Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Human iPSC Culture and Expansion
2.2. Generation of CMs from iPSCs
2.3. Digestion of iPSC-CMs
2.4. Purification of iPSC-CMs
2.5. Maturation of iPSC-CMs
2.6. Immunofluorescence Staining
2.7. Morphological Analysis
2.8. Transmission Electron Microscopy
2.9. RNA Isolation and RT-qPCR
2.10. Protein Quantification
2.11. Western Blot
2.12. Mitochondrial Bioenergetic Analysis
2.13. Glycolysis Analysis
2.14. Statistical Analysis
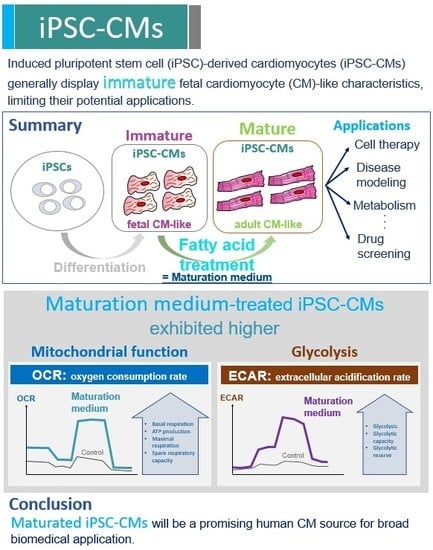
3. Results
3.1. Characterization of iPSCs and iPSC-derived CMs
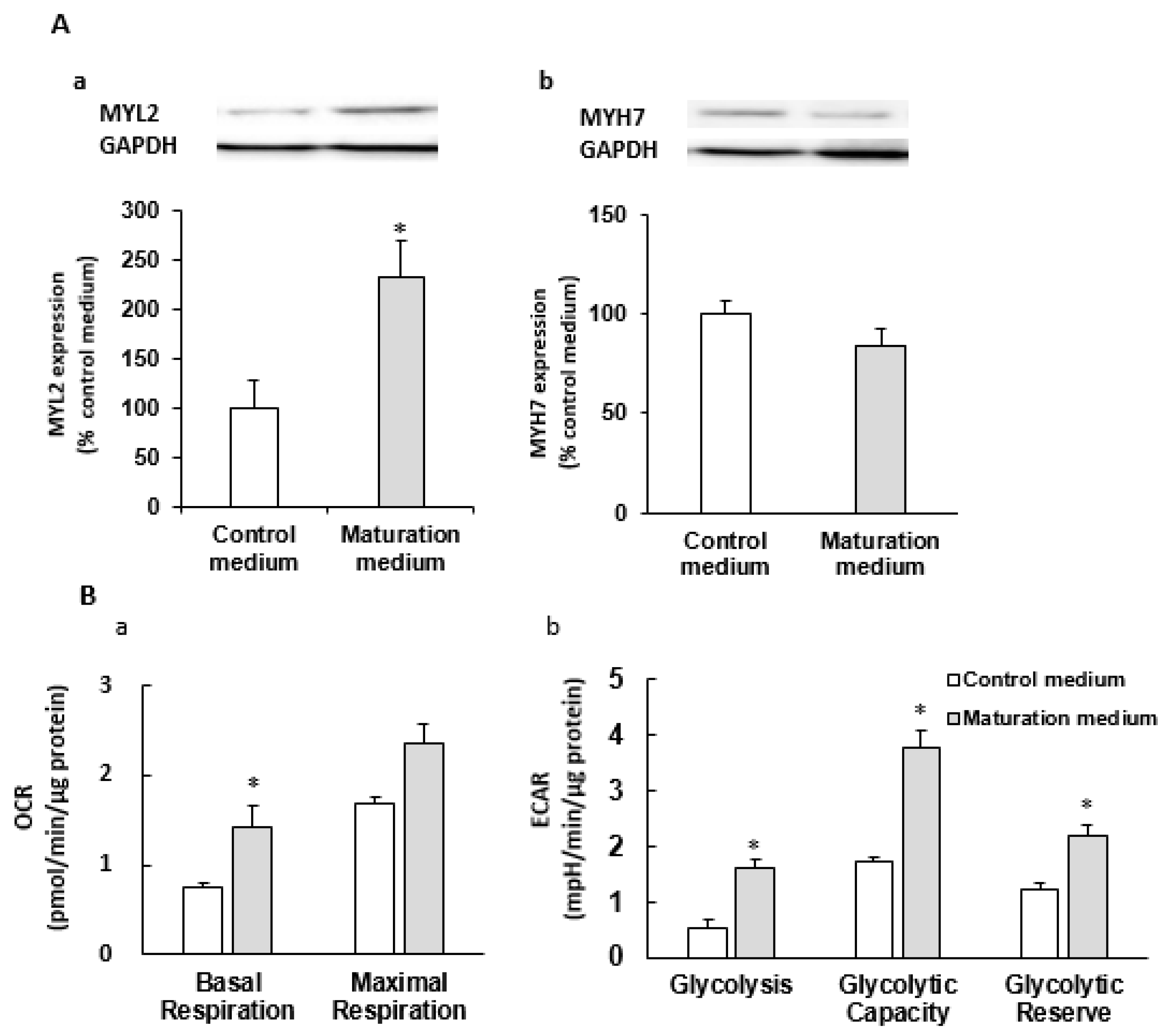
3.2. The Effect of Maturation Medium on Morphological and Ultrastructural Change, and CM-related Gene and Protein Expression in iPSC-CMs
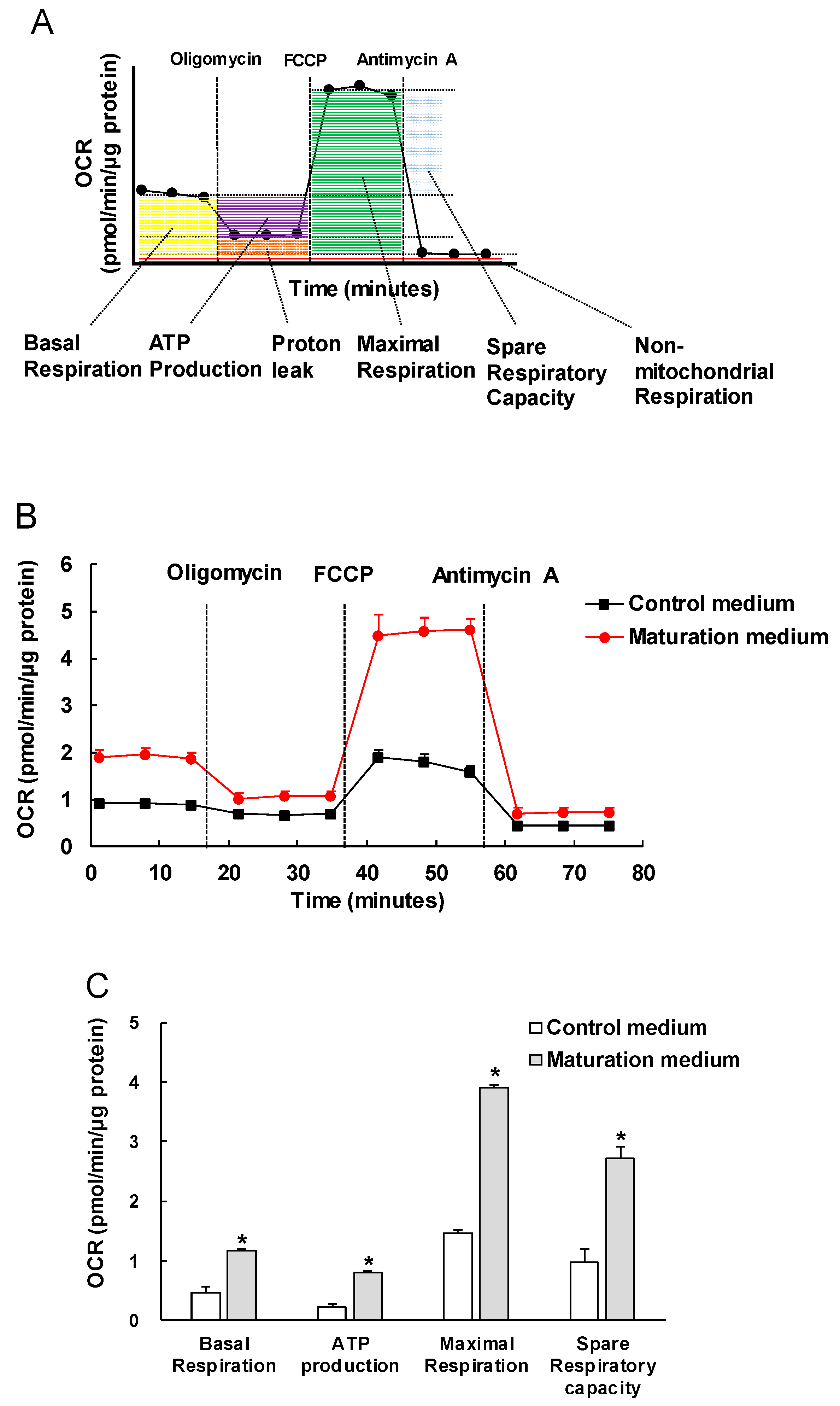
3.3. The Effect of Maturation Medium on the Mitochondrial Oxidation of iPSC-CMs
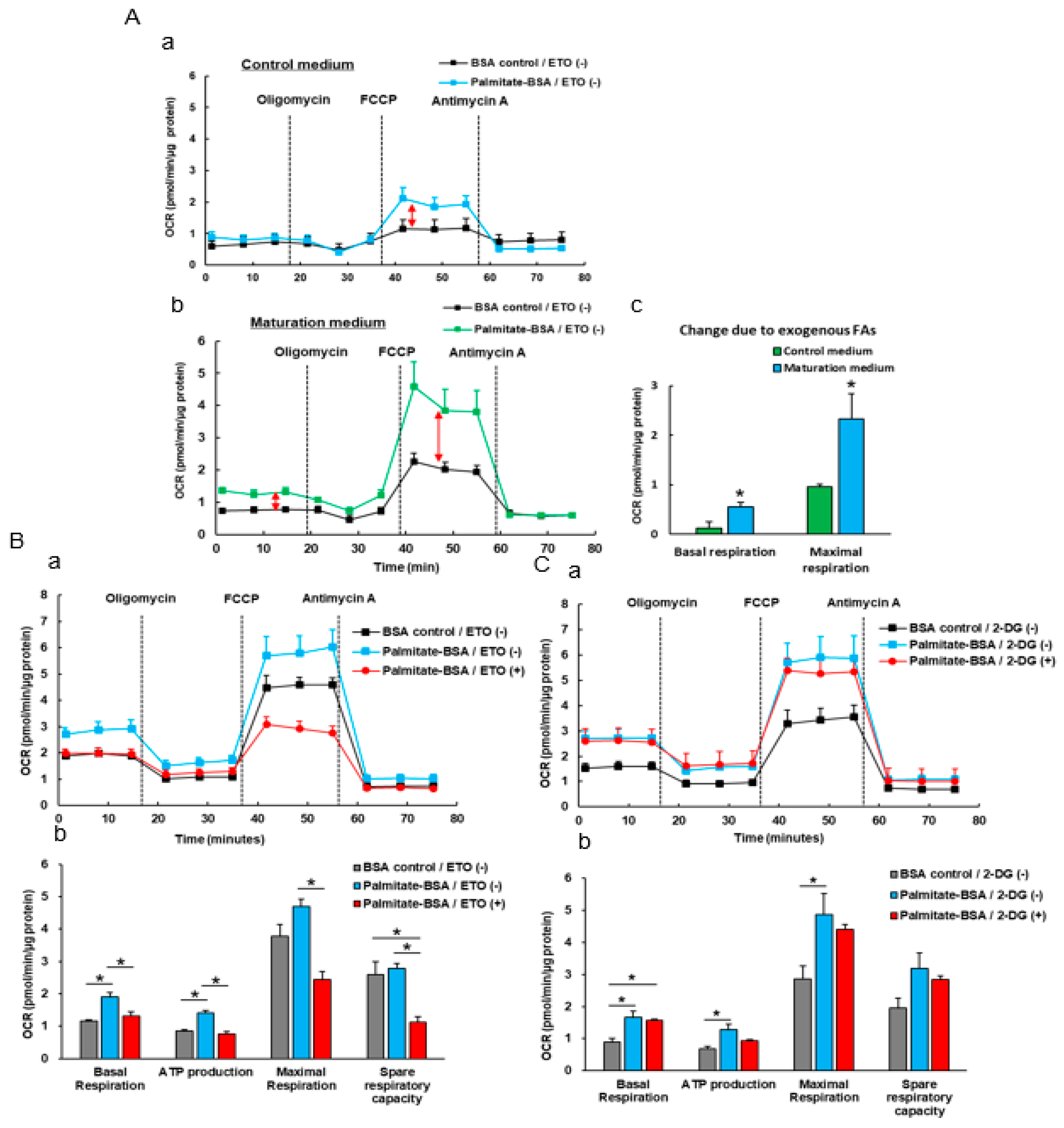
3.4. The Effect Of Exogenous Fatty Acids (Palmitate) on the OCR of iPSC-CMs
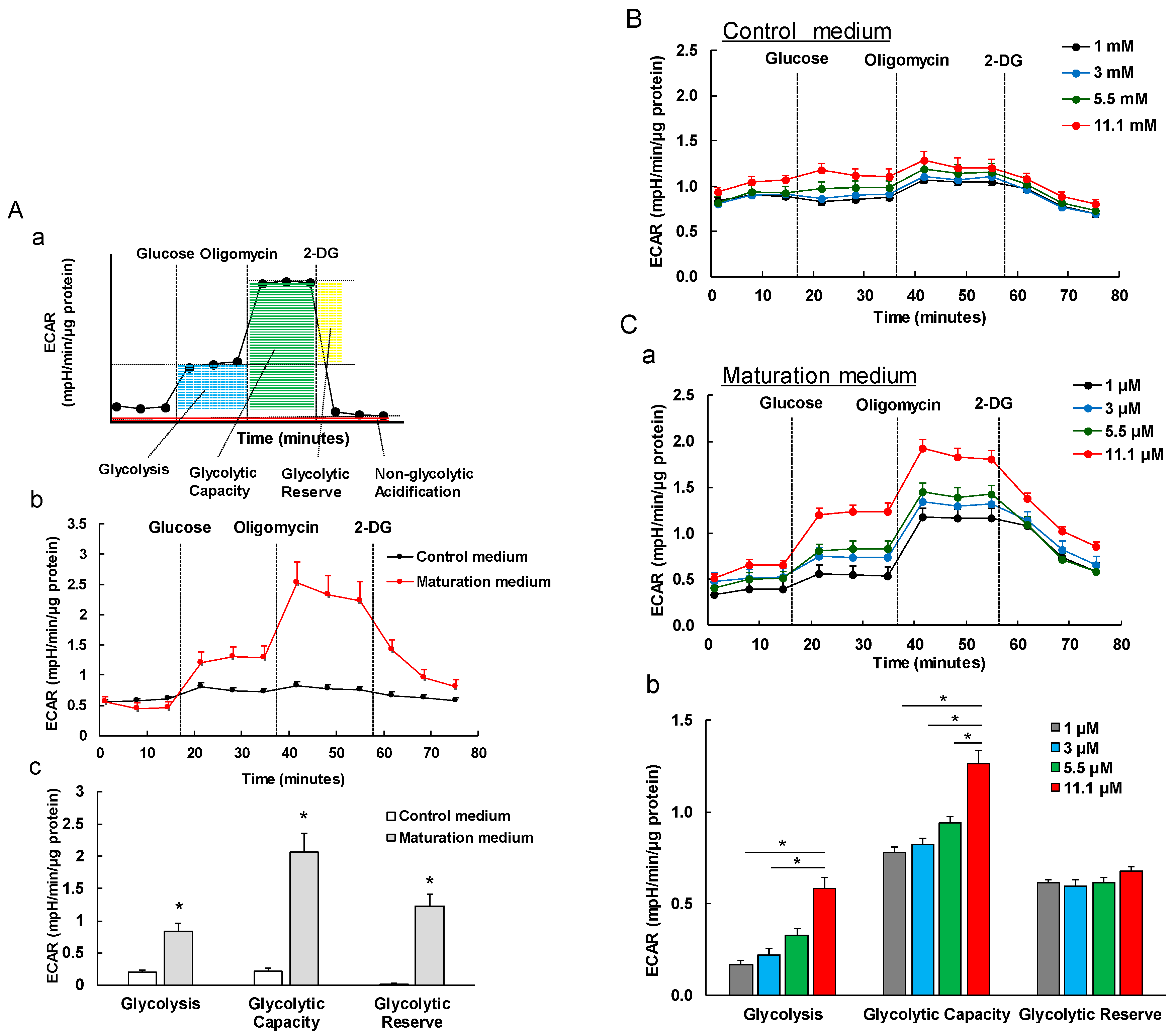
3.5. The Effect of Maturation Medium on the Glycolysis of iPSC-CMs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rubart, M.; Field, L.J. Cardiac regeneration: Repopulating the heart. Annu. Rev. Physiol. 2006, 68, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahid, E.; Siminiak, T.; Guarita-Souza, L.C.; Teixeira de Carvalho, K.A.; Gallo, P.; Shim, W.; Condorelli, G. Stem cell therapy in heart diseases: A review of selected new perspectives, practical considerations and clinical applications. Curr. Cardiol. Rev. 2011, 7, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Alt, E. Myocardial regeneration potential of adipose tissue-derived stem cells. Biochem Biophys. Res. Commun. 2010, 401, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yan, Y.; Song, Y.H.; Seidensticker, M.; Rabinovich, B.; Metzele, R.; Bankson, J.A.; Vykoukal, D.; Alt, E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur. Heart J. 2010, 31, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.J.; Lee, D.S.; Hsieh, P.C.H. Solving the puzzle of pluripotent stem cell-derived cardiomyocyte maturation: Piece by piece. Ann. Transl. Med. 2017, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, E.G.; Liang, P.; Lan, F.; Sanchez-Freire, V.; Simmons, C.; Gong, T.; Sharma, A.; Burridge, P.W.; Patlolla, B.; Lee, A.S.; et al. Screening drug-induced arrhythmia [corrected] using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation 2013, 128, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Ito, E.; Harada, A.; Matsuura, R.; Iseoka, H.; Sougawa, N.; Mochizuki-Oda, N.; et al. Development of In Vitro Drug-Induced Cardiotoxicity Assay by Using Three-Dimensional Cardiac Tissues Derived from Human Induced Pluripotent Stem Cells. Tissue Eng. Part C Methods 2018, 24, 56–67. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Wang, H.; Zhao, M.; Wang, C. Current status of induced pluripotent stem cells in cardiac tissue regeneration and engineering. Regen. Med. Res. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Bayzigitov, D.R.; Medvedev, S.P.; Dementyeva, E.V.; Bayramova, S.A.; Pokushalov, E.A.; Karaskov, A.M.; Zakian, S.M. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Afford New Opportunities in Inherited Cardiovascular Disease Modeling. Cardiol. Res. Pr. 2016, 2016, 3582380. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying Human Neurological Disorders Using Induced Pluripotent Stem Cells: From 2D Monolayer to 3D Organoid and Blood Brain Barrier Models. Compr. Physiol. 2019, 9, 565–611. [Google Scholar] [PubMed]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Canfield, S.G.; Zaja, I.; Godshaw, B.; Twaroski, D.; Bai, X.; Bosnjak, Z.J. High Glucose Attenuates Anesthetic Cardioprotection in Stem-Cell-Derived Cardiomyocytes: The Role of Reactive Oxygen Species and Mitochondrial Fission. Anesth. Analg. 2016, 122, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Canfield, S.G.; Sepac, A.; Sedlic, F.; Muravyeva, M.Y.; Bai, X.; Bosnjak, Z.J. Marked hyperglycemia attenuates anesthetic preconditioning in human-induced pluripotent stem cell-derived cardiomyocytes. Anesthesiology 2012, 117, 735–744. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Kikuchi, C.; Bienengraeber, M.; Canfield, S.; Koopmeiner, A.; Schafer, R.; Bosnjak, Z.J.; Bai, X. Comparison of Cardiomyocyte Differentiation Potential Between Type 1 Diabetic Donor-and Nondiabetic Donor-Derived Induced Pluripotent Stem Cells. Cell Transplant. 2015, 24, 2491–2504. [Google Scholar] [CrossRef]
- Chung, S.; Dzeja, P.P.; Faustino, R.S.; Perez-Terzic, C.; Behfar, A.; Terzic, A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pr. Cardiovasc. Med. 2007, 4 (Suppl. 1), S60–S67. [Google Scholar] [CrossRef]
- Cho, Y.M.; Kwon, S.; Pak, Y.K.; Seol, H.W.; Choi, Y.M.; Park, D.J.; Park, K.S.; Lee, H.K. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys. Res. Commun. 2006, 348, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- St John, J.C.; Ramalho-Santos, J.; Gray, H.L.; Petrosko, P.; Rawe, V.Y.; Navara, C.S.; Simerly, C.R.; Schatten, G.P. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells 2005, 7, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Jaswal, J.S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharm. 2010, 56, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, B.M.; Stoehr, A.; Schulze, M.L.; Patel, S.; Gucek, M.; Mannhardt, I.; Funcke, S.; Murphy, E.; Eschenhagen, T.; Hansen, A. Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes. Stem Cell Rep. 2018, 10, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Minami, I.; Braas, D.; Pappoe, H.; Wu, X.; Sagadevan, A.; Vergnes, L.; Fu, K.; Morselli, M.; Dunham, C.; et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. eLife 2017, 6, e29330. [Google Scholar] [CrossRef] [PubMed]
- Drawnel, F.M.; Boccardo, S.; Prummer, M.; Delobel, F.; Graff, A.; Weber, M.; Gerard, R.; Badi, L.; Kam-Thong, T.; Bu, L.; et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014, 9, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Ban, K.; Bae, S.; Yoon, Y.S. Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Theranostics 2017, 7, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Metzele, R.; Alt, C.; Bai, X.; Yan, Y.; Zhang, Z.; Pan, Z.; Coleman, M.; Vykoukal, J.; Song, Y.H.; Alt, E. Human adipose tissue-derived stem cells exhibit proliferation potential and spontaneous rhythmic contraction after fusion with neonatal rat cardiomyocytes. FASEB J. 2011, 25, 830–839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bosnjak, Z.J.; Yan, Y.; Canfield, S.; Muravyeva, M.Y.; Kikuchi, C.; Wells, C.W.; Corbett, J.A.; Bai, X. Ketamine induces toxicity in human neurons differentiated from embryonic stem cells via mitochondrial apoptosis pathway. Curr. Drug Saf. 2012, 7, 106–119. [Google Scholar] [CrossRef]
- Olson, J.M.; Yan, Y.; Bai, X.; Ge, Z.D.; Liang, M.; Kriegel, A.J.; Twaroski, D.M.; Bosnjak, Z.J. Up-regulation of microRNA-21 mediates isoflurane-induced protection of cardiomyocytes. Anesthesiology 2015, 122, 795–805. [Google Scholar] [CrossRef]
- Twaroski, D.M.; Yan, Y.; Zaja, I.; Clark, E.; Bosnjak, Z.J.; Bai, X. Altered Mitochondrial Dynamics Contributes to Propofol-induced Cell Death in Human Stem Cell-derived Neurons. Anesthesiology 2015, 123, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Qiao, S.; Kikuchi, C.; Zaja, I.; Logan, S.; Jiang, C.; Arzua, T.; Bai, X. Propofol Induces Apoptosis of Neurons but Not Astrocytes, Oligodendrocytes, or Neural Stem Cells in the Neonatal Mouse Hippocampus. Brain Sci. 2017, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Birket, M.J.; Mummery, C.L. Pluripotent stem cell derived cardiovascular progenitors—A developmental perspective. Dev. Biol. 2015, 400, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.J.; Zhang, J.H.; Azarin, S.M.; Zhu, K.X.; Hazeltine, L.B.; Bao, X.P.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [PubMed]
- Talkhabi, M.; Aghdami, N.; Baharvand, H. Human cardiomyocyte generation from pluripotent stem cells: A state-of-art. Life Sci. 2016, 145, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Halloin, C.; Schwanke, K.; Lobel, W.; Franke, A.; Szepes, M.; Biswanath, S.; Wunderlich, S.; Merkert, S.; Weber, N.; Osten, F.; et al. Continuous WNT Control Enables Advanced hPSC Cardiac Processing and Prognostic Surface Marker Identification in Chemically Defined Suspension Culture. Stem Cell Rep. 2019, 13, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Ye, L. Maturation of Pluripotent Stem Cell-Derived Cardiomyocytes: A Critical Step for Drug Development and Cell Therapy. J. Cardiovasc. Transl. Res. 2018, 11, 375–392. [Google Scholar] [CrossRef]
- Scuderi, G.J.; Butcher, J. Naturally Engineered Maturation of Cardiomyocytes. Front. Cell Dev. Biol. 2017, 5, 50. [Google Scholar] [CrossRef]
- Bedada, F.B.; Chan, S.S.K.; Metzger, S.K.; Zhang, L.Y.; Zhang, J.Y.; Garry, D.J.; Kamp, T.J.; Kyba, M.; Metzger, J.M. Acquisition of a Quantitative, Stoichiometrically Conserved Ratiometric Marker of Maturation Status in Stem Cell-Derived Cardiac Myocytes. Stem Cell Rep. 2014, 3, 594–605. [Google Scholar] [CrossRef]
- Rana, P.; Anson, B.; Engle, S.; Will, Y. Characterization of human-induced pluripotent stem cell-derived cardiomyocytes: Bioenergetics and utilization in safety screening. Toxicol. Sci. 2012, 130, 117–131. [Google Scholar] [CrossRef]
- Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Teixeira, A.; Domian, I.; Serra, M.; Alves, P.M. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep. 2017, 7, 8590. [Google Scholar] [CrossRef] [PubMed]
- Piquereau, J.; Caffin, F.; Novotova, M.; Lemaire, C.; Veksler, V.; Garnier, A.; Ventura-Clapier, R.; Joubert, F. Mitochondrial dynamics in the adult cardiomyocytes: Which roles for a highly specialized cell? Front. Physiol. 2013, 4, 102. [Google Scholar] [CrossRef] [PubMed]
- Hom, J.R.; Quintanilla, R.A.; Hoffman, D.L.; de Mesy Bentley, K.L.; Molkentin, J.D.; Sheu, S.S.; Porter, G.A., Jr. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev. Cell 2011, 21, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Keung, W.; Boheler, K.R.; Li, R.A. Developmental cues for the maturation of metabolic, electrophysiological and calcium handling properties of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Linders, A.; Yamak, A.; Correia, C.; Kijlstra, J.D.; Garakani, A.; Xiao, L.; Milan, D.J.; van der Meer, P.; Serra, M.; et al. Metabolic Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes by Inhibition of HIF1alpha and LDHA. Circ. Res. 2018, 123, 1066–1079. [Google Scholar] [CrossRef]
- Wen, J.Y.; Wei, C.Y.; Shah, K.; Wong, J.; Wang, C.; Chen, H.S. Maturation-Based Model of Arrhythmogenic Right Ventricular Dysplasia Using Patient-Specific Induced Pluripotent Stem Cells. Circ. J. 2015, 79, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Gaull, G.E. Taurine in pediatric nutrition: Review and update. Pediatrics 1989, 83, 433–442. [Google Scholar]
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; van Zanten, A.R.H. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. 2019, 38, 982–995. [Google Scholar] [CrossRef]
- Saito, Y.; Yoshida, Y.; Takahashi, K.; Niki, E. Cell death caused by selenium deficiency and protective effect of antioxidants. Free Radic. Biol. Med. 2003, 35, S46. [Google Scholar] [CrossRef]
- Wanet, A.; Arnould, T.; Najimi, M.; Renard, P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 2015, 24, 1957–1971. [Google Scholar] [CrossRef]



| Medium Name | Component | Supplier | Cetology Number | Final Concentration |
|---|---|---|---|---|
| iPSC culture medium | mTeSR1 | STEMCELL Technologies | 85850 | 1× |
| Penicillin-Streptomycin | Thermo Fisher Scientific | 15140122 | 100× | |
| Control medium | RPMI 1640 Medium | Thermo Fisher Scientific | 11875093 | 1× |
| B-27 Supplement (50X), serum free | Thermo Fisher Scientific | 17504044 | 50× | |
| Penicillin-Streptomycin | Thermo Fisher Scientific | 15140122 | 100× | |
| Lactate purification medium | DMEM, no Glucose | Thermo Fisher Scientific | 11966025 | 1× |
| Sodium L-lactate | Sigma-Aldrich | 71718 | 4 mM | |
| Penicillin-Streptomycin | Thermo Fisher Scientific | 15140122 | 100× | |
| Maturation medium | DMEM, no Glucose | Thermo Fisher Scientific | 11966025 | 1× |
| HEPES (1M) | Thermo Fisher Scientific | 15630080 | 10 mM | |
| L-carnitine inner salt | Sigma-Aldrich | C0158 | 2 mM | |
| Creatine | Sigma-Aldrich | C0780 | 5 mM | |
| Taurine | Sigma-Aldrich | T0625 | 5 mM | |
| MEM (non-essential amino acids) Solution | Thermo Fisher Scientific | 11140076 | 100× | |
| Insulin, Transferrin, Selenium Solution (ITS-G) | Thermo Fisher Scientific | 41400045 | 100× | |
| Linoleic Acid-Oleic Acid-albumin | Sigma-Aldrich | L9655 | 100× | |
| Penicillin-Streptomycin | Thermo Fisher Scientific | 15140122 | 100× |
| Gene | Forward Primer Sequence (5′to 3′) | Reverse Primer Sequence (5′to 3′) | PCR Product Length (bp) |
|---|---|---|---|
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA | 131 |
| TNNT2 | AGCATCTATAACTTGGAGGCAGAG | TGGAGACTTTCTGGTTATCGTTG | 112 |
| TNNI1 | CAGCTCCACGAGGACTGAAC | CTCTTCAGCAAGAGTTTGCG | 101 |
| TNNI3 | CCTCAAGCAGGTGAAGAAGG | CAGTAGGCAGGAAGGCTCAG | 134 |
| MYL2 | ACATCATCACCCACGGAGAAGAGA | ATTGGAACATGGCCTCTGGATGGA | 247 |
| MYL3 | GCCCTAAGGAGGTCGAGTTT | ACACTGCCCGTAGGTGATCT | 137 |
| MYL4 | GACTTCACTGCCGACCAGAT | CTCGGCATTGGTAGGGTTCT | 90 |
| MYL7 | CCGTCTTCCTCACGCTCTT | TGAACTCATCCTTGTTCACCAC | 120 |
| MYH6 | GCTGGCCCTTCAACTACAGA | CTTCTCCACCTTAGCCCTGG | 106 |
| MYH7 | GAGGACAAGGTCAACACCCT | CGCACCTTCTTCTCTTGCTC | 95 |
| MYBPC3 | GGCATGCTAAAGAGGCTCAA | TCTTGTGGCCTTTGCTCAC | 103 |
| SCN5A | ACTGCACAATGACCAGCAGGA | GTGAGAAGTGCTCGATTAGTTCAGACA | 77 |
| KCNJ4 | CCTTGGGATGTAGCGCC | GTCCGTGCATGTCCTGAAG | 109 |
| KCNJ12 | TGGATCCTTTCCAGTTGGTG | CGGCTCCTCTTGAGTTCTATCTT | 114 |
| KCNH2 | CACCGCCCTGTACTTCATCT | AGGCCTTGCATACAGGTTCA | 118 |
| KCNQ1 | CGCCTGAACCGAGTAGAAGA | TGAAGCATGTCGGTGATGAG | 71 |
| KCND2 | CTACCTGTTCCGGTGATTGTATCC | TCTTTTGTGCCCTTCGTTTGT | 82 |
| KCND3 | CCAATTCTAACCTGCCAGCTAC | CTGCTTTCAAATTAAGGCTGGA | 122 |
| CACNA1C | CAATCTCCGAAGAGGGGTTT | TCGCTTCAGACATTCCAGGT | 78 |
| GJA1 | CAATCACTTGGCGTGACTTC | AAAGGCAGACTGCTCATCTC | 211 |
| SERCA2A | TTGGCAGGAGCGGAACGCAG | CAACCCGCAGCGTGGTGGAT | 209 |
| RYR2 | AAGCCCTCTCGTCTGAAACA | CCACCCAGACATTAGCAGGT | 193 |
| PPARα | ATTACGGAGTCCACGCGTGTG | TTGTCATACACCAGCTTGAGT | 76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horikoshi, Y.; Yan, Y.; Terashvili, M.; Wells, C.; Horikoshi, H.; Fujita, S.; Bosnjak, Z.J.; Bai, X. Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells 2019, 8, 1095. https://doi.org/10.3390/cells8091095
Horikoshi Y, Yan Y, Terashvili M, Wells C, Horikoshi H, Fujita S, Bosnjak ZJ, Bai X. Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells. 2019; 8(9):1095. https://doi.org/10.3390/cells8091095
Chicago/Turabian StyleHorikoshi, Yuichi, Yasheng Yan, Maia Terashvili, Clive Wells, Hisako Horikoshi, Satoshi Fujita, Zeljko J. Bosnjak, and Xiaowen Bai. 2019. "Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes" Cells 8, no. 9: 1095. https://doi.org/10.3390/cells8091095
APA StyleHorikoshi, Y., Yan, Y., Terashvili, M., Wells, C., Horikoshi, H., Fujita, S., Bosnjak, Z. J., & Bai, X. (2019). Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells, 8(9), 1095. https://doi.org/10.3390/cells8091095