.; Penzo, M.A.; Tai, L.-H.; Wilbrecht, L.; Li, B. A basal ganglia circuit for evaluating action outcomes. Nature 2016, 539, 289–293. [Google Scholar] [CrossRef]
- Vincent, S.R.; Brown, J.C. Somatostatin immunoreactivity in the entopeduncular projection to the lateral habenula in the rat. Neurosci. Lett. 1986, 68, 160–164. [Google Scholar] [CrossRef]
- Benhamou, L.; Cohen, D. Electrophysiological characterization of entopeduncular nucleus neurons in anesthetized and freely moving rats. Front. Syst. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Nakahishi, H.; Kita, H.; Kitai, S. Intracellular study of rat entopeduncular nucleus neurons in an in vitro slice preparation: electrical membrane properties. Brain Res. 1990, 527, 81–88. [Google Scholar] [CrossRef]
- Kita, H. Neostriatal and globus pallidus stimulation induced inhibitory postsynaptic potentials in entopeduncular neurons in rat brain slice preparations. Neuroscience 2001, 105, 871–879. [Google Scholar] [CrossRef]
- Bevan, M.D.; Clarke, N.P.; Bolam, J.P. Synaptic Integration of Functionally Diverse Pallidal Information in the Entopeduncular Nucleus and Subthalamic Nucleus in the Rat. J. Neurosci. 1997, 17, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Lavian, H.; Korngreen, A. Inhibitory short-term plasticity modulates neuronal activity in the rat entopeduncular nucleus in vitro. Eur. J. Neurosci. 2016, 43, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Lavian, H.; Almog, M.; Madar, R.; Loewenstern, Y.; Bar-Gad, I.; Okun, E.; Korngreen, A. Dopaminergic Modulation of Synaptic Integration and Firing Patterns in the Rat Entopeduncular Nucleus. J. Neurosci. 2017, 37, 7177–7187. [Google Scholar] [CrossRef] [PubMed]
- Lavian, H.; Loewenstern, Y.; Madar, R.; Almog, M.; Bar-Gad, I.; Okun, E.; Korngreen, A. Dopamine receptors in the rat entopeduncular nucleus. Brain Struct. Funct. 2018, 223, 2673–2684. [Google Scholar] [CrossRef] [PubMed]
- Ferre, S.; O’Connor, W.T.; Svenningsson, P.; Bjorklund, L.; Lindberg, J.; Tinner, B.; Stromberg, I.; Goldstein, M.; Ogren, S.O.; Ungerstedt, U.; et al. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur. J. Neurosci. 1996, 8, 1545–1553. [Google Scholar] [CrossRef]
- Nauta, H.J.W.; Cole, M. Efferent projections of the subthalamic nucleus: An autoradiographic study in monkey and cat. J. Comp. Neurol. 1978, 180, 1–16. [Google Scholar] [CrossRef]
- Gorodetski, L.; Zeira, R.; Lavian, H.; Korngreen, A. Long-term plasticity of glutamatergic input from the subthalamic nucleus to the entopeduncular nucleus. Eur. J. Neurosci. 2018, 48, 2139–2151. [Google Scholar] [CrossRef]
- Delong, M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990, 13, 281–285. [Google Scholar] [CrossRef]
- Bergman, H.; Wichmann, T.; Karmon, B.; DeLong, M.R. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 1994, 72, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Magill, P.; Bolam, J.P.; Bevan, M.; Magill, P. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus–globus pallidus network. Neuroscience 2001, 106, 313–330. [Google Scholar] [CrossRef]
- Tseng, K.Y.; Kasanetz, F.; Kargieman, L.; Pazo, J.H.; Murer, M.; A Riquelme, L. Subthalamic nucleus lesions reduce low frequency oscillatory firing of substantia nigra pars reticulata neurons in a rat model of Parkinson’s disease. Brain Res. 2001, 904, 93–103. [Google Scholar] [CrossRef]
- Wichmann, T.; Bergman, H.; Starr, P.A.; Delong, M.R.; Watts, R.L.; Subramanian, T. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp. Brain Res. 1999, 125, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.A.; Boraud, T.; Maraton, S.; Haber, S.N.; Vaadia, E.; Bergman, H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson’s disease. J. Neurosci. 2002, 22, 4639–4653. [Google Scholar] [CrossRef] [PubMed]
- Nini, A.; Feingold, A.; Slovin, H.; Bergman, H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J. Neurophysiol. 1995, 74, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Ellens, D.J.; Leventhal, D.K. Electrophysiology of Basal Ganglia and Cortex in Models of Parkinson Disease. J. Park. Dis. 2013, 3, 241–254. [Google Scholar]
- Nelson, A.B.; Kreitzer, A.C. Reassessing Models of Basal Ganglia Function and Dysfunction. Annu. Rev. Neurosci. 2014, 37, 117–135. [Google Scholar] [CrossRef]
- Plotkin, J.L.; Goldberg, J.A. Thinking Outside the Box (and Arrow): Current Themes in Striatal Dysfunction in Movement Disorders. Neuroscientist 2018, 1073858418807887. [Google Scholar] [CrossRef]
- Sharott, A.; Gulberti, A.; Zittel, S.; Jones, A.A.T.; Fickel, U.; Münchau, A.; Köppen, J.A.; Gerloff, C.; Westphal, M.; Buhmann, C.; et al. Activity Parameters of Subthalamic Nucleus Neurons Selectively Predict Motor Symptom Severity in Parkinson’s Disease. J. Neurosci. 2014, 34, 6273–6285. [Google Scholar] [CrossRef]
- Sanders, T.H.; Clements, M.A.; Wichmann, T. Parkinsonism-related features of neuronal discharge in primates. J. Neurophysiol. 2013, 110, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Ketzef, M.; Spigolon, G.; Johansson, Y.; Bonito-Oliva, A.; Fisone, G.; Silberberg, G. Dopamine Depletion Impairs Bilateral Sensory Processing in the Striatum in a Pathway-Dependent Manner. Neuron 2017, 94, 855–865.e5. [Google Scholar] [CrossRef] [PubMed]
- Mallet, N.; Ballion, B.; Le Moine, C.; Gonon, F. Cortical Inputs and GABA Interneurons Imbalance Projection Neurons in the Striatum of Parkinsonian Rats. J. Neurosci. 2006, 26, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.G.; Marshall, J.D.; Ahanonu, B.; Wu, Y.-W.; Kim, T.H.; Grewe, B.F.; Zhang, Y.; Li, J.Z.; Ding, J.B.; Ehlers, M.D.; et al. Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 2018, 557, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Deffains, M.; Iskhakova, L.; Katabi, S.; Haber, S.N.; Israel, Z.; Bergman, H. Subthalamic, not striatal, activity correlates with basal ganglia downstream activity in normal and parkinsonian monkeys. eLife 2016, 5, e16443. [Google Scholar] [CrossRef] [PubMed]
- Heimer, G.; Bar-Gad, I.; Goldberg, J.A.; Bergman, H. Dopamine Replacement Therapy Reverses Abnormal Synchronization of Pallidal Neurons in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Primate Model of Parkinsonism. J. Neurosci. 2002, 22, 7850–7855. [Google Scholar] [CrossRef]
- Mallet, N.; Pogosyan, A.; Sharott, A.; Csicsvari, J.; Bolam, J.P.; Brown, P.; Magill, P.J. Disrupted Dopamine Transmission and the Emergence of Exaggerated Beta Oscillations in Subthalamic Nucleus and Cerebral Cortex. J. Neurosci. 2008, 28, 4795–4806. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, T.; Bergman, H.; Delong, M.R. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J. Neurophysiol. 1994, 72, 521–530. [Google Scholar] [CrossRef]
- Pasquereau, B.; DeLong, M.R.; Turner, R.S. Primary motor cortex of the parkinsonian monkey: Altered encoding of active movement. Brain 2016, 139, 127–143. [Google Scholar] [CrossRef]
- Freeze, B.S.; Kravitz, A.V.; Hammack, N.; Berke, J.D.; Kreitzer, A.C. Control of Basal Ganglia Output by Direct and Indirect Pathway Projection Neurons. J. Neurosci. 2013, 33, 18531–18539. [Google Scholar] [CrossRef]
- Roseberry, T.K.; Lee, A.M.; Lalive, A.L.; Wilbrecht, L.; Bonci, A.; Kreitzer, A.C. Cell-Type-Specific Control of Brainstem Locomotor Circuits by Basal Ganglia. Cell 2016, 164, 526–537. [Google Scholar] [CrossRef]
- Lemos, J.C.; Friend, D.M.; Kaplan, A.R.; Shin, J.H.; Rubinstein, M.; Kravitz, A.V.; Alvarez, V.A. Enhanced GABA transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron 2016, 90, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Okuno, H.; Bito, H. A new era for functional labeling of neurons: Activity-dependent promoters have come of age. Front. Neural Circuits 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Fourreau, L.; Bloch, B.; Fredholm, B.; Gonon, F.; Le Moine, C. Opposite tonic modulation of dopamine and adenosine on c-fos gene expression in striatopallidal neurons. Neuroscience 1999, 89, 827–837. [Google Scholar] [CrossRef]
- Day, M.; Wang, Z.; Ding, J.; An, X.; A Ingham, C.; Shering, A.F.; Wokosin, D.; Ilijic, E.; Sun, Z.; Sampson, A.R.; et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006, 9, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Gittis, A.H.; Hang, G.B.; LaDow, E.S.; Shoenfeld, L.R.; Atallah, B.V.; Finkbeiner, S.; Kreitzer, A.C. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron 2011, 71, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ke, Y.; Chan, D.C.; Qian, Z.-M.; Yung, K.K.; Ko, H.; Arbuthnott, G.W.; Yung, W.-H. Therapeutic Deep Brain Stimulation in Parkinsonian Rats Directly Influences Motor Cortex. Neuron 2012, 76, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A.; Tachibana, Y.; Chiken, S. Cause of parkinsonian symptoms: Firing rate, firing pattern or dynamic activity changes? Basal Ganglia 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Quiroga-Varela, A.; Walters, J.; Brazhnik, E.; Marin, C.; Obeso, J. What basal ganglia changes underlie the parkinsonian state? The significance of neuronal oscillatory activity. Neurobiol. Dis. 2013, 58, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Liang, L.; Kaneoke, Y.; Cao, X.; Papa, S.M. Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J. Neurophysiol. 2015, 113, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Bergman, H.; Wichmann, T.; Delong, M. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 1990, 249, 1436–1438. [Google Scholar] [CrossRef] [PubMed]
- Brown, P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 2007, 17, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Dostrovsky, J.; Bergman, H. Oscillatory activity in the basal ganglia--relationship to normal physiology and pathophysiology. Brain 2004, 127, 721–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neumann, W.-J.; Degen, K.; Schneider, G.-H.; Brücke, C.; Huebl, J.; Brown, P.; Kühn, A.A. Subthalamic Synchronized Oscillatory Activity Correlates With Motor Impairment in Patients With Parkinson’s Disease. Mov. Disord. 2016, 31, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Beudel, M.; Oswal, A.; Jha, A.; Foltynie, T.; Zrinzo, L.; Hariz, M.; Limousin, P.; Litvak, V. Oscillatory Beta Power Correlates With Akinesia-Rigidity in the Parkinsonian Subthalamic Nucleus. Mov. Disord. 2017, 32, 174–175. [Google Scholar] [CrossRef]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting Cortical Activity at Beta-Band Frequencies Slows Movement in Humans. Curr. Boil. 2009, 19, 1637–1641. [Google Scholar] [CrossRef]
- Chen, C.C.; Litvak, V.; Gilbertson, T.; Kühn, A.; Lu, C.S.; Lee, S.T.; Tsai, C.H.; Tisch, S.; Limousin, P.; Hariz, M. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease. Exp. Neurol. 2007, 205, 214–221. [Google Scholar] [CrossRef]
- Ermentrout, B.; Pascal, M.; Gutkin, B. The Effects of Spike Frequency Adaptation and Negative Feedback on the Synchronization of Neural Oscillators. Neural Comput. 2001, 13, 1285–1310. [Google Scholar] [CrossRef]
- Pavlides, A.; Hogan, S.J.; Bogacz, R. Computational Models Describing Possible Mechanisms for Generation of Excessive Beta Oscillations in Parkinson’s Disease. PLoS Comput. Boil. 2015, 11, e1004609. [Google Scholar] [CrossRef]
- Rubin, J.E. Computational models of basal ganglia dysfunction: The dynamics is in the details. Curr. Opin. Neurobiol. 2017, 46, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Shouno, O.; Tachibana, Y.; Nambu, A.; Doya, K. Computational Model of Recurrent Subthalamo-Pallidal Circuit for Generation of Parkinsonian Oscillations. Front. Neuroanat. 2017, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Plenz, D.; Kital, S.T. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 1999, 400, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Magill, P.J.; Bolam, J.P.; Bevan, M.D. Relationship of Activity in the Subthalamic Nucleus–Globus Pallidus Network to Cortical Electroencephalogram. J. Neurosci. 2000, 20, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.S.; Brown, P. Oscillations and the basal ganglia: Motor control and beyond. NeuroImage 2014, 85, 637–647. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Moore-Kochlacs, C.; Gu, X.; Boyden, E.S.; Han, X.; Kopell, N. Striatal origin of the pathologic beta oscillations in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2011, 108, 11620–11625. [Google Scholar] [CrossRef] [PubMed]
- Corbit, V.L.; Whalen, T.C.; Zitelli, K.T.; Crilly, S.Y.; Rubin, J.E.; Gittis, A.H. Pallidostriatal Projections Promote β Oscillations in a Dopamine-Depleted Biophysical Network Model. J. Neurosci. 2016, 36, 5556–5571. [Google Scholar] [CrossRef]
- Leblois, A.; Boraud, T.; Meissner, W.; Bergman, H.; Hansel, D. Competition between Feedback Loops Underlies Normal and Pathological Dynamics in the Basal Ganglia. J. Neurosci. 2006, 26, 3567–3583. [Google Scholar] [CrossRef]
- Nambu, A.; Tachibana, Y. Mechanism of parkinsonian neuronal oscillations in the primate basal ganglia: Some considerations based on our recent work. Front. Syst. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Tachibana, Y.; Iwamuro, H.; Kita, H.; Takada, M.; Nambu, A. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur. J. Neurosci. 2011, 34, 1470–1484. [Google Scholar] [CrossRef]
- Baufreton, J.; Atherton, J.F.; Surmeier, D.J.; Bevan, M.D. Enhancement of Excitatory Synaptic Integration by GABAergic Inhibition in the Subthalamic Nucleus. J. Neurosci. 2005, 25, 8505–8517. [Google Scholar] [CrossRef] [PubMed]
- Terman, D.; Rubin, J.E.; Yew, A.C.; Wilson, C.J. Activity Patterns in a Model for the Subthalamopallidal Network of the Basal Ganglia. J. Neurosci. 2002, 22, 2963–2976. [Google Scholar] [CrossRef] [PubMed]
- Degos, B.; Deniau, J.M.; Chavez, M.; Maurice, N. Chronic but not acute dopaminergic transmission interruption promotes a progressive increase in cortical beta frequency synchronization: Relationships to vigilance state and akinesia. Cereb. Cortex 2009, 19, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Galati, S.; Stanzione, P.; D’Angelo, V.; Fedele, E.; Marzetti, F.; Sancesario, G.; Procopio, T.; Stefani, A. The pharmacological blockade of medial forebrain bundle induces an acute pathological synchronization of the cortico–subthalamic nucleus–globus pallidus pathway. J. Physiol. 2009, 587, 4405–4423. [Google Scholar] [CrossRef] [PubMed]
- Leblois, A.; Meissner, W.; Bioulac, B.; Gross, C.E.; Hansel, D.; Boraud, T. Late emergence of synchronized oscillatory activity in the pallidum during progressive parkinsonism. Eur. J. Neurosci. 2007, 26, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Bar-Gad, I.; Heimer, G.; Ritov, Y.; Bergman, H. Functional Correlations between Neighboring Neurons in the Primate Globus Pallidus Are Weak or Nonexistent. J. Neurosci. 2003, 23, 4012–4016. [Google Scholar] [CrossRef]
- Avila, I.; Parr-Brownlie, L.C.; Brazhnik, E.; Castaneda, E.; Bergstrom, D.A.; Walters, J.R. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp. Neurol. 2010, 221, 307–319. [Google Scholar] [CrossRef]
- Brazhnik, E.; McCoy, A.J.; Novikov, N.; Hatch, C.E.; Walters, J.R. Ventral Medial Thalamic Nucleus Promotes Synchronization of Increased High Beta Oscillatory Activity in the Basal Ganglia–Thalamocortical Network of the Hemiparkinsonian Rat. J. Neurosci. 2016, 36, 4196–4208. [Google Scholar] [CrossRef]
- Sharott, A.; Magill, P.J.; Harnack, D.; Kupsch, A.; Meissner, W.; Brown, P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur. J. Neurosci. 2005, 21, 1413–1422. [Google Scholar] [CrossRef]
- Deffains, M.; Iskhakova, L.; Katabi, S.; Israel, Z.; Bergman, H. Longer β oscillatory episodes reliably identify pathological subthalamic activity in Parkinsonism. Mov. Disord. 2018, 33, 1609–1618. [Google Scholar] [CrossRef]
- Lobb, C.J.; Zaheer, A.K.; Smith, Y.; Jaeger, D. In vivo electrophysiology of nigral and thalamic neurons in alpha-synuclein-overexpressing mice highlights differences from toxin-based models of parkinsonism. J. Neurophysiol. 2013, 110, 2792–2805. [Google Scholar] [CrossRef] [PubMed]
- Guridi, J.; Alegre, M. Oscillatory activity in the basal ganglia and deep brain stimulation. Mov. Disord. 2017, 32, 64–69. [Google Scholar] [CrossRef] [PubMed]
- McConnell, G.C.; So, R.Q.; Hilliard, J.D.; Lopomo, P.; Grill, W.M. Effective deep brain stimulation suppresses low frequency network oscillations in the basal ganglia by regularizing neural firing patterns. J. Neurosci. 2012, 32, 15657–15668. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.; Ammari, R.; Bioulac, B.; García, L. Latest view on the mechanism of action of deep brain stimulation. Mov. Disord. 2008, 23, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.; Bergman, H.; Brown, P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007, 30, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, T.; Delong, M.R. Deep Brain Stimulation for Movement Disorders of Basal Ganglia Origin: Restoring Function or Functionality? Neurotherapeutics 2016, 13, 264–283. [Google Scholar] [CrossRef] [PubMed]
- Ranck, J.B. Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Res. 1975, 98, 417–440. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallet, N.; Delgado, L.; Chazalon, M.; Miguelez, C.; Baufreton, J. Cellular and Synaptic Dysfunctions in Parkinson’s Disease: Stepping Out of the Striatum. Cells 2019, 8, 1005. https://doi.org/10.3390/cells8091005
Mallet N, Delgado L, Chazalon M, Miguelez C, Baufreton J. Cellular and Synaptic Dysfunctions in Parkinson’s Disease: Stepping Out of the Striatum. Cells. 2019; 8(9):1005. https://doi.org/10.3390/cells8091005
Chicago/Turabian StyleMallet, Nicolas, Lorena Delgado, Marine Chazalon, Cristina Miguelez, and Jérôme Baufreton. 2019. "Cellular and Synaptic Dysfunctions in Parkinson’s Disease: Stepping Out of the Striatum" Cells 8, no. 9: 1005. https://doi.org/10.3390/cells8091005
APA StyleMallet, N., Delgado, L., Chazalon, M., Miguelez, C., & Baufreton, J. (2019). Cellular and Synaptic Dysfunctions in Parkinson’s Disease: Stepping Out of the Striatum. Cells, 8(9), 1005. https://doi.org/10.3390/cells8091005




